Abstract
Background
Recent advances in multidetector computed tomography (MDCT) facilitate acquiring important clinical information for managing patients with COPD. MDCT can detect the loss of lung tissue associated with emphysema as a low-attenuation area (LAA) and the thickness of airways as the wall area percentage (WA%). The percentage of small pulmonary vessels <5 mm2 (% cross-sectional area [CSA] <5) has been recently recognized as a parameter for expressing pulmonary perfusion. We aimed to analyze the longitudinal changes in structural abnormalities using these CT parameters and analyze the effect of exacerbation and smoking cessation on structural changes in COPD patients.
Methods
We performed pulmonary function tests (PFTs), an MDCT, and a COPD assessment test (CAT) in 58 patients with COPD at the time of their enrollment at the hospital and 2 years later. We analyzed the change in clinical parameters including CT indices and examined the effect of exacerbations and smoking cessation on the structural changes.
Results
The CAT score and forced expiratory volume in 1 second (FEV1) did not significantly change during the follow-up period. The parameters of emphysematous changes significantly increased. On the other hand, the WA% at the distal airways significantly decreased or tended to decrease, and the %CSA <5 slightly but significantly increased over the same period, especially in ex-smokers. The parameters of emphysematous change were greater in patients with exacerbations and continued to progress even after smoking cessation. In contrast, the WA% and %CSA <5 did not change in proportion to emphysema progression.
Conclusion
The WA% at the distal bronchi and the %CSA <5 did not change in parallel with parameters of LAA over the same period. We propose that airway disease and vascular remodeling may be reversible to some extent by smoking cessation and appropriate treatment. Optimal management may have a greater effect on pulmonary vascularity and airway disease than parenchymal deconstruction in the early stage of COPD.
Background
COPD is the third leading cause of death globally and is associated with increasing economic costs and social burdens.Citation1 Inflammation from cigaret smoking, the major cause of COPD, starts in the small airways and progresses to the lung parenchyma with alveolar destruction (lung emphysema) and then to the central airways, resulting in bronchiolitis (airway diseases).Citation2,Citation3 Pulmonary function tests (PFTs) are physiologically the gold standard for the diagnosis and staging of COPD.
Recent advances in multidetector computed tomography (MDCT) facilitate the acquisition of important clinical information for managing patients with COPD. According to emphysematous changes, it can detect the loss of lung tissue associated with emphysema as a low-attenuation area (LAA). Mishima et al explored emphysema progression using an elastic spring network model and described that neighboring, small, low-attenuation clusters tend to coalesce and break under tension.Citation4 In addition, a recent study analyzed the mean size of LAA clusters (LAS) and the number of low-attenuation clusters (LAN) by their sizes (small LAN, medium LAN, and large LAN).Citation5,Citation6 Focusing on the airway disease, it was possible to analyze the inner luminal area and the wall thickness of airways at the level of the distal bronchi (from the third to the fifth or sixth bronchi) as inner luminal area (Ai) and wall area percentage (WA%), respectively.Citation7,Citation8 These indices were associated with airflow limitation, and such correlations were significant at the distal (small) rather than the proximal (large) airways. Moreover, airway wall percentage and airway wall thickness were greater in the subjects with chronic respiratory symptoms (cough, excessive mucous secretion, dyspnea, and wheezing) than those in the subjects without respiratory symptoms.Citation9 They also associate with the clinical features that may represent chronic bronchitis, which has been reported to result in poor outcomes in COPD.Citation10,Citation11
Finally, a new CT marker for expressing the area of small pulmonary vessels, the percentage of small pulmonary vessels (% cross-sectional area [CSA]), has been introduced to reflect pulmonary vascular change and to correlate inversely with airflow limitation as well as with pulmonary hypertension and radiographic emphysema in COPD.Citation12,Citation13 We previously described that the percentage of small pulmonary vessels <5 mm2 (%CSA <5) was lower in COPD patients with the emphysema phenotype as compared with non-COPD smoker and COPD patients with the no/mild emphysema phenotype.Citation14
Although longitudinal studies about health status or lung function in COPD have been performed,Citation15 the longitudinal changes in structural abnormalities in COPD have not been fully explored. Studies on emphysema progression have been addressed,Citation16–Citation18 but studies about the changes in airway diseaseCitation19 or vascular remodeling are rare.Citation20 The association between alveolar destruction, airway disease, and pulmonary vascular change also remains unknown. Moreover, the data on structural changes after exacerbation or smoking cessation in COPD patients are not established.
Therefore, the aims of this study were to explore the longitudinal change in structural abnormalities in COPD subjects using MDCT; to clarify the change in pulmonary emphysema, airway disease, and pulmonary vasculature; and to clarify the effect of an intervention such as smoking cessation and exacerbations on these structural abnormalities in COPD patients.
Methods
Subjects
We enrolled 112 subjects who presented to Chiba University Hospital from May 2012 to July 2014 for management of mild-to-very-severe COPD in a prospective, observational study investigating COPD exacerbations. COPD was diagnosed on the basis of past history, physical examination, and spirometric data according to the American Thoracic Society/European Respiratory Society recommendations.Citation21 Spirometric measurements were performed using a Fudac-60 (Fukuda Denshi, Tokyo, Japan). Forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) were examined, and their predicted values were calculated according to the Japanese Respiratory Society guidelines.Citation22
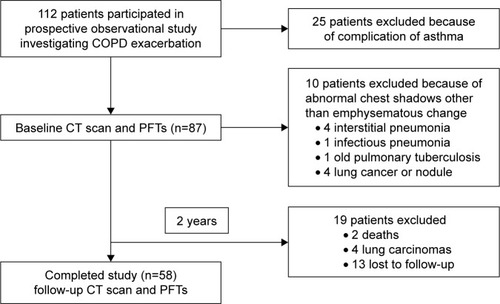
Twenty-five patients were excluded because of complications of asthma. We performed PFTs, an MDCT, and a COPD assessment test (CAT) in 87 patients with COPD at the time of their enrollment at the hospital and 2 years later. Ten patients were excluded because of abnormal chest shadows other than emphysematous changes: interstitial pneumonia (n=4), infectious pneumonia (n=1), old pulmonary tuberculosis (n=1), lung cancer, or pulmonary nodule (n=4). Finally, 58 patients completed this study (). We defined an exacerbation as the situation of deterioration of COPD that requires antibiotics and/or systemic steroid use.Citation17
Figure 1 Patient disposition and reason for exclusion.
This study was approved by the ethics committee of the Chiba University School of Medicine (approval number: 857), and written informed consent was obtained from all study participants.
MDCT scanning
All patients underwent 64-MDCT scanning (Aquilion ONE; Toshiba Medical Systems, Tokyo, Japan) from the thoracic inlet to diaphragm while at full inspiration. No contrast medium was used. MDCT scan parameters were as follows: collimation of 0.5 mm; 120 kV; autoexposure control; gantry rotation time of 0.5 second; and beam pitch of 0.83. All images were reconstructed using standard reconstruction algorithms with a slice thickness of 0.5 mm and a reconstruction interval of 0.5 mm. The voxel size was 0.63×0.63×0.5 mm.
MDCT measurements
We selected three CT slices for the measurements of LAA, LAN, and CSA:Citation12,Citation14 1) the level at 1 cm above the upper margin of the aortic arch (upper lung fields); 2) 1 cm below the carina (middle lung fields); and 3) 1 cm below the right inferior pulmonary vein (lower lung fields).
Measurements of LAA, LAN, LAS, and CSA were performed using a free open-source software, ImageJ Version 1.44 (imagej.nih.gov/ij/download/).Citation13,Citation19
LAA measurements were performed according to the following steps: 1) the threshold technique for the total lung area (TLA) was adopted with attenuation between −500 and −1,024 Hounsfield units (HU). 2) These images were converted into binary images at a window level of −950 HU. 3) The range of circularity was set from 0 to infinity, and the LAA was summed using the “analyze particles” function of ImageJ software on each slice. We counted the number of LAA clusters (LAN) on each slice, and the LAN were divided into three categories by size of cluster (small LAN [sLAN], medium LAN [mLAN], and large LAN [lLAN]). The size of the clusters was as follows: small cluster, 0.4–8 mm2; medium, 8.4–40 mm2; and large, >40 mm2.Citation5 LAAs on the three selected slices were summed, and the average of these values was determined. The LAS was obtained semiautomatically.
The CSA measurements were performed according to the following steps:Citation12,Citation14 1) the threshold technique was adopted with attenuation between −500 and −1,024 HU; 2) these images were converted into binary images at a window level of −720 HU; 3) the range of circularity was set from 0.9 to 1.0 using the “analyze particles” function of ImageJ software; and 4) CSA was measured separately by the size of each vessel (<5 mm2; CSA <5). CSAs on the three selected slices were summed, and the average of these values was determined.
Measurements of WA% were performed using a free open-source software Airway Inspector (Brigham and Women’s Hospital).Citation23 1) We measured WA% according to the method of Yamashiro et al.Citation24 2) The WA% was measured semiautomatically with the full width at a half-maximum method by the software. 3) We identified the third, fourth, and fifth generations of airway bronchi (B1 and B10 in the right lung). We measured bronchi on the images that were peripheral and next to the branching point.
These data were confirmed independently by two pulmonologists (ST and YM) who were blinded to all clinical information.
Statistical analysis
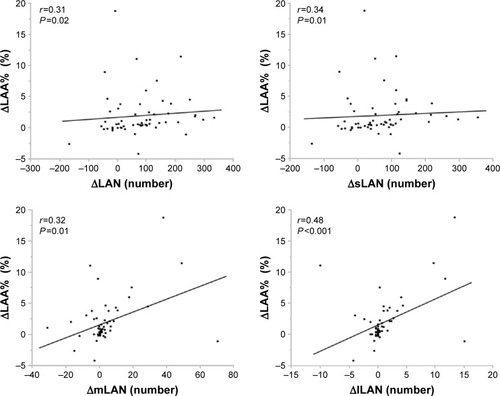
Data are expressed as mean (±SD). Comparisons between the quantitative measurements of the initial and follow-up CT scans, PFTs, and CAT scores were performed using the Wilcoxon signed-rank test. And comparisons of data between current smokers and ex-smokers were evaluated using the Mann–Whitney U test. Correlations of ΔLAA% with ΔLAN (ΔsLAN, ΔmLAN, and ΔlLAN) were evaluated by Spearman’s rank correlation analysis. For all statistical analyses, the level of significance was set at P<0.05. All statistical analyses were performed using JMP 10.0 software (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
Patient characteristics are presented in . There were 58 patients (51 males and 7 females; mean age 69.7±7.3 years; mean FEV1/FVC 55.9%±12.0%). The number of patients with COPD in each Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage was as follows: stage I, n=14 (24.1%); stage II, n=31 (53.4%); stage III, n=9 (15.5%); and stage IV, n=4 (6.9%). The sum of GOLD I and II, defined as a mild-to-moderate form, was 77.5%. Forty-six patients (79.3%) were ex-smokers, and 12 patients (20.7%) were current smokers. Fourteen patients had exacerbations, and the frequency of exacerbations per 2 years was 1.5±0.9 times. The number of patients who received COPD treatment was 21 (36%) at enrollment and 40 (69%) 2 years later.
Table 1 Characteristics of study participants with COPD
Change of CAT score and PFTs
The CAT score and FEV1 did not significantly change during the follow-up period. FEV1/FVC (%) decreased slightly but significantly, and the V50/V25 slightly increased from the initial to the follow-up PFTs ().
Table 2 Longitudinal changes in CAT score, pulmonary function tests, and computed tomography measurements in the follow-up period
Change in CT parameters
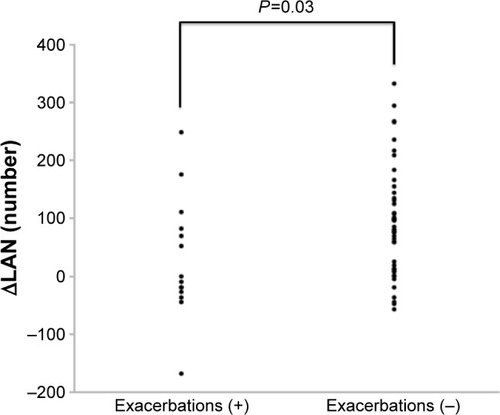
The CT parameters measured on the initial and follow-up CT scans are shown in . LAA, LAA%, LAN, sLAN, and mLAN significantly increased, and lLAN tended to increase from the initial to the follow-up CT scans. The changes in TLA or LAS were not significant. ΔLAA, ΔLAN and ΔsLAN, ΔmLAN, and ΔlLAN positively correlated with each other (). On the other hand, the WA% significantly decreased or tended to decrease at the distal airways (the fourth and fifth bronchi), and the CSA <5 and %CSA <5 slightly but significantly increased during the 2 years of follow-up. Interestingly, the WA% in the ex-smokers significantly decreased in the distal bronchi, and the %CSA <5 of ex-smokers slightly but significantly increased (). In contrast, neither the WA% or %CSA <5 in current smokers changed. In patients in the exacerbation group, the WA% and %CSA <5 did not change during the follow-up period. In contrast, the WA% of patients without exacerbations significantly decreased, and %CSA significantly increased () over the same period. The ΔLAN of the patients who had exacerbations significantly decreased in comparison with that of subjects without exacerbations (). However, the change in the number of LAA grouped by size was not significantly different in patients with or without exacerbations ().
Table 3 Longitudinal changes of WA%, %CSA <5, and LAA% in the patients with current smoking, and smoking cessation
Table 4 Longitudinal changes of WA%, %CSA <5, and LAA% in the patients with/without exacerbations
Table 5 Longitudinal changes of ΔLAN and ΔsLAN, mLAN, and lLAN in the patients with/without exacerbations
Discussion
The key findings in the present study were first that the LAA and the number of clusters by size simultaneously increased during the follow-up period. The LAA clusters increased and coalesced from the initial to the follow-up CT scan. On the other hand, the percentage of the small pulmonary vessels and the bronchial wall thickness did not change in parallel with the parameters of emphysema progression. We found a difference in changes between three structural abnormalities in COPD. Second, the changes in the CT parameters of emphysema were greater in patients with exacerbations than in patients without exacerbations. Moreover, they also progressed even after smoking cessation. In contrast, the parameters of airway disease and pulmonary vasculature did not change in proportion to emphysema progression with exacerbations or smoking cessation. These findings suggest that the progression of emphysema and the change in pulmonary vasculature and airway disease do not always occur with each other after intervention or treatment for COPD.
Although the CAT score and FEV1 did not significantly decrease, both the LAA and LAN increased during the follow-up period consistent with previous imaging studies.Citation5,Citation16 Parenchymal destruction extended during the 2 years, and the number of clusters in each size also increased as emphysema extended. The changes of LAA% and the changes of LAN (including small, medium, and large LAN) were positively correlated in the present study. Parenchymal inflammation, mechanical forces, and coalescence of the neighboring LAA clusters and others are regarded as the mechanism that makes the size of LAA clusters larger.Citation25–Citation27 Mishima et al modeled emphysema progression using an elastic spring network model and described that neighboring, small, low-attenuation clusters tend to coalesce and break under tension.Citation4 They suggested the concept of the D exponent. The cumulative frequency distribution of LAA sizes, Y, could be described by a power law of LAA size X of the form: Y = K × X−D. The D value reflects the fractal dimension of the terminal airspace geometry and sensitively detects alveolar structural changes that were not reflected by the changes in LAA%. They analyzed longitudinal emphysema progression with the original simulation model in the subsequent studies.Citation17 Another cross-sectional study described that the degree of coalescence of the clusters and the extent of emphysema depends on the size of clusters in the subjects with severe emphysema.Citation6 They concluded that the coalescence of LAA requires the medium or large LAN, not the small LAN. Because our data are from a longitudinal study, we did not refer to the difference between our results and the previous study. However, we speculate that coalescence and the new generation of clusters may happen simultaneously regardless of the size of clusters in mild-to-moderate COPD or mild emphysema. Seventy-five percent of our subjects belong to the mild-to-moderate stage, and emphysematous changes were mild as compared with those of previous studies.
The changes in the emphysema area was greater in patients with exacerbations of COPD than in those without exacerbations, consistent with previous studies.Citation17,Citation25 LAN increased in both of the groups, but the change of LAN was greater in patients without exacerbations than in those with exacerbations (). However, the changes in small, medium, or large LANs were not different between the two groups. It may also suggest that exacerbations have an impact on the progression of emphysema and that coalescence and new formation of LAA might simultaneously happen.
In addition, the LAA% increased in both the current and past smokers, consistent with a previous analysis.Citation16 A more rapid decline in lung function was reported in participants with emphysema than in those without emphysema.Citation28 Once lung inflammation and parenchyma destruction have been established by smoking,Citation21,Citation29 the emphysema progression might extend to some extent regardless of smoking cessation.
Some cross-sectional studies reported that the %CSA <5 decreased with emphysematous change or airflow limitation.Citation13,Citation14,Citation30 In addition, the %CSA <5 significantly correlated with pulmonary perfusion and reflected microvascular remodeling in the lung.Citation31 A recent advanced study using gadolinium-enhanced MRI showed reductions in the pulmonary microvascular blood flow in the lung without clear evidence of emphysema, suggesting the presence of distinct pathological processes associated with emphysema change and pulmonary vascular remodeling in COPD.Citation32 In the present study, the mean WA% at the distal bronchi slightly decreased, and the %CSA <5 slightly but significantly increased during the follow-up period, despite emphysema progression. The airway caliber change in proportion to the improvement in airflow limitation by inhaled anticholinergic agents has been shown in COPD patients.Citation18,Citation33 Saruya et al reported that there was a progressive increase in the extent of emphysema over time, but no significant decrease in small pulmonary vessels over the same time period.Citation20 They described that a decrease in the %CSA <5 may be associated with various improvements in the condition of the patients, such as smoking cessation, exacerbation risk, and pharmacotherapies, in comparison with emphysematous progression. Very recently, the morphological changes in small pulmonary vessels were reported to be associated independently with severe acute exacerbations.Citation34 These findings support our results.
In the present study, the %CSA <5 and the WA% at the distal bronchi in ex-smokers did not change in parallel with the progression of emphysema. Smoking cessation might decrease the inflammation of the bronchus, which led to a decrease in the WA% of the bronchi. In the ex-smokers group, the WA% decreased or tended to decrease in the distal bronchi, and the %CSA <5 significantly increased. In contrast, the WA% and %CSA <5 in current smokers did not significantly change. This may suggest that smoking cessation not only influences airway inflammation but also improves the pulmonary vasculature.
Quality of life is related to exacerbation frequency,Citation35,Citation36 and mortality increases with the frequency of severe exacerbations in COPD.Citation37 Exacerbations also occur in mild COPD, although at a lower frequency compared with moderate-to-severe disease,Citation38,Citation39 and exacerbations are involved in emphysema progression.Citation17,Citation25 In the present study, some patients with mild-to-moderate airflow limitation underwent exacerbations. Exacerbations cause inflammation of the airway, but smoking cessation and inhaled pharmacotherapies, such as β-agonists, anticholinergics, and steroids, reduce inflammation of the airways and the risk of exacerbations of COPD.Citation40–Citation44 In our study, the WA% and the %CSA <5 slightly changed in patients without exacerbations. We suppose that the WA% may be improved because of decreasing inflammation of the small airways due to the intervention, such as smoking cessation and appropriate treatment, which will lead to no exacerbations. Then, inflammation of the blood vessel accompanying the bronchial wall may be improved, and the %CSA <5 may be improved. The changes in the morphology of the small pulmonary vessels in COPD follow airway remodeling, and the complications of both airway and vascular change are associated with each other.Citation45
Interestingly, we previously reported that the WA% at the distal bronchi and the %CSA <5 simultaneously decreased after 3 months of inhaled therapy in asthma–COPD overlap syndrome (ACOS) subjects.Citation46 In the present study, the %CSA in subjects with smoking cessation and in subjects without exacerbations increased in accordance with the decrease of the WA% in COPD-alone subjects. We could not clarify the point in this study, but we speculate that it may be caused by the difference in the pathogenesis and responsiveness to intervention between COPD and asthma.Citation47,Citation48 In COPD, there is a well-known hypothesis that depleting vascular endothelial growth factor induces the apoptosis of parenchymal epithelial and endothelial cells and results in emphysematous changes.Citation49,Citation50 On the contrary, airway inflammation in asthma is accompanied by vascular endothelial growth factor and an increase in subepithelial microvessels.Citation48 Based on these situations, the responsiveness to intervention will differ between the COPD-alone and ACOS subjects. In ACOS subjects, pharmacotherapies could improve the remodeling of both the airways and pulmonary vasculature by suppressing inflammation and leakage from immature microvessels, in addition to decreasing the local vascular constriction. We are conducting the next study to clarify the difference in structural changes between COPD-alone and ACOS subjects.
The frequency of exacerbations also contributes to the long-term decline in lung function.Citation51 We conclude that there is a possibility that airway remodeling and small pulmonary vascular remodeling may react to interventions, such as smoking cessation and appropriate treatment, in particular in mild-to-moderate COPD patients. An aggressive smoking intervention program significantly reduces the age-related decline in FEV1.Citation52 Further research focusing on the effects of early intervention (smoking cession, inhaled therapy, and more) in mild-to-moderate COPD patients will be needed.Citation53
There were several limitations of our study. First, our sample size was relatively small, and most of the patients were male. We could not analyze the gender differences for longitudinal changes. A recent study mentioned the association of male gender with clinical features of chronic bronchitis in COPD.Citation11 Second, the observational period of 2 years was not long.Citation17 Although this study was conducted at a single institute, our data were accurate and reproducible because all the parameters were assessed using the same equipment during the follow-up period. Third, we could not evaluate the effect of the pharmacotherapies on CT parameters because of our sample size and the short follow-up period. However, a large cohort study showed that inhaled therapy could decrease the exacerbation risk and reduce the decline of pulmonary function during 4 years of follow-up.Citation40,Citation54 We would like to elucidate these points in a follow-up study. Finally, 78.5% of patients were in the mild-to-moderate stage of COPD in this study; therefore, it may turn out differently with the reports focusing on patients in stages that are severe to very severe. We could not analyze the subjects according to the disease severity because of our sample size. However, there are few reports focusing on a relatively early stage of COPD; therefore, this study is useful for managing COPD patients in clinical practice.
Conclusion
In conclusion, the WA% at the distal bronchi and the %CSA <5 did not change in parallel with emphysema progression during the follow-up period. By using WA% and %CSA <5, we could evaluate the change in airway remodeling and pulmonary vasculature in addition to emphysematous progression. Optimal management may have an effect on the pulmonary vasculature and airway disease in the early stage of COPD.
Author contributions
ST, NK, and KT conceived and designed the paper. ST, NK, YM, and JI collected the data. ST, NK, and YM analyzed the data. ST, NK, S Matsuoka, S Matsushita, NY, YM, YT, and YK contributed reagents/materials/analysis tools. ST, NK, and KT contributed to the writing of the manuscript. All authors read and approved the final manuscript. All authors contributed toward data analysis and drafting the paper, and they agree to be accountable for all aspects of the work.
Acknowledgments
We thank Dr Toshihiko Sugiura, Dr Toshio Suzuki, Dr Misuzu Yahaba, and Dr Yoriko Sakurai for their technical assistance and general support.
This research was partially supported by the Ministry of Education, Science, Sports and Culture, Grant-in-Aid for Scientific Research (C) (16K01407).
KT reports grants from Boehringer Ingelheim Japan, Inc., GlaxoSmithKline, Pfizer, and Astellas, Inc. outside the submitted work.
Disclosure
The authors report no conflicts of interest in this work.
References
- LozanoRNaghaviMForemanKGlobal and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010Lancet201238098592095212823245604
- VestboJHurdSSAgustíAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- HoggJCChuFUtokaparchSThe nature of small-airway obstruction in chronic obstructive pulmonary diseaseN Engl J Med2004350262645265315215480
- MishimaMHiraiTItohHComplexity of terminal airspace geometry assessed by lung computed tomography in normal subjects and patients with chronic obstructive pulmonary diseaseProc Natl Acad Sci U S A199996168829883410430855
- MatsuokaSKuriharaYYagihashiKNakajimaYMorphological progression of emphysema on thin-section CT: analysis of longitudinal change in the number and size of low-attenuation clustersJ Comput Assist Tomogr200630466967416845301
- MatsuokaSKuriharaYYagihashiKQuantitative thin-section CT analysis of the enlargement and coalescence of low-attenuation clusters in patients with emphysemaRespiration200774213614117008789
- NakanoYMuroSSakaiHComputed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung functionAm J Respir Crit Care Med20001623 Pt 11102110810988137
- HasegawaMNasuharaYOnoderaYAirflow limitation and airway dimensions in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2006173121309131516556695
- XieXDijkstraAEVonkJMOudkerkMVliegenthartRGroenHJChronic respiratory symptoms associated with airway wall thickening measured by thin-slice low-dose CTAJR Am J Roentgenol20142034W383W39025247967
- PatelBDCoxsonHOPillaiSGAirway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2008178550050518565956
- KimVDaveyAComellasAPCOPDGene® InvestigatorsClinical and computed tomographic predictors of chronic bronchitis in COPD: a cross sectional analysis of the COPD Gene studyRespir Res2014155224766722
- MatsuokaSWashkoGRYamashiroTNational Emphysema Treatment Trial Research GroupPulmonary hypertension and computed tomography measurement of small pulmonary vessels in severe emphysemaAm J Respir Crit Care Med2010181321822519875683
- MatsuokaSWashkoGRDransfieldMTQuantitative CT measurement of cross-sectional area of small pulmonary vessel in COPD: correlations with emphysema and airflow limitationAcad Radiol2010171939919796970
- MatsuuraYKawataNYanagawaNQuantitative assessment of cross-sectional area of small pulmonary vessels in patients with COPD using inspiratory and expiratory MDCTEur J Radiol201382101804181023769190
- JonesPWAndersonJACalverleyPMTORCH investigatorsHealth status in the TORCH study of COPD: treatment efficacy and other determinants of changeRespir Res2011127121627828
- SoejimaKYamaguchiKKohdaELongitudinal follow-up study of smoking-induced lung density changes by high-resolution computed tomographyAm J Respir Crit Care Med20001614 Pt 11264127310764322
- TanabeNMuroSHiraiTImpact of exacerbations on emphysema progression in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2011183121653165921471102
- TanabeNMuroSOgumaTComputed tomography assessment of pharmacological lung volume reduction induced by bronchodilators in COPDCOPD20129440140822509949
- OharaTHiraiTSatoSLongitudinal study of airway dimensions in chronic obstructive pulmonary disease using computed tomographyRespirology200813337237818399859
- SaruyaSMatsuokaSYamashiroTQuantitative CT measurements of small pulmonary vessels in chronic obstructive pulmonary disease: do they change on follow-up scans?Clin Physiol Funct Imaging201636321121725393655
- American Thoracic Society European Respiratory Society Task ForceStandards for the Diagnosis and Management of Patients with COPD. Version 1.2New York, NYAmerican Thoracic Society2004 [updated September 8, 2005]. Available from: http://www.thoracic.org/go/copdAccessed August 1, 2016
- Committee of Pulmonary Physiology. The Japanese Respiratory SocietyGuidelines for pulmonary function tests: spirometry, flow-volume curve, diffusion capacity of the lung1st edTokyoMedical Review Co, Ltd2004 Nihon Kokyuki Gakkai Zasshi2004Suppl156 Japanese
- WashkoGRDransfieldMTEsteparRSAirway wall attenuation: a biomarker of airway disease in subjects with COPDJ Appl Physiol (1985)2009107118519119407254
- YamashiroTMatsuokaSEsteparRSQuantitative assessment of bronchial wall attenuation with thin-section CT: an indicator of airflow limitation in chronic obstructive pulmonary diseaseAJR Am J Roentgenol2010195236336920651191
- KiyokawaHMuroSOgumaTImpact of COPD exacerbations on osteoporosis assessed by chest CT scanCOPD20129323524222360380
- SukiBLutchenKRIngenitoEPOn the progressive nature of emphysema: roles of proteases, inflammation, and mechanical forcesAm J Respir Crit Care Med2003168551652112941655
- WinklerTSukiBEmergent structure-function relations in emphysema and asthmaCrit Rev Biomed Eng201139426328022011233
- VestboJEdwardsLDScanlonPDECLIPSE InvestigatorsChanges in forced expiratory volume in 1 second over time in COPDN Engl J Med2011365131184119221991892
- BarnesPJCOPD: is there light at the end of the tunnel?Curr Opin Pharmacol20044326327215140418
- AndoKKuraishiHNagaokaTPotential role of CT metrics in chronic obstructive pulmonary disease with pulmonary hypertensionLung2015193691191826453478
- MatsuokaSYamashiroTMatsushitaSRelationship between quantitative CT of pulmonary small vessels and pulmonary perfusionAJR Am J Roentgenol2014202471972424660697
- HueperKVogel-ClaussenJParikhMAPulmonary microvascular blood flow in mild chronic obstructive pulmonary disease and emphysema. The MESA COPD StudyAm J Respir Crit Care Med2015192557058026067761
- HasegawaMMakitaHNasuharaYRelationship between improved airflow limitation and changes in airway calibre induced by inhaled anticholinergic agents in COPDThorax200964433233819074932
- YoshimuraKSuzukiYUtoTSatoJImokawaSSudaTMorphological changes in small pulmonary vessels are associated with severe acute exacerbation in chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis2016111435144527418816
- SeemungalTADonaldsonGCPaulEABestallJCJeffriesDJWedzichaJAEffect of exacerbation on quality of life in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19981575 Pt 1141814229603117
- HurstJRVestboJAnzuetoAEvaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- Soler-CatalunaJJMartinez-GarciaMARoman SanchezPSalcedoENavarroMOchandoRSevere acute exacerbations and mortality in patients with chronic obstructive pulmonary diseaseThorax2005601192593116055622
- BurgeSWedzichaJACOPD exacerbations: definitions and classificationsEur Respir J Suppl20034146s53s12795331
- GreenbergSBAllenMWilsonJAtmarRLRespiratory viral infections in adults with and without chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2000162116717310903237
- TashkinDPCelliBSennSA 4-year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med2008359151543155418836213
- BarrRGBourbeauJCamargoCARamFSTiotropium for stable chronic obstructive pulmonary disease: a meta-analysisThorax2006611085486216844726
- RennardSIAndersonWZuWallackRUse of a long-acting inhaled beta2-adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200116351087109211316640
- RossiAKristufekPLevineBEFormoterol in Chronic Obstructive Pulmonary Disease (FICOPD) II Study GroupComparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPDChest200212141058106911948033
- JonesPWWillitsLRBurgePSCalverleyPMInhaled Steroids in Obstructive Lung Disease in Europe study investigatorsDisease severity and the effect of fluticasone propionate on chronic obstructive pulmonary disease exacerbationsEur Respir J2003211687312570111
- PeinadoVIPizarroSBarberaJAPulmonary vascular involvement in COPDChest2008134480881418842913
- SuzukiTTadaYKawataNClinical, physiological, and radiological features of asthma-chronic obstructive pulmonary disease overlap syndromeInt J Chron Obstruct Pulmon Dis20151094795426028967
- BarnesPJMechanisms in COPD: differences from asthmaChest2000117Suppl 210S14S10673467
- ZaniniAChettaAImperatoriASSpanevelloAOlivieriDThe role of the bronchial microvasculature in the airway remodelling in asthma and COPDRespir Res20101113220920222
- KasaharaYTuderRMTaraseviciene-StewartLInhibition of VEGF receptors causes lung cell apoptosis and emphysemaJ Clin Invest2000106111311131911104784
- KasaharaYTuderRMCoolCDLynchDAFloresSCVoelkelNFEndothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysemaAm J Respir Crit Care Med20011633 Pt 173774411254533
- DonaldsonGCSeemungalTABhowmikAWedzichaJARelationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax2002571084785212324669
- AnthonisenNRConnettJEKileyJPEffects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health StudyJAMA199427219149715057966841
- GagnonPCasaburiRSaeyDCluster analysis in patients with GOLD 1 chronic obstructive pulmonary diseasePLoS One2015104e012362625906326
- DecramerMCelliBKestenSLystigTMehraSTashkinDPUPLIFT investigatorsEffect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trialLancet200937496961171117819716598