Abstract
Background
Early diagnosis of COPD is often not achieved due to limited recognition and limited access to the pulmonary function test. Our hypothesis was that lung function decline may be different between populations with mild COPD and those who are at high risk and do not receive treatment.
Patients and methods
Subjects with mild COPD and those from a high-risk COPD population were recruited from a community-based COPD epidemiological study after obtaining consent. Baseline clinical characteristics, symptom questionnaire, spirometry, low-dose computed tomography (LDCT) chest scan, and blood plasma biomarker data were collected initially and then 1 year later.
Results
A total of 617 participants were recruited, and 438 eventually completed the first-year follow-up visit; 72 participants (46 males) were in the mild COPD group, and 225 participants (165 males) were in the high-risk group. The mean forced expiratory volume in the first second of expiration (FEV1) decline in the mild COPD group was 129 mL, which was significantly higher than the 30 mL decline in the high-risk population group (P=0.005). Group category (odds ratio [OR] =0.230) and COPD Assessment Test (CAT) score (OR =9.912) were independent risk factors for an FEV1% predicted decline of >15% for all participants. In the mild COPD group, patients with a higher CAT (OR =5.310) and Emphysema Index (OR =5.681) were associated with a FEV1% predicted decline of >15% at the first-year follow-up. No factor showed a significantly predictive effect on FEV1 decline in the high-risk COPD group.
Conclusion
Group category was an independent influential factor associated with FEV1 decline.
Introduction
COPD is a leading cause of morbidity and mortality worldwide.Citation1 The prevalence of physiologically defined COPD in adults older than 40 years is ~9%–10%, with variation due to differences in survey methods, diagnostic criteria, and statistical methods.Citation2 In a study from China, the overall prevalence of COPD (males, 12.4%; females, 5.1%) among 20,245 participants aged 40 years or older from seven provinces/cities was 8.2%.Citation3 Among the participants, 35.5% were asymptomatic and only 6.5% had been tested with spirometry. Most of the underdiagnosed COPD patients had no clinical symptoms and no awareness of the lung function test as a medical examination. Owing to the lack of awareness about the high prevalence of COPD among both doctors and patients, underdiagnosis is a severe problem.Citation3–Citation6 Many patients are diagnosed in the moderate-to-severe stage and even in the very severe stage, when they come to the hospital for help with respiratory symptoms. Patients with high-grade COPD impose a high economic burden on the health care system. The severity of COPD is positively correlated with total direct costs, including costs of medications, ventilation therapy, beds, and nursing care during hospitalization, as well as with the cost and duration of ICU care.Citation7,Citation8 Early detection may allow for appropriate medical intervention to slow down lung function decline in patients.Citation9 The value of spirometry screening is still being debated to date, and it is not supported by the Global Initiative for Chronic Obstructive Lung Disease (GOLD).Citation10
As being reported, mean forced expiratory volume in the first second of expiration (FEV1) loss is higher in moderate than that in severe–very severe COPD patients,Citation11,Citation12 but there is very limited information on FEV1 decline in patients with mild COPD and high-risk population. Our hypothesis was that lung function decline may be different between patients with mild COPD and those in the high-risk COPD population who do not receive treatment. The annual FEV1 changes in patients with mild COPD and high-risk population in the real world are to be investigated. Identifying the clinical profile of lung function decline together with laboratory examinations may provide a new screening approach for rapid FEV1 decline.
Patients and methods
Trial organization
Five tertiary hospitals in Shanghai participated in this study from September 20, 2013, to May 20, 2014. Participants were either individuals from a community-based COPD epidemiological study or patients with a high-risk of COPD seeking medical services at a participating institution.
The study was approved by the institutional review board of Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China, and the study was registered in the Chinese Clinical Trials Registry (http://www.chictr.org.cn/index.aspx; ChiCTR-OCS-13003610). All the subjects signed written informed consent for participating in this study. And the informed consent was approved by the institution review board.
Inclusion criteria
The subjects were required to meet the following inclusion criteria: 1) 45–80 years old; 2) history of cigarette smoking or a history of exposure to second-hand smoke for >10 years; 3) post-bronchodilator FEV1/forced vital capacity (FVC) ratio <0.70 and FEV1% predicted value >80% in the absence of a bronchodilator or an inhaled corticosteroid treatment, defined as the mild COPD group, or an FEV1/FVC ratio >0.70 and FEV1% predicted value <95%, defined as the high-risk group; 4) predicted to finish the 2-year follow-up; and 5) ability and willingness to undergo low-dose computed tomography (LDCT) at the end of the expiration period.
Exclusion criteria
The exclusion criteria included asthma, bronchiectasis, psychiatric disease, cognitive disorders, malignancies, severe kidney or liver dysfunction, and life expectancy of <2 years. Participants who could not undergo the spirometry test and/or LDCT, as well as those with restrictive ventilation dysfunction, were also excluded from the study.
Measurements
After enrollment, participants completed the chest LDCT scan and blood was collected for the biomarker test. Sociodemographic characteristics, such as age, gender, body mass index (BMI), smoking history, allergy history, family history, occupational/bioaerosol exposure, and comorbidities, were recorded. All participants completed the COPD Assessment Test (CAT) and the modified British Medical Research Council (mMRC) questionnaire.
Spirometry was used as the GOLD criteria for COPD diagnosis according to the American Thoracic Society (ATS) operation standard,Citation13 which was performed by the Jaeger machine (Master Screen PFT, Hoechberg, Germany). All chest LDCT scans were acquired using a 16-slice scanner (Siemens, Erlangen, Germany and GE, Milwaukee, WI, USA) at end expiration with the participants in the supine position. Before the scan began, the technologist let the participants perform breathing exercises. The standard scan parameters used for all examinations were as follows: slice thickness of 1 mm, increment of 0.5 mm, tube current of ~20–40 mAs, tube voltage of 120 kV, and lung window algorithm of “Lung” for the GE machine and “kernel” for the Siemens machine. All images were evaluated at a digital reading workstation using the “Lung Emphysema” application (Myrian Series Software, Paris, France). For each participant, the following parameters were calculated: total lung volume (TLV, liters) and emphysema volume (EV, liters) of both lungs. The EV/TLV ratio (%) was calculated as the Emphysema Index (EI). For the diagnosis of emphysema, a threshold of −950 Hounsfield units (HU) was used. Participants did the spirometry test (pre- and post-bronchodilators) first and then LDCT scan on the same day.
The concentrations of blood biomarkers such as leptin, matrix metalloproteinase-10 (MMP-10), carbon tetrachloride-4 (CCL-4), carbon tetrachloride-2 (CCL-2), vascular endothelial growth factor (VEGF), interleukin-1 (IL-1), interleukin-8 (IL-8), and insulin-like growth factor 1 (IGF-1) screened by protein chip technique in the preliminary test were detected in triplicate by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Inc., Minneapolis, MN, USA) in 40 patients. The demographics of the patients in the slow-decline group were matched with those of the patients in the fast-decline group for blood biomarker detection. No intervention was given to the participants during the observation periods.
Statistical analysis
Mean and standard deviation for normally distributed differences were calculated. Differences in the baseline characteristics of patients in the mild COPD group and in the high-risk group were evaluated using the independent t-test or chi-square test as appropriate. Univariate and multivariate logistic regressions were performed for the mild COPD and high-risk groups, respectively, to evaluate the effects of baseline characteristics and serum biomarkers on the FEV1 decline. Age, gender, BMI, occupational exposure, bioaerosol exposure, family history, CAT score, mMRC score, and EI were evaluated as covariates. A stepwise backward procedure was used to derive a final model of the variables that had a significant effect on rapid FEV1 decline. The Youden Index was calculated to identify the cutoff point of the CAT score and EI. A P-value <0.05 was considered as statistically significant. Statistical analysis was performed using the SAS9.4 software (SAS Institute Inc., Cary, NC, USA).
Results
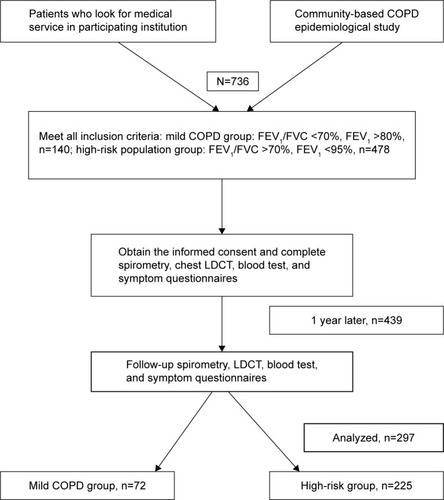
In total, 617 participants were screened and enrolled in the study for investigation at baseline. After 1 year, 438 (71%) participants returned for the first follow-up visit. Based on the completed data collection in both visits, 297 participants (72 in the mild COPD group and 225 in the high-risk group) were ultimately analyzed in this study. All of them had no medical intervention and smoking status change due to COPD. The flow chart of the study is presented in . All participants with restrictive lung function were excluded after the follow-up spirometry.
Figure 1 Flow diagram of the study.
Fast-decline group definition
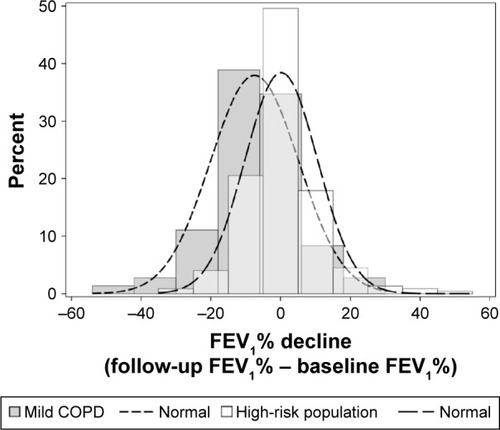
A larger proportion of patients in the mild COPD group presented with a reduced FEV1% predicted at follow-up than that in the high-risk group. In addition, the FEV1% predicted for the mild COPD group of patients was significantly lower than that for the high-risk group (). Based on the histogram of FEV1% predicted decline, 15% was chosen as the cutoff value to define the fast-decline group, ie, an individual with an FEV1% predicted decline >15% at the follow-up was included in the fast-decline group (n=34), while individuals with a <15% decline were included in the slow-decline group (n=263).
Baseline characteristics
Participant demographic information is presented in . The mean age of the mild COPD group was 57.23 years, which was less than that of the high-risk group (61.74 years). Males comprised 63.89% of the mild COPD group and 73.33% of the high-risk group. There was no statistically significant difference between these two groups with respect to BMI, mean cigarette smoking years, exposure frequency, or family disease history. The symptom evaluation showed that the CAT and mMRC scores of the mild COPD group were higher than those of the high-risk group. The FEV1/FVC% ratio in the mild COPD group was significantly lower than that in the high-risk group (P<0.001).
Table 1 Patient demographics and baseline characteristics
Follow-up analysis
The results showed that group category (odds ratio [OR] =0.230) and CAT score (OR =9.912, with 6 defined as the cutoff value) were the independent risk factors for an FEV1% predicted decline of >15% (fast decline) for all participants at the follow-up. The mean FEV1% predicted decline was 7.46% (95% confidence interval [CI]: 4.49–10.43) in the mild COPD group and 0.19% (95% CI: 1.18–1.56) in the high-risk group. The difference in the declines in FEV1% predicted between these two groups was statistically significant (P<0.0001). Meanwhile, the mean absolute value of FEV1 decline in the mild COPD group was 129 mL (95% CI: 73–186), which was significantly greater than the 30 mL decline in the high-risk group (95% CI: 5–65; P=0.005). Furthermore, in the fast-decline group, the mean absolute value of FEV1 decline was 380 mL (95% CI: 290–460) and the mean predicted decline of FEV1% was 21.54% (95% CI: 18.95–24.13). In contrast, in the slow-decline group, the mean predicted declines of FEV1 and FEV1% were 10 mL (95% CI: −20–40) and −0.82% (95% CI: −1.94–0.3), respectively. The differences in the FEV1 absolute and FEV1% predicted declines between the fast- and slow-decline groups were statistically significant (P<0.0001). Other factors, including age, gender, BMI, exposure frequency, family disease history, mMRC score, smoking, and EI, were not risk factors for fast FEV1 decline at the first-year follow-up.
In the fast-decline group, 19 of 34 patients were in the mild CODP group and 15 of 34 patients were in the high-risk group. The mild COPD group presented a higher proportion of individuals with fast FEV1 decline at the follow-up (P<0.01). Considering that the prognostic characteristics of patients with a low FEV1/FVC ratio at baseline may be different from those of patients with a normal FEV1/FVC ratio, the participants were stratified into the mild COPD group and high-risk group according to their baseline FEV1/FVC ratio, and prognostic effect analysis was performed separately for these two groups. In the analysis of the mild COPD group, CAT score (OR =5.310, with 6 defined as the cutoff value) and EI (OR =5.681, with 12.3 defined as the cutoff value) were predictive factors for FEV1% predicted decline >15% (). Combining the CAT score with the EI, the model Akaike information criterion was 53.6 and its consistency rate was 63.3%, which was significantly better than those for the CAT score or EI alone. However, in the analysis of the high-risk group, there were no factors, including CAT score and EI, that showed a significant effect on rapid FEV1% decline, both in the univariate and multivariate analyses (). This finding indicates that as soon as the patients met the criteria for early COPD diagnosis, they were more likely to undergo rapid FEV1 decline. For these patients, the risk of developing rapid FEV1% decline was associated with an increased CAT score and/or EI.
Table 2 The factors influencing FEV1% predicted decline in the mild COPD group
Table 3 The factors influencing FEV1% predicted decline in the high-risk group
Blood biomarker measurement
The results showed that the levels of MMP-10, CCL-4, CCL-2, and IL-1 were all higher in the fast-decline group than in the slow-decline group (), although there were no significant differences between the groups in the levels of the eight cytokines that were detected at baseline.
Table 4 Cytokine levels in the fast-decline and slow-decline groups
Discussion
It has been reported that age, smoking, and exposure to inhaled particles are the main recognized COPD risk factors.Citation14–Citation16 The incidence of COPD is associated with age,Citation17 and COPD is less frequently diagnosed in adults younger than 45 years.Citation18 Most studies target populations older than 40 or 45 years.Citation4,Citation5,Citation19,Citation20 Pulmonary function progressively deteriorates and inflammation increases with airway/parenchyma structural changes, which are characteristics of senile emphysema.Citation16 Emphysema in computed tomography (CT) imaging is more frequently seen in elderly participants with a history of smoking. Chest CT has been recognized as the most sensitive method to show pulmonary lesions. Several studies used −900 HU, −910 HU, and −950 HU as the cutoff points for distinguishing normal lung inflation and emphysema.Citation21–Citation24 Although it is still debated whether an end-inspiration or end-expiration scan is better at reflecting air trapping and lung overinflation, more studies supported the use of the end-expiration scan.Citation25–Citation27 In a previous study, it was found that the EI derived from CT 3D reconstruction was significantly and negatively correlated with FEV1, FEV1% predicted, FEV1/FVC, and TLC% predicted when an end-expiration scan was performed with a −950 HU threshold setting.Citation28 From lung function testing, it has been interpreted that year-to-year changes in FEV1 exceeding 15% over 1 year could be considered a clinically meaningful change.Citation29 According to the histogram of FEV1% predicted decline in our study, the cutoff values of 10%, 15%, 20%, and 25% were all analyzed for their possibility in defining the fast-decline group. As a result, a 15% FEV1% predicted decline was chosen as the best cut-off point based on clinical significance. In addition, COPD is a systemic inflammatory disease causing persistent airflow limitation. Symptom assessments and serum inflammatory biomarker evaluations are considered very important for disease evaluation and exacerbation prediction.Citation30–Citation32 These parameters may help to determine what type of population requires immediate intervention to slow down the lung function decline process.
There were several interesting findings in our study. First, the lung function variation between the mild COPD patients and the high-risk population was observed at the first-year follow-up. The results demonstrated that the group category was an independent influential factor for a FEV1% predicted decline >15%. A previous study showed that FEV1 declined faster in current smokers and in moderate or severe COPD patients with a lower BMI.Citation33 FEV1 mean loss was higher in moderate COPD (stage II) patients than in stage III and stage IV patients.Citation11,Citation12 With treatment, the mean decline in FEV1 in the moderate COPD patients was reported to be 35 mL per yearCitation12 or 112 mL per year.Citation11 Obviously, the data for FEV1 decline in mild COPD patients were limited. In our study, the stage of disease itself had an effect on lung function decline in mild COPD, without the influences of age, gender, BMI, exposure frequency, family disease history, and smoking. Without any intervention, the FEV1 of mild COPD patients may decline rapidly. The mean annual decline of 129 mL in mild COPD patients is far greater than the 30 mL decline in the high-risk group under the natural state Therefore, when patients are diagnosed with mild COPD, medical intervention should be recommended immediately to slow down lung function decline. Second, a new cutoff value of the CAT score for predicting FEV1 decline in the mild COPD group was defined. The CAT score consists of eight items covering common COPD symptoms ranging from 0 to 40 and is broadly used for assessing COPD symptoms with a cutoff point of 10. A CAT score <10 indicates fewer symptoms, whereas a CAT score ≥10 indicates a greater number of symptoms.Citation34 Additionally, a recent review noted that the CAT may be used as a complementary tool for predicting COPD exacerbation, depression, acute deterioration of health status, and mortality.Citation35 A CAT score ≥13.5 is a significant risk factor for a composite event (new ambulatory or emergency exacerbation, hospitalization, or death).Citation36 The probability cutoffs for a CAT score to identify a mild COPD exacerbation and moderate–severe exacerbation were 11 and 17, respectively.Citation37 It is clear that the cutoff value may differ according to different diagnostic goals. In our study, the target population had mild COPD and a high risk for rapid FEV1 decline, so they had fewer symptoms than patients with more severe COPD. The results verified that 6 was the best cutoff point; it was an independent influential factor for FEV1% predicted decline >15% for all participants and also had a good predictive effect on rapid FEV1 decline in the mild COPD group. Third, imaging examination and reconstruction are useful for detecting emphysema and predicting rapid lung function decline. Previous studies have shown that worsened lung function, airflow obstruction, and larger increases in residual lung volume existed in patients with emphysema based on CT scanning compared with patients with radiological evidence of bronchiectasis or bronchial wall thickening.Citation38 In one study, the emphysema group exhibited a faster decline in FVC with a low attenuation areas less than −950 Hounsfield Unit (%LAA−950) median of 13.3.Citation39 Jairam et alCitation40 reported that emphysema and airway thickening in COPD detected by CT scans were strong independent predictors of severe COPD exacerbation. Emphysema is an important pathophysiological manifestation and is considered a particular phenotype of COPD. In our opinion, patients with mild COPD and emphysema may be more likely to have rapid FEV1 decline with an EI >12.3 and, therefore, require more clinical attention.
To our knowledge, no recognized blood biomarker can predict the current risk of COPD or the probability of rapid lung function decline in early stage COPD without symptoms. Recent literature showed that a lower plasma leptin/adiponectin ratio was significantly associated with an annual decline in COPD.Citation41 Inflammatory biomarker detection was mostly based on GOLD stages II–IV COPD patients in the stable or exacerbation phase compared with one another or compared with healthy subjects.Citation42,Citation43 IL-8, interleukin-6 (IL-6), tumor necrosis factor-α, fibrinogen, and white cell count are generally used to predict the exacerbation and mortality of COPD in clinical trials.Citation44,Citation45 Our study analyzed the relationships between inflammatory biomarkers and early COPD from a different perspective. According to our findings, there was no significant difference in blood biomarker levels between the baseline and follow-up in either the mild COPD group or high-risk group. In addition, there was an increased trend of inflammatory biomarkers only in the fast-decline group and not in the slow-decline group.
One limitation of this study is that the follow-up period was only 1 year. The process of pathophysiological changes in COPD is long, from normal lung function to emphysema and/or chronic airway inflammation. It would be more meaningful to extend the investigation time to understand the exact magnitude of lung function decline and verify the predictive effect of risk factors on lung function decline in the long term. Inflammatory biomarkers may show significant changes with disease progression. It is assumed that continuous follow-up in these two populations may provide additional insight into the pathophysiological development of COPD in order to explain the reasons of differences in FEV1 decline between mild COPD patients and high-risk population.
Conclusion
Airway obstruction was found to be an independent risk factor for FEV1 decline. The patients with emphysema and a high CAT score were more likely to have rapid FEV1 decline. Therefore, as soon as patients are diagnosed with mild COPD, medical intervention must be recommended to slow down lung function decline.
Acknowledgments
The authors would like to acknowledge the community health service centers, such as Gangyan and Xinhe Community Health Service Center of Chongming District, Lingyun and Tianping Community Health Service Center of Xuhui District, Jiangdong Town Community Health Service Center, and Jinze Community Health Service Center of Qingpu District, for their assistance with participant selection and follow-up visits. The authors would also like to acknowledge the doctors who recruited the participants into the study, such as Xiaolian Song, Yang Chen, Yi Guo, and Yiliang Su. They would like to give thanks to Heng Fan for the help in data analysis. This work was funded by the Science and Technology Commission of Shanghai Municipality (12411950100 and 134119a4900).
Disclosure
The authors report no conflicts of interest in this work.
References
- VestboJHurdSSAgustiAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: gold executive summaryAm J Respir Crit Care Med2013187434736522878278
- HalbertRJNatoliJLGanoABadamgaravEBuistASManninoDMGlobal burden of COPD systematic review and meta-analysisEur Respir J200628352353216611654
- ZhongNWangCYaoWPrevalence of chronic obstructive pulmonary disease in china: a large, population-based surveyAm J Respir Crit Care Med2007176875376017575095
- LamprechtBSorianoJBStudnickaMBOLD Collaborative Research Group, the EPI-SCAN Team, the PLATINO Team, and the PREPOCOL Study Group. Determinants of underdiagnosis of COPD in national and international surveysChest2015148497198525950276
- MunSYHwangYIKimJHAwareness of chronic obstructive pulmonary disease in current smokers: a nationwide surveyKorean J Intern Med201530219119725750560
- de QueirozMCMoreiraMAJardimJRKnowledge about COPD among users of primary health care servicesInt J Chron Obstruct Pulmon Dis2014101625565794
- ChenSBaiCGuYSocioeconomics of chronic obstructive pulmonary disease in a three-A level hospital in ShanghaiShanghai Med J2010339849852
- KimJRheeCKYooKHThe health care burden of high grade chronic obstructive pulmonary disease in Korea: analysis of the Korean Health Insurance Review and Assessment Service dataInt J Chron Obstruct Pulmon Dis2013856156824277985
- AnthonisenNRConnettJEMurrayRPSmoking and lung function of Lung Health Study participants after 11 yearsAm J Respir Crit Care Med2002166567567912204864
- DirvenJATangeHJMurisJWvan HaarenKMVinkGvan SchayckOCEarly detection of COPD in general practice: patient or practice managed? A randomised controlled trial of two strategies in different socioeconomic environmentsPrim Care Respir J201322333133723966214
- CasanovaCde TorresJPAguirre-JaimeAThe progression of chronic obstructive pulmonary disease is heterogeneous: the experience of the BODE cohortAm J Respir Crit Care Med201118491015102121836135
- VestboJEdwardsLDScanlonPDChanges in forced expiratory volume in 1 second over time in COPDN Engl J Med2011365131184119221991892
- MillerMRHankinsonJBrusascoVStandardisation of spirometryEur Respir J200526231933816055882
- TanWCLoCJongAVancouver Burden of Obstructive Lung Disease (BOLD) Research Group. Marijuana and chronic obstructive lung disease: a population-based studyCMAJ2009180881482019364790
- MaheshPAJayarajBSPrabhakarAKChayaSKVijaysimhaRIdentification of a threshold for biomass exposure index for chronic bronchitis in rural women of Mysore district, Karnataka, IndiaIndian J Med Res20131371879423481056
- ItoKBarnesPJCOPD as a disease of accelerated lung agingChest2009135117318019136405
- XuGChenZCaoXWangYYangPAnalysis of pulmonary function test results in a health check-up populationJ Thorac Dis2015791624162926543610
- CerveriICorsicoAGAccordiniSUnderestimation of airflow obstruction among young adults using FEV1/FVC <70% as a fixed cut-off: a longitudinal evaluation of clinical and functional outcomesThorax200863121040104518492741
- TeoWSTanWSChongWFEconomic burden of chronic obstructive pulmonary diseaseRespirology201217112012621954985
- HanMKMuellerovaHCurran-EverettDGOLD 2011 disease severity classification in COPD gene: a prospective cohort studyLancet Respir Med201311435024321803
- CerveriIDoreRCorsicoAAssessment of emphysema in COPD: a functional and radiologic studyChest200412551714171815136381
- IwanoSOkadaTSatakeHNaganawaS3D-CT volumetry of the lung using multidetector row CT: comparison with pulmonary function testsAcad Radiol200916325025619201353
- MullerNLStaplesCAMillerRRAbboudRT“Density mask”. An objective method to quantitate emphysema using computed tomographyChest19889447827873168574
- ParkYSSeoJBKimNTexture-based quantification of pulmonary emphysema on high-resolution computed tomography: comparison with density-based quantification and correlation with pulmonary function testInvest Radiol200843639540218496044
- CamiciottoliGBartolucciMMaluccioNMSpirometrically gated high-resolution CT findings in COPD: lung attenuation vs lung function and dyspnea severityChest2006129355856416537852
- ZaporozhanJLeySEberhardtRPaired inspiratory/expiratory volumetric thin-slice CT scan for emphysema analysis: comparison of different quantitative evaluations and pulmonary function testChest200512853212322016304264
- ChenHChenRCGuanYBLiWLiuQZengQSCorrelation of pulmonary function indexes determined by low-dose MDCT with spirometric pulmonary function tests in patients with chronic obstructive pulmonary diseaseAJR Am J Roentgenol2014202471171824660696
- ChenSGuYZhangJCorrelation between the parameters quantified by chest low-dose CT scan and airflow limitation examined by spirometryInt J Respir20123211833837
- PellegrinoRViegiGBrusascoVInterpretative strategies for lung function testsEur Respir J200526594896816264058
- BroekhuizenRWoutersEFCreutzbergECScholsAMRaised CRP levels mark metabolic and functional impairment in advanced COPDThorax2006611172216055618
- SariogluNAlpaydinAOCoskunASCelikPOzyurtBCYorganciogluARelationship between BODE index, quality of life and inflammatory cytokines in COPD patientsMultidiscip Respir Med201052849122958780
- TuYHZhangYFeiGHUtility of the CAT in the therapy assessment of COPD exacerbations in ChinaBMC Pulm Med2014144224618290
- CelliBRThomasNEAndersonJAEffect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH studyAm J Respir Crit Care Med2008178433233818511702
- Lopez-CamposJLTanWSorianoJBGlobal burden of COPDRespirology2016211142326494423
- KarlohMFleig MayerAMauriciRPizzichiniMMJonesPWPizzichiniEThe COPD Assessment Test: what do we know so far? A systematic review and meta-analysis about clinical outcomes prediction and classification of patients into GOLD stagesChest2016149241342526513112
- MiravitllesMGarcia-SidroPFernandez-NistalAThe chronic obstructive pulmonary disease assessment test improves the predictive value of previous exacerbations for poor outcomes in COPDInt J Chron Obstruct Pulmon Dis2015102571257926664105
- LeeSDHuangMSKangJThe COPD assessment test (CAT) assists prediction of COPD exacerbations in high-risk patientsRespir Med2014108460060824456695
- BafadhelMUmarIGuptaSThe role of CT scanning in multidimensional phenotyping of COPDChest2011140363464221454400
- KooHKJinKNKimDKChungHSLeeCHAssociation of incidental emphysema with annual lung function decline and future development of airflow limitationInt J Chron Obstruct Pulmon Dis20161116116626893550
- JairamPMvan der GraafYLammersJWMaliWPde JongPAPROVIDI Study groupIncidental findings on chest CT imaging are associated with increased COPD exacerbations and mortalityThorax201570872573126024687
- SuzukiMMakitaHOstlingJLower leptin/adiponectin ratio and risk of rapid lung function decline in chronic obstructive pulmonary diseaseAnn Am Thorac Soc201411101511151925372271
- ChenHWangYBaiCWangXAlterations of plasma inflammatory biomarkers in the healthy and chronic obstructive pulmonary disease patients with or without acute exacerbationJ Proteomics201275102835284322343073
- ThomsenMIngebrigtsenTSMarottJLInflammatory biomarkers and exacerbations in chronic obstructive pulmonary diseaseJAMA2013309222353236123757083
- AgustiAEdwardsLDRennardSIPersistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotypePLoS One201275e3748322624038
- CelliBRLocantoreNYatesJInflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2012185101065107222427534