 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
SERPINA1 gene has been implicated in the pathogenesis of chronic obstructive pulmonary disease (COPD), while smoking is a known risk factor for COPD. Little is known on the effect of SERPINA1 gene and its interaction with smoking in the Chinese population. In this study, the effect of SERPINA1 gene polymorphisms on COPD risk and its interaction with smoking status has been investigated.
Method
A total of 120 COPD patients and 481 healthy controls were recruited at The Armed Police Corps Hospital. Data on demographic variables, smoking status, history of occupational dust exposure, and allergies were collected. Genotyping for single nucleotide polymorphism’s (SNP) rs1243160, rs2854254, and rs8004738 was performed in all participants.
Results
SNP rs8004738 genotype was associated with a significantly higher risk for COPD (odds ratio (OR) =1.835, 95% confidence interval (CI): 1.002–3.360), whereas SNPs rs1243160 and rs2854254 did not exhibit such an association. Smoking habit also significantly increased the risk for COPD (OR =2.306, 95% CI: 1.537–3.459). On stepwise logistic regression analysis, advanced age, smoking, and SNP rs8004738 variant were associated with increased risk for COPD, while female gender and higher educational status decreased the risk. On additive interaction analysis, a significant interactive effect of SNP rs8004738 and smoking was observed in this population (relative excess risk due to interaction =0.478; attributable proportion due to interaction (AP) =0.123; S=1.197).
Conclusion
SNP rs8004738 of SERPINA1 gene significantly interacted with smoking status and was associated with a higher risk for COPD in the Chinese population.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by a progressive decrease in pulmonary ventilation due to obstruction of small airways. Multiple genetic and environmental factors are implicated in the causation of COPD. Genome-wide association studies have implicated genetic polymorphisms including that of SERPINA1 gene in its pathogenesis. SERPINA1 gene, located on chromosome 14q32.1, encodes for a serine protease inhibitor (alpha 1 antitrypsin, AAT), which targets elastase, plasmin, thrombin, trypsin, chymotrypsin, and plasminogen activator, and has been shown to play a role in the development of COPD.Citation1 A null mutation of SERPINA1 gene was shown to significantly increase the risk of severe emphysema.Citation2 Single nucleotide polymorphisms (SNPs) of SERPINA1 have also linked to a heightened susceptibility to COPD.Citation3,Citation4 However, majority of these studies were performed in Caucasians, definitive evidence of such an association is yet to be demonstrated in Asian population.Citation5,Citation6
An earlier study that investigated potential associations of different SNPs found that only rs8004738 allele frequency was significantly different between COPD and control after adjusting for confounders.Citation7 Smoking is a leading environmental risk factor for COPD with 2–4 times higher risk in smokers compared to that in nonsmokers.Citation8,Citation9 As there is a high prevalence of smokers in China,Citation10 smoking would continue to be a predominant risk factor for COPD. Of note, a synergistic effect between genetic polymorphisms and environmental factors has also been implicated in the pathogenesis of COPD.Citation11,Citation12 Therefore, this study aimed to investigate rs8004738, which only has not been studied in Chinese population and other two nearby SNPs, rs1243160 and rs2854254, were also selected to assesse their potential interactions with smoking in the association with the risk of COPD.
Materials and methods
Study participants
Patients diagnosed with COPD and admitted to The Armed Police Corps Hospital between September 2014 and September 2015 were eligible for participation. Patients who fulfilled the diagnostic criteria for COPD laid down in the GOLD (Global initiative for chronic Obstructive Lung Disease) Guidelines 2015 were included.Citation13 The criteria included 1) cough, expectoration, and/or wheeze for >3 months in a year; 2) FEV1/FVC <70% and FEV1 <80% on lung function tests after administration of bronchodilator drug.
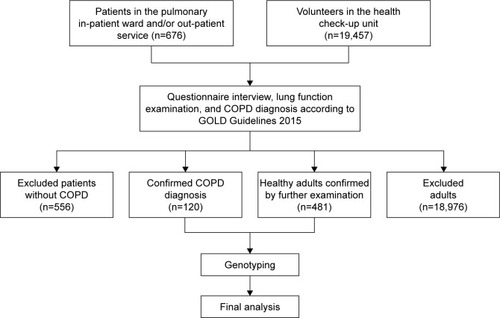
The exclusion criteria included 1) patients unable to perform lung function tests; 2) history of bronchial asthma, interstitial lung disease, bronchiectasis, pulmonary embolism, lung cancer, active tuberculosis, or any type of chest surgery or mental disease which would affect lung function. A total of 676 patients met the inclusion criteria, whereas 556 patients met the exclusion criteria. A total of 120 patients were included in the case group for final analysis.
Healthy volunteers who underwent routine health checkup at The Armed Police Corps Hospital between September 2014 and September 2015 were recruited as controls (n=19,457). Exclusion criteria for controls was individuals suffering from any organic disease based on clinical examination, chest X-ray, laboratory investigations (complete blood counts, biochemical tests, urine or feces test, and those who were unable to undergo lung function test). Therefore, a total of 481 healthy controls were included in the final analysis (). All participants were Han Chinese and were unrelated to each other. This study was approved by the Ethic Committee of General Hospital of Chinese People’s Armed Police Forces, People’s Republic of China. Each participant signed a consent form before enrolment in this study.
Figure 1 The flow chart of population selection.
Abbreviation: GOLD, Global initiative for chronic Obstructive Lung Disease.
Collection of demographic data
A standard questionnaire was administered to participants by trained personnel for data collection on following demographic variables: age, gender, body mass index (BMI), smoking status, education level, type of utensils and fuel used for cooking, occupational exposure to dust, and history of allergies.
Smoking index
Smoking status of participants was collected during their clinical visits. Smokers were defined as use of at least one cigarette a day, continuously for more than 1 year. Smoking index was calculated as follows:
DNA extraction, polymerase chain reaction, and genotyping
Approximately 2 mL of fasting blood was collected from each participant and stored at −80°C. DNA was extracted using DNA extraction kit (Tiangen Biotech Co. Ltd, Beijing, China). Primer pairs for each SNP were shown in . Primers were designed by Beijing Microread Genetics Co. Ltd (Beijing, China) (). Genotype was determined using SNaPshot assay (SNaPshot™ Multiplex Kit; Ukzybiotech. Ltd, Beijing, China) and sequencing was performed by Beijing Double Gene Technology Co., Ltd (Beijing, China). Polymerase chain reaction buffer system included 4.0 μL 2.5× buffer, 0.2 μL polymerase chain reaction primer (10 μM, F + R), 0.1 μL Taq DNA polymerase, 1.0 μL DNA templates (50–100 ng/μL), and 4.7 μL double distilled water. Genotyping data were analyzed using software GeneMapper v3.2 (Applied Biosystems Inc., Foster City, CA, USA).
Table 1 SNPs and primers design
Statistical analyses
Data analyses were performed using SPSS software, ver 17.0 (SPSS Inc., Chicago, IL, USA). Quantitative data are presented as mean ± standard deviation. Independent t-test was used to compare the differences between age. Between-group differences with respect to baseline demographic variables and SNP genotypes were assessed by chi-squared test (χ2). Further, Hardy–Weinberg genetic equilibrium test was performed. Stepwise logistic regression analysis was performed to identify independent risk factors. Odds ratios (ORs) and confidence intervals (CIs) were presented by multivariable logistic regression analysis. Additive interactions between SNP genotypes and smoking habit in association with COPD was accessed using following equations:
Synergy index
A value of P<0.05 was considered statistically significant.
Results
Baseline characteristics of study participants
COPD group consisted of 92 males and 28 females (mean age: 65.3±11.3 years); the control group consisted of 322 males and 159 females (mean age: 49.3±10.4 years) (). Statistically significant between-group differences were observed with respect to age and gender distribution (P<0.001 and P=0.040, respectively). Smoking status, BMI, and educational level were also significantly different between the two groups (higher rate for smokers, higher BMI, and lower educational level in the COPD group; P<0.001 for all). No significant difference was observed with respect to type of utensils and fuel used for cooking, and for occupational exposure to dust, or history of allergy ().
Table 2 Baseline characteristics of subjects by study group
Risk factors for COPD
On stepwise logistic regression analysis, advanced age (OR: 1.121, 95% CI: 1.088–1.155, P<0.001), current smoking status (OR: 1.011, 95% CI: 1.006–1.016, P<0.001), and mutation at rs8004738 of SERPINA1 (OR: 3.948, 95% CI: 1.688–9.232, P=0.002) was significantly associated with a higher risk for COPD (). Female gender (OR: 0.348, 95% CI: 0.154–0.787, P=0.011) and higher educational status (OR: 0.323, 95% CI: 0.186–0.561, P<0.001) showed a significant negative association with COPD ().
Table 3 Results of stepwise logistic regression analyses showing risk factors for COPD
Association between smoking index and COPD
Compared to nonsmokers, smokers had a significantly higher risk of COPD after adjusting for age, gender, educational levels, and SNP variations (OR: 2.306, 95% CI: 1.537–3.459, P<0.001) (). Subgroup analysis by smoking index showed a dose response effect of smoking on COPD. Participants who had a smoking index >800 had four times higher risk for COPD compared to people who barely smoked (OR: 4.336, 95% CI: 2.094–8.981, P<0.001).
Table 4 Association between smoking index and COPD risk
SNP genotypes
Genotype frequency of SNP rs8004738, rs2854254, and rs1243160 fulfilled the Hardy–Weinberg genetic equilibrium test (all P>0.05; data not shown). There was no difference between allele frequencies for SNP rs2854254 and rs1243160. However, there was a significant difference in frequency of rs8004738 genotype between cases and controls; a higher frequency of A allele was observed in the COPD group (P=0.042) (). This indicates that mutations of A allele were associated with a higher risk of COPD (OR =1.373, 95% CI =1.028–1.832, P=0.031).
Table 5 SNP genotypes frequencies in COPD case and control groups
Interaction between smoking status and rs8004738 genotype
In nonsmoking participants, carriers of either G/A or A/A had a higher risk for COPD; the difference was statistically significant (OR: 1.835, 95% CI: 1.002–3.360). Among GG carriers, smokers showed a modestly higher risk for COPD compared to nonsmokers (OR: 2.582, 95% CI: 1.303–5.117). Among smokers, with non-GG genotype, the risk of COPD was significantly higher than that of nonsmokers with GG genotype (OR: 3.895, 95% CI: 2.120–7.157), which was indicative of a significant interactive effect (RERI =0.478; AP =0.123; S=1.197) ().
Table 6 Interactions between smoking and rs8004738 genotypes in association with chronic obstructive pulmonary disease
Discussion
In this study, we selected three SNPs, rs8004738, rs1243160, and rs2854254. rs8004738 was selected based on Chappel et al where they found it was the only SNP with a significantly different allele frequency between COPD and controls.Citation7 In this study, due to the limited budget, we could only assess two more SNPs, therefore, we picked rs1243160 and rs2854254, which is closest to rs8004738 in genetic sequence. Our results showed that a G→A allele mutation of SNP rs8004738 in SERPINA1 gene was associated with a higher risk for COPD. More importantly, this mutation may also interact with an individual’s smoking habit. The results suggest that both risk factors may contribute synergistically to COPD. Other two SNPs, rs1243160 and rs2854254, were not associated with COPD. To our knowledge, this is the first study to investigate the interrelation between genetic susceptibility and a modifiable life style factor associated with COPD in Chinese population.
The most important reported mutation of gene SERPINA1 was the null mutation, which is known to cause a severe deficiency of AATCitation2 and enhances the susceptibility of the lungs to potential damage caused by proteases. The cross-sectional studies involving the heterozygotes for of this mutation (PiMZ) also suggested a two times higher risk for COPD.Citation3 In this study, we did not examine the Pi genotype or the level of AAT, which is also our limitation. A previous study from Hong Kong demonstrated a very low prevalence of PiMZ mutation in Chinese population,Citation14 indicating that this mutation has limited effect on the development of COPD in Chinese. Besides this mutation, other polymorphisms of SERPINA1 gene were also reported to be associated with emphysema or COPD.Citation15,Citation16 Gene polymorphism associated with COPD or poor lung function does not infer association with altered serum AAT level. Thun et al did not find association of rs8004738 with serum AAT level conditioned for M allele mutation.Citation17 Therefore, whether SNP found in our study is an independent factor associated with serum AAT levels needs to be determined. Based on our results, SNP rs8004738 was associated with higher risk for COPD, which is consistent with previous findings in Caucasian population.Citation7 However, a previous study in a Japanese population demonstrated a lack of association between rs8004738 homozygotes and risk for emphysema.Citation18 The disparity between their results and ours are probably due to the difference in disease category and ethnic background. Future studies with larger sample size and simultaneously tested serum AAT levels can further elucidate the role of rs8004738 in the development of COPD.
The potential interaction between smoking and genetic mutations in the causation of COPD has been reported. De Jong et al reported that in utero and adult exposure to tobacco modified the protective effect of glutathione S transferase omega gene polymorphism on lung function.Citation12 Furthermore, a study conducted on European population revealed a significant interaction between SERPINA1 PiMZ genotype and occupational exposure to vapors, dust, gases, and fumes in association with decrease in lung function.Citation11 In this study, we also observed that the interaction between SNP rs8004738 and smoking contributed significantly to the risk of COPD in Chinese. The findings highlight the need for a ban on use of tobacco and concerted efforts at health education for management and prevention of COPD in China. Taken together, these data provided scientific evidence on the synergistic effects of genetic and environmental factors on the development of COPD.
Our study also ruled out the potential association of SNP rs1243160 and rs2854254 with the risk of COPD. The association of either rs1243160 or rs2854254 with COPD has not been reported earlier. It remained possible that the lack of association of rs2854254 and rs1243160 was due to our limited sample size. Therefore, further study with large sample size is required to further elucidate the role of these two SNPs.
The case–control study design and retrospective nature of the analysis are key study limitations. There is a lack of data on serum AAT levels and limited information of participant’s with respect to exposure to passive smoking.
Conclusion
This study demonstrated that SNP rs8004738 in SERPINA1 gene significantly interacted with smoking status, and that both in association lead to an increased risk for COPD in the Chinese population. These results increased our knowledge regarding the interaction between genetic and environmental factors contributing to COPD. It emphasizes the need for educating COPD patients on quitting tobacco during COPD management.
Acknowledgments
This work was supported by National Natural Science Foundation of China (grant no 81402471) and Basic Research Program of General Hospital of Chinese People’s Armed Police Forces (grant no WZ2014026).
Disclosure
The authors report no conflicts of interest in this work.
References
- LomasDASilvermanEKThe genetics of chronic obstructive pulmonary diseaseRespir Res200121202611686861
- FregoneseLStolkJFrantsRRVeldhuisenBAlpha-1 antitrypsin null mutations and severity of emphysemaRespir Med2008102687688418353624
- HershCPDahlMLyNPBerkeyCSNordestgaardBGSilvermanEKChronic obstructive pulmonary disease in alpha1-antitrypsin PI MZ heterozygotes: a meta-analysisThorax2004591084384915454649
- DendenSKhelilAHKnaniJAlpha-1 antitrypsin gene polymorphism in Chronic Obstructive Pulmonary Disease (COPD)Genet Mol Biol2010331232621637600
- SeyamaKNukiwaTSoumaSShimizuKKiraSAlpha 1-antitrypsin-deficient variant Siiyama (Ser53[TCC] to Phe53[TTC]) is prevalent in Japan. Status of alpha 1-antitrypsin deficiency in JapanAm J Respir Crit Care Med19951526 Pt 1211921268520784
- KoDHChangHESongSHYoonHParkKUSongJIdentification of compound heterozygous mutation in a Korean patient with alpha 1-antitrypsin deficiencyKorean J Lab Med201131429429722016686
- ChappellSDalyLMoranKCryptic haplotypes of SERPINA1 confer susceptibility to chronic obstructive pulmonary diseaseHum Mutat 2006. Cryptic haplotypes of SERPINA1 confer susceptibility to chronic obstructive pulmonary disease71103109
- CunninghamTJFordESRolleIVWheatonAGCroftJBAssociations of self-reported cigarette smoking with chronic obstructive pulmonary disease and comorbid chronic conditions in the United StatesCOPD201512327628625207639
- WangBXiaoDWangCSmoking and chronic obstructive pulmonary disease in Chinese population: a meta-analysisClin Respir J20159216517524517735
- LvJChenWSunDGender-specific association between tobacco smoking and central obesity among 0.5 million Chinese people: the China Kadoorie Biobank StudyPLoS One2015104e012458625897789
- MehtaAJThunGAImbodenMInteractions between SERPINA1 PiMZ genotype, occupational exposure and lung function declineOccup Environ Med201471423424024213563
- de JongKBoezenHMHackenNHPostmaDSVonkJMLifeLines cohort studyGST-omega genes interact with environmental tobacco smoke on adult level of lung functionRespir Res2013148323937118
- VestboJHurdSSAgustiAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- KwokJSLawtonJWYewWWChauCHLeeJWongPCProtease inhibitor phenotypes and serum alpha-1-antitrypsin levels in patients with COPD: a study from Hong KongRespirology20049226527015182280
- KongXChoMHAndersonWGenome-wide association study identifies BICD1 as a susceptibility gene for emphysemaAm J Respir Crit Care Med20111831434920709820
- ObeidatMWainLVShrineNA comprehensive evaluation of potential lung function associated genes in the SpiroMeta general population samplePLoS One201165e1938221625484
- ThunGAImbodenMFerrarottiICausal and synthetic associations of variants in the SERPINA gene cluster with alpha1-antitrypsin serum levelsPLoS Genet201398e100358523990791
- FujimotoKIkedaSAraiTPolymorphism of SERPINE2 gene is associated with pulmonary emphysema in consecutive autopsy casesBMC Med Genet20101115921067581
