Abstract
Introduction
Intergenerational associations in chronic obstructive pulmonary disease (COPD) have been well recognized and may result from genetic, gene environment, or exposure to life course factors. Consequently, adult offspring of parents with COPD may be at a greater risk of developing COPD. The aim of this study was to review the prevalence of co-occurrence of COPD in adult offspring with one or both parents having COPD independent of specific genetic variations.
Methods
In total, five databases were searched for original studies in which prevalence of COPD was reported in both offspring (children) and one or both parents. Studies were excluded if COPD was not clearly defined, COPD was linked to specific genetic variations, COPD was combined with other chronic respiratory conditions, or estimates included other first-degree relatives. Data extraction (ie, sample characteristics, prevalence of COPD, and odds ratio [OR] if reported) was completed by two independent reviewers. A meta-analysis of prevalence and OR was conducted, where possible.
Results
Of the 3,382 citations, 129 full texts were reviewed to include eight studies (six case–control, one cross-sectional, and one cohort) reflecting either prevalence of COPD in offspring of parents with COPD (descendent approach, n=3), which ranged from 0% to 17.3%, or prevalence of people with COPD reporting positive parental history of COPD (antecedent approach, n=5), for which the pooled prevalence was 28.6%. Offspring of people with COPD had 1.57 times greater odds (95% confidence interval =1.29–1.93; P<0.001) of having COPD compared with people not having a parental history of COPD.
Conclusion
The prevalence of COPD in adult offspring of people with COPD is greater than population-based estimates, and the ORs indicate a higher risk in this group. This offers clinicians a potential strategy for opportunistic screening, early identification, and intervention in this at-risk group.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common condition and is estimated to become the third leading cause of death by 2030.Citation1,Citation2 Globally, the prevalence of COPD has been estimated to be between 9% and 10%.Citation3 Although COPD is a progressive disease, early intervention strategies such as optimizing medication, exercise, and pulmonary rehabilitation have been shown to reduce the rate of disease progression and improve symptom control in people diagnosed with COPD.Citation4–Citation8 The high prevalence of undiagnosed COPD has been reported internationally.Citation9,Citation10 Underdiagnosis of COPD is problematic as symptoms of COPD may hinder an individual’s daily activities.Citation11,Citation12 Generalized population level screening for COPD is controversial and, currently, is not recommended for asymptomatic individuals as it is unclear whether early detection improves clinical outcomes.Citation13 Individuals may, however, present asymptomatic because of modification in activities that trigger symptoms.Citation14 Targeted case findings in the community and in primary care commonly focus on people with recognized COPD risk factors or exposures such as smokers and consider them as an at-risk population.Citation15,Citation16
Although cigarette smoking is a key risk factor for COPD development, not all COPD cases can be explained by smoking.Citation17 Lifetime exposure to smoke or pollution has been shown to increase a person’s risk of developing COPD.Citation18,Citation19 In addition, early life factors such as low birth weight, prematurity, nutrition, childhood respiratory illness, and exposure to passive smoking are associated with reduced lung function in later life.Citation20–Citation24
Familial or intergenerational associations for chronic respiratory disease have been observed with an increased risk of chronic bronchitis in people with family members having bronchitis.Citation25,Citation26 Strong familial associations have been reported for increased respiratory symptoms or conditions including wheezing, asthma, and lung cancer among twins and individuals living in the same household.Citation23,Citation27–Citation29 Currently, although alpha-1-antitrypsin deficiency is the only robustly defined inherited form of COPD that can explain familial aggregation of COPD,Citation30 <3% of people with COPD have this deficiency.Citation31 Intergenerational associations overlap with the risk factors associated with the development of COPD, including smoking behavior of parents and offspring, educational achievement, lung function, and conditions or diseases such as asthma and lung cancer.Citation29,Citation32–Citation35 Concepts, knowledge, and behaviors about health can be transmitted from one generation to the next.Citation36 Thus, offspring of people with COPD are considered as an at-risk group for the development of COPD, as they may be exposed to a greater number of these intergenerational factors that could trigger or have a cumulative effect that shapes the trajectory of lung health.Citation37
Apart from studies that focus on associations between genetic forms of COPD, specific genes, and lung function in families,Citation38,Citation39 there seem to be little direct data concerning the likelihood or specific risk of COPD in offspring of parents with COPD. The odds of having COPD have been shown to be 1.7–2.7 times higher in people with family history of COPD (all first-degree relatives included) than in those without family history.Citation40,Citation41 Adult offspring of people with COPD might present with a different risk profile as they are more likely to cohabitate during formative early life periods. It is hypothesized that offspring of people with COPD through exposure to intergenerational and life course factors will have higher rates or an increased risk of COPD compared with people without a parental history of COPD. The overall aim of this systematic review was to collate the reported evidence for co-occurrence and risk of COPD, especially in adult offspring with one or both parents having COPD independent of specific genetic variations.
The following were the two objectives of this review:
To report the prevalence of COPD in offspring of people with COPD and/or prevalence of a positive parental history of COPD in people with COPD.
To report the strength of the association between parental and offspring COPD status.
Methods
This review was designed and reported according to the recommendations in the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines, and the protocol was registered (PROSPERO no: CRD42015025228).Citation42
Study selection
Electronic databases (ie, Medline, CINAHL, EMBASE, SCOPUS, and Cochrane Library) were systematically searched from inception to mid-September 2015. Search terms () were adapted from previous systematic reviews on COPD and the effects of family history.Citation5,Citation8,Citation23,Citation29 Two researchers independently performed the systematic search following a prospectively planned search protocol that was developed with the aid of an academic librarian.
Table 1 Search strategy
Inclusion and exclusion criteria
Studies were included if data were reported or could be inferred for the prevalence of COPD, chronic bronchitis, and/or emphysema (through self-report, physician-diagnosed, spirometry-diagnosed, or symptom profile) in parents and adult offspring of people with COPD. No year or language limits were set for inclusion of studies in this review.
Studies were excluded: 1) if they did not define family history or specifically report offspring/parents within first-degree relative groups (due to possible inclusions of other family members such as siblings); 2) if they reported specific lung function parameters without a COPD classification definition; 3) if COPD, emphysema, or chronic bronchitis was combined with other respiratory conditions; 4) if data were limited to familial investigation of known genetic variations in COPD (alpha-1-antitrypsin deficiency); and 5) if studies were on participants from specific occupations/living environment (eg, coal miners, coke oven workers, and living close to asbestos factory).
Screening of studies
Two reviewers independently screened the titles and abstracts of citations returned from searches. Full texts of studies were reviewed by two independent reviewers for inclusion or exclusion. Reasons for excluding the studies were recorded, and discrepancies were discussed until consensus was reached. Reference lists of relevant articles were searched, and citation tracking was performed to retrieve any potential additional citations.
Data extraction and appraisal of methodological bias
By using a predetermined template (Table S1), two reviewers independently extracted data from the included studies with disagreements resolved by consensus. A ten-item methodological appraisal checklist for studies reporting prevalence data was used.Citation43 Interpretations specific to the review questions (Table S2) were used by two independent reviewers; when consensus was not reached, then a third reviewer was consulted. No studies were excluded on the basis of the methodological bias.
Data analysis
Study design, participant characteristics, and the method of COPD diagnosis were descriptively reported. Studies were collated and analyzed separately: 1) studies that considered people with confirmed COPD and the prevalence of COPD in their offspring (descendent approach); and 2) studies that considered prevalence of positive parental history of COPD in people with confirmed COPD (antecedent approach). If odds ratios (ORs) were not reported in the study but sufficient data were provided, then the ORs were calculated. The prevalence and ORs were compiled from the reported statistics or calculated when sufficient information was provided, the pooled prevalence and OR were calculated, and forest plots were created by using MedCalc for Windows, Version 15.11.4 (MedCalc Software, Ostend, Belgium). I2 statistics were used to assess the heterogeneity of the studies. The random-effects model was used due to the high level of heterogeneity between the studies.
Results
Search results
One hundred and twenty-nine full-text articles were reviewed (including 18 non-English articles; ). Studies were excluded for two main reasons: first (51% of the excluded studies), despite reporting data for family history of COPD in people with COPD, studies either did not define family history as a parental historyCitation44,Citation45 or reported a pooled analysis of family history that included various combinations of siblings, parents, offspring, and first-degree relatives.Citation46,Citation47 Second (33% of the excluded), studies reported on the prevalence of respiratory problems/symptoms or on measures of lung function in offspring of people with COPD without a reference to a COPD classification.Citation48–Citation50
Figure 1 Flow chart for identification of studies.
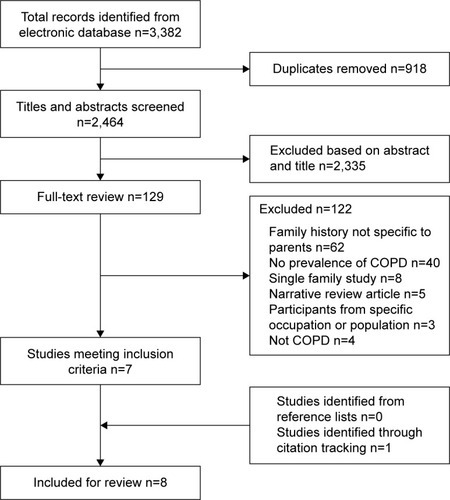
Seven studies were included after full-text review, and one additional study was identified through citation tracking, resulting in a total of eight studies eligible for inclusion in this review.
Study characteristics
All the included studies were observational in design. Of the eight studies, three studies reported on the prevalence of COPD in offspring of people with COPD (descendent approach, ), with a total of 204 cases and 161 controls in two case–control studies and 5,054 participants in a cross-sectional study,Citation51–Citation53 and five studies reported on the prevalence of a parental history of COPD in people with COPD (antecedent approach, ), with 1,545 cases and 1,995 controls in four case–control studies and 80,214 participants in a cohort study.Citation54–Citation58 None of the studies included in this review were primarily designed as prevalence studies, and all the studies included had a variable risk of bias when appraised using the prevalence appraisal checklist (Table S2).Citation43
Table 2 Summary of studies reporting on COPD prevalence in offspring of people with COPD (descendent approach)
Table 3 Summary of studies reporting prevalence of parental history of COPD in people with COPD (antecedent approach)
Prevalence
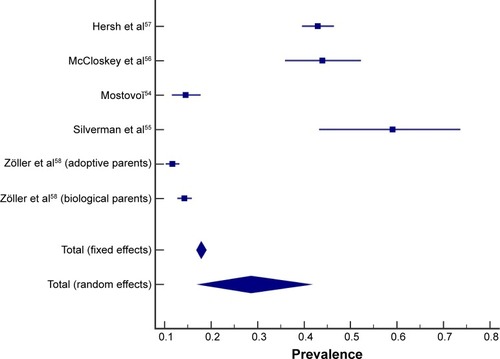
The prevalence of COPD in offspring of one or more parents with a diagnosis of COPD ranged from 0% to 17.3% in studies that used a descendent approach (). Studies that used the antecedent approach reported prevalence estimates ranging from 11.7% to 58% in people with COPD with a parental history of COPD ().
Pooled prevalence estimate
A pooled prevalence of COPD in offspring of people with COPD could not be calculated across the three studies that employed the descendent approach (), as one study reported prevalence of COPD in offspring for different age groups and a second study essentially excluded offspring with respiratory conditions.Citation52,Citation53
The calculated pooled prevalence of people with COPD with a positive parental history of COPD (antecedent approach) was 28.6% (random-effects model, 95% confidence interval [CI] =17.4–41.3; , ). One study provided prevalence of COPD in both biological and adoptive parents of people with COPD (both included in the pooled prevalence calculation).Citation58 Evidence of heterogeneity between the studies was found (I2=98.8%; P<0.0001). A study by Silverman et al (1998) included a specific group of participants (severe early-onset COPD).Citation55 When excluding Silverman et al (1998), the prevalence of people with COPD with a positive parental history of COPD was 24.0% (random-effects model, 95% CI =13.2–36.8; I2=99.0%; P<0.0001).Citation55
Pooled OR
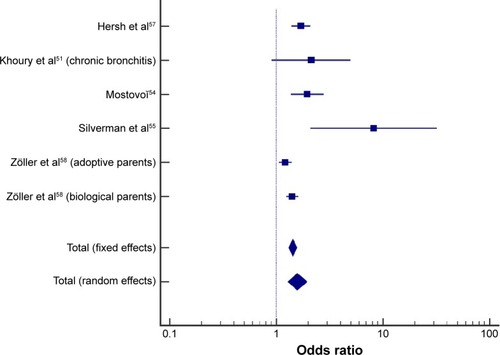
Of the eight included studies, three studies reported an OR (of the increased likelihood of offspring of people with COPD to develop COPD),Citation51,Citation55,Citation57 two studies reported enough information to calculate comparable ORs,Citation54,Citation58 and three studies did not provide sufficient information for OR calculation.Citation52,Citation53,Citation56 A study by Khoury et al reported the prevalence of chronic bronchitis and airway obstruction in the offspring of people with COPD.Citation51 However, this study defined airway obstruction (forced expiratory volume in 1 s [FEV1]/forced vital capacity [FVC] <68%) differently from the conventional ratio used for COPD diagnosis (FEV1/FVC <70%).Citation1 To maintain consistency across diagnosis methods through the studies, only the data on chronic bronchitis from the study by Khoury et al were used in the pooled analysis.Citation51
Meta-analysis of crude OR from five studies indicated that offspring of people with COPD had 57% higher odds of having COPD compared with people without a parental history of COPD (random-effects model, pooled OR =1.57; 95% CI =1.29–1.93; P<0.001; ). Evidence of heterogeneity between studies was found (I2=72%; P=0.0029). When the study by Silverman et al (in severe early-onset COPD) was excluded from the pooled analysis,Citation55 the heterogeneity decreased (I2=66%; P=0.0198), and offspring of people with COPD had 50% higher odds of having COPD compared with people without a parental history of COPD (random-effects model, pooled OR =1.50; 95% CI =1.26–1.77).
Discussion
This review specifically focused on the co-occurrence of COPD in parents and offspring. The pooled prevalence of people with COPD and a positive parental history of COPD was 28.6%, and offspring of people with COPD had 1.57 times greater odds of having COPD compared with people without a parental history of COPD. Although there is evidence to support familial associations in lung function decline in parent and offspring pairs,Citation59 this review identified a paucity of studies (especially recent publications) that provided or enabled an estimate of the prevalence of COPD, especially in offspring of people with COPD, to be obtained.
Due to the inconsistencies in the definition of family history and reporting of outcome measures, only a small number of studies met the inclusion criteria of this review. Lower values for pulmonary function in offspring of COPD probands compared with reference or non-COPD probands have been reported,Citation49,Citation60,Citation61 but in the absence of categorizing offspring with or without COPD or sufficient information to calculate COPD prevalence within offspring, these studies were excluded.
Variation in the original intent of the included studies resulted in differences in study design, source and nature of participants, and classification of COPD. Index cases in the case–control studies varied from people with chronic bronchitis to people with severe early-onset COPD,Citation54,Citation55 whereas index cases in the control groups varied from matched individuals to smokers.Citation52,Citation57 Apart from two older studies (published in 1975 and 1987), all other studies employed spirometry to classify COPD in offspring;Citation53,Citation54 only one study directly assessed both parents and offspring with spirometry.Citation56 Parental COPD history was self-reported by participants in all but one of the antecedent studies,Citation54–Citation57 with the remaining study using medical records to identify COPD in parents and offspring.Citation58 High prevalence of undiagnosed COPD has been reported worldwide;Citation10,Citation62 thus, identifying COPD by self-report or medical record screening may be an underestimation and not reveal the true prevalence of positive parental COPD history.Citation11,Citation59
Three of the eight studies used a descendent approach and reported a wide range of COPD prevalence (0%–17.3%) in offspring of people with COPD.Citation51–Citation53 Amra et al represent an outlier within this group as none of the offspring (n=54) met spirometric criteria for COPD (FEV1/FVC <0.7).Citation52 However, young mean age of offspring (34.3 years) and exclusion criteria applied to offspring cases (smokers, employed in high-risk jobs for the respiratory system, or had a pulmonary disease) biased the case group toward including only those with lower risk profile for COPD. The other two studies reported prevalence ranging between 6% and 17.3% which was higher than contemporaneous published prevalence estimates of COPD in the general population for a comparable age-group (4% in 25–44 years).Citation51,Citation53,Citation63
Across five studies that used the antecedent approach, between 11.7% and 58.0% of people with COPD reported a positive parental history.Citation54–Citation58 No comparable reviews or population-based data were found to compare these estimates of parental history of COPD; however, this prevalence appears high when compared to the estimate of prevalence of COPD in the general population (9%–10%).Citation3 Studies that recruited participants with moderate-to-severe COPD reported a relatively higher positive parental history of COPD (43%–58%) and alluded that the increased risk of COPD in families was likely to be the result of genetic factors.Citation55–Citation57 Although there are ongoing studies on genome-wide association exploring genotypes of COPD, there remain unanswered questions on COPD heritability and susceptibility.Citation64 Another possible explanation for the higher prevalence of positive parental COPD history reported by participants with more severe COPD may be an increased awareness of respiratory problems in their immediate family and greater vigilance or likelihood of reporting.Citation65
The risk of developing COPD in people with a parental history of COPD (OR =1.57) was comparable to those exposed to passive smoking (OR =1.48), maternal smoking (OR =1.7), and childhood pneumonia (OR =1.4) and lower compared with the effects of personal smoking in the development of COPD (OR =6.3).Citation18,Citation66–Citation68 Further epidemiological studies are required to promote understanding of the mechanism of interactions between various risk factors and differentiate shared lifestyle factors from true genetic risk in parents with COPD and their adult offspring.
Early identification of those at a risk of developing COPD is commonly based on modifiable behavior such as smoking or symptom profiles. Smokers are a common target population in COPD case-finding studies as smoking cessation is a well-recognized early intervention that slows the progression of the disease.Citation16,Citation69,Citation70 As universal screening of asymptomatic individuals for COPD is not recommended,Citation13 offspring of people with COPD may be considered as an at-risk group due to their shared genetic and environment factors, which presents an opportunity for case finding. By capitalizing on the increased prevalence and the risk of developing COPD in adult offspring of people with confirmed COPD, health practitioners working with people with COPD could use opportunistic screening of adult offspring to enable early lifestyle intervention strategies such as smoking cessation, physical activity, appropriate pharmacologic support, and pulmonary rehabilitation to reduce the rate of lung function decline, improve symptom control, and improve quality of life.Citation7,Citation71,Citation72
Limitations
This review included studies that were published over a 40-year period (1975–2015). The two oldest studies included in this review reported on the prevalence of COPD in offspring of people with COPD, and unsurprisingly the diagnostic criteria used differed from the current standard.Citation1,Citation51,Citation53 Diagnostic criteria and terminology for COPD have changed over time.Citation73 As no time limit was set for the systematic search strategy, studies were eligible for inclusion in this review if participants were classified as having emphysema, chronic bronchitis, or COPD. The prevalence of co-occurrence of COPD reported in this review may differ from the present day due to changes in the definition of COPD, lifestyle, and increased education and health promotion on the health consequences of smoking.Citation74
A critical appraisal checklist for studies reporting on prevalence data was used even though the included studies were not specifically designed to report prevalence.Citation43 As no studies were excluded from the review, modifications were required to adapt the checklist to determine the representativeness of the study sample and interpretation of the results specific to the review question.
Meta-analysis was performed despite significant heterogeneity among the studies. The pooled prevalence and OR calculated provided a summary of the available evidence but may not be generalizable due to the heterogeneity and limited populations of the included studies.
Conclusion
Although familial associations with COPD have been generally well recognized, the results of this review indicate that there were few studies that specifically reported on the co-occurrence of COPD diagnosis in both parents and offspring. Nevertheless, the findings of this review indicate that offspring of people with COPD are at an increased risk of developing COPD. Targeted screening in this at-risk group (adult offspring of people with COPD) may lead to opportunities to capitalize on familial links and promote early identification and intervention.
Author contributions
All the authors (LSKL, CP, KJ, and MTW) contributed substantially to the study design, data collection, data analysis and interpretation, and the writing and critical revision of the manuscript.
Acknowledgments
This systematic review abstract was presented at the Thoracic Society of Australia and New Zealand Annual Scientific Meeting (April 1–5, 2016) in Perth, Australia (poster). This project received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Supplementary materials
Table S1 Data extraction table
Table S2 Methodology quality assessment of the included studies
References
- HigginsMKellerJFamilial occurrence of chronic respiratory disease and familial resemblance in ventilatory capacityJ Chron Dis19752842392511127070
- KhouryMJBeatyTHTockmanMSSelfSGCohenBHFamilial aggregation in chronic obstructive pulmonary disease: use of the loglinear model to analyze intermediate environmental and genetic risk factorsGenet Epidemiol1985221551663876967
- MostovoĭIClinico-genetic research in chronic bronchitisTer Arkh19876035255 Russian
- SilvermanEKChapmanHADrazenJMGenetic epidemiology of severe, early-onset chronic obstructive pulmonary disease: risk to relatives for airflow obstruction and chronic bronchitisAm J Respir Crit Care Med19981576177017789620904
- McCloskeySCPatelBDHinchliffeSJReidEDWarehamNJLomasDASiblings of patients with severe chronic obstructive pulmonary disease have a significant risk of airflow obstructionAm J Respir Crit Care Med20011648 Pt 11419142411704589
- HershCPHokansonJELynchDAFamily history is a risk factor for COPDChest2011140234335021310839
- AmraBBorougeniVBGolshanMSoltaninejadFPulmonary function tests and impulse oscillometry in severe chronic obstructive pulmonary disease patients’ offspringJ Res Med Sci201520769770026622261
- ZöllerBLiXSundquistJSundquistKFamilial transmission of chronic obstructive pulmonary disease in adoptees: a Swedish nationwide family studyBMJ open201554e007310
- MunnZMoolaSLisyKRiitanoDThe Joanna Briggs Institute Reviewers’ Manual 2014. The Systematic Review of Prevalence and Incidence DataAdelaide, SAThe Joanna Briggs Institute2014
Disclosure
The authors report no conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Lung DiseasePocket Guide to COPD Diagnosis, Management and Prevention 20152015 Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Pocket_2015_Feb18.pdfAccessed March 25, 2015
- World Health OrganizationWorld Health Statistics: 20082008 Available from: http://www.who.int/gho/publications/world_health_statistics/EN_WHS08_Full.pdfAccessed March 30, 2015
- HalbertRNatoliJGanoABadamgaravEBuistAManninoDMGlobal burden of COPD: systematic review and meta-analysisEur Respir J200628352353216611654
- AbramsonMCrockettAJDabscheckEThe COPDX Plan: Australian and New Zealand Guidelines for the Management of Chronic Obstructive Pulmonary Disease 20142014 Available from: http://2014-new.copdx.org.au/wp-content/uploads/2011/08/COPDX-V2.39-October-2014_FINAL.pdfAccessed July 23, 2015
- NgaiSPJonesAYTamWTai Chi for chronic obstructive pulmonary disease (COPD)Cochrane Database Syst Rev20167CD00995327272131
- WelteTVogelmeierCPapiACOPD: early diagnosis and treatment to slow disease progressionInt J Clin Pract201569333634925363328
- McCarthyBCaseyDDevaneDMurphyKMurphyELacasseYPulmonary rehabilitation for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20152CD00379325705944
- MaltaisFDennisNChanCKRationale for earlier treatment in COPD: a systematic review of published literature in mild-to-moderate COPDCOPD20131017910323272663
- ToelleBGXuanWBirdTERespiratory symptoms and illness in older Australians: the Burden of Obstructive Lung Disease (BOLD) studyMed J Aust2013198314414823418694
- MoreiraGLManzanoBMGazzottiMRPLATINO, a nine-year follow-up study of COPD in the city of São Paulo, Brazil: the problem of underdiagnosisJ Bras Pneumol2014401303724626267
- SeymourJSpruitMHopkinsonNThe prevalence of quadriceps weakness in COPD and the relationship with disease severityEur Respir J2010361818819897554
- KesslerRPartridgeMRMiravitllesMSymptom variability in patients with severe COPD: a pan-European cross-sectional studyEur Respir J201137226427221115606
- Guirguis-BlakeJMSengerCAWebberEMMularskiRAWhitlockEPScreening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task ForceJAMA2016315131378139327046366
- LabakiWWMartinezCHHanMKCOPD in 2016: some answers, more questionsLancet Respir Med201641294194327890500
- SansoresRHRamírez-VenegasAHernández-ZentenoRPrevalence and diagnosis of chronic obstructive pulmonary disease among smokers at risk. A comparative study of case-finding vs screening strategiesRespir Med2013107458058623313037
- StratelisGJakobssonPMolstadSZetterstromOEarly detection of COPD in primary care: screening by invitation of smokers aged 40 to 55 yearsBr J Gen Pract20045450020120615006126
- EisnerMDAnthonisenNCoultasDAn official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2010182569371820802169
- YinPJiangCChengKPassive smoking exposure and risk of COPD among adults in China: the Guangzhou Biobank Cohort StudyLancet2007370958975175717765524
- HuGZhouYTianJRisk of COPD from exposure to biomass smoke: a metaanalysisChest20101381203120139228
- SvanesCOmenaasEJarvisDChinnSGulsvikABurneyPParental smoking in childhood and adult obstructive lung disease: results from the European Community Respiratory Health SurveyThorax200459429530215047948
- KouzounaAGilchristFBallVA systematic review of early life factors which adversely affect subsequent lung functionPaediatr Respir Rev201620677527197758
- EdwardsCAOsmanLMGoddenDJDouglasJGWheezy bronchitis in childhood: a distinct clinical entity with lifelong significance?Chest20031241182412853497
- BurkeHLeonardi-BeeJHashimAPrenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysisPediatrics2012129473574422430451
- PikeKPillowJJLucasJSLong term respiratory consequences of intrauterine growth restrictionSemin Fetal Neonatal Med2012172929822277109
- OswaldNHaroldJMartinWClinical pattern of chronic bronchitisLancet19532626787639643
- OgilvieAGChronic bronchitis in Newcastle upon TyneJ R Soc Promot Health19597918087
- TarnokiDLTarnokiALazarZA possible genetic influence in parenchyma and small airway changes in COPD: a pilot study of twins using HRCTActa Physiol Hung2014101216717524901078
- TagerIBRosnerBTishlerPVSpeizerFEKassEHHousehold Aggregation of Pulmonary Function and Chronic BronchitisAm Rev Respir Dis19761143485492970729
- MatakidouAEisenTHoulstonRSystematic review of the relationship between family history and lung cancer riskBr J Cancer200593782583316160696
- CastaldiPJChoMHCohnMThe COPD genetic association compendium: a comprehensive online database of COPD genetic associationsHum Mol Genet201019352653419933216
- IoachimescuOCStollerJKA review of alpha-1 antitrypsin deficiencyCOPD20052226327517136953
- BurkeWFesinmeyerMReedKHampsonLCarlstenCFamily history as a predictor of asthma riskAm J Prev Med200324216016912568822
- PalmerLKnuimanMDivitiniMFamilial aggregation and heritability of adult lung function: results from the Busselton Health StudyEur Respir J200117469670211401066
- DustmannCParental background, secondary school track choice, and wagesOxf Econ Pap2004562209230
- BaileySLEnnettSTRingwaltCLPotential mediators, moderators, or independent effects in the relationship between parents’ former and current cigarette use and their children’s cigarette useAddic Behav1993186601621
- TaylorJPriceKBraunack-MayerAHarenMTMcdermottRIntergenerational learning about keeping health: a qualitative regional Australian studyHealth Prom Int2014292361368
- Ben-ShlomoYKuhDA life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectivesInt J Epidemiol200231228529311980781
- JoostOWilkJBAdrienne CupplesLGenetic loci influencing lung function: a genomewide scan in the Framingham studyAm J Respir Crit Care Med2002165679579911897646
- CastaldiPDeMeoDKentDDevelopment of predictive models for airflow obstruction in alpha-1-antitrypsin deficiencyAm J Epidemiol200917081005101319726494
- NihlénUNybergPMontnémeryPLöfdahlC-GInfluence of family history and smoking habits on the incidence of self-reported physician’s diagnosis of COPDRespir Med200498326327015002763
- HooperRBurneyPVollmerWMRisk factors for COPD spiro-metrically defined from the lower limit of normal in the BOLD projectEur Respir J20123961343135322183479
- LiberatiAAltmanDGTetzlaffJThe PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaborationAnn Intern Med200915146594
- MunnZMoolaSLisyKRiitanoDThe Joanna Briggs Institute Reviewers’ Manual 2014. The Systematic Review of Prevalence and Incidence DataAdelaide, SAThe Joanna Briggs Institute2014
- PallasahoPKainuASovijärviALindqvistAPiiriläPLCombined effect of smoking and occupational exposure to dusts, gases or fumes on the incidence of COPDCOPD2014111889524111617
- ÖzgeAÖzgeCKaleagasiHYalinOÖÜnalÖÖzgürESHeadache in patients with chronic obstructive pulmonary disease: effects of chronic hypoxaemiaJ Headache Pain200671374316408153
- CeledonJSpeizerFDrazenJBronchodilator responsiveness and serum total IgE levels in families of probands with severe early-onset COPDEur Respir J19991451009101410596682
- ZhouYWangCYaoWCOPD in Chinese nonsmokersEur Respir J200933350951819251797
- LebowitzMHolbergCMartinezFA longitudinal study of risk factors in asthma and chronic bronchitis in childhoodEur J Epidemiol1990643413472091933
- KawakamiYIrieTKishiFFamilial aggregation of abnormal ventilatory control and pulmonary function in chronic obstructive pulmonary diseaseEur J Respir Dis198162156647227484
- HansenJGaoWDupuisJAssociation of 25-hydroxyvitamin D status and genetic variation in the vitamin D metabolic pathway with FEV1 in the Framingham Heart StudyRespir Res20151611825567521
- KhouryMJBeatyTHTockmanMSSelfSGCohenBHFamilial aggregation in chronic obstructive pulmonary disease: use of the loglinear model to analyze intermediate environmental and genetic risk factorsGenet Epidemiol1985221551663876967
- AmraBBorougeniVBGolshanMSoltaninejadFPulmonary function tests and impulse oscillometry in severe chronic obstructive pulmonary disease patients’ offspringJ Res Med Sci201520769770026622261
- HigginsMKellerJFamilial occurrence of chronic respiratory disease and familial resemblance in ventilatory capacityJ Chron Dis19752842392511127070
- MostovoĭIClinico-genetic research in chronic bronchitisTer Arkh19876035255 Russian
- SilvermanEKChapmanHADrazenJMGenetic epidemiology of severe, early-onset chronic obstructive pulmonary disease: risk to relatives for airflow obstruction and chronic bronchitisAm J Respir Crit Care Med19981576177017789620904
- McCloskeySCPatelBDHinchliffeSJReidEDWarehamNJLomasDASiblings of patients with severe chronic obstructive pulmonary disease have a significant risk of airflow obstructionAm J Respir Crit Care Med20011648 Pt 11419142411704589
- HershCPHokansonJELynchDAFamily history is a risk factor for COPDChest2011140234335021310839
- ZöllerBLiXSundquistJSundquistKFamilial transmission of chronic obstructive pulmonary disease in adoptees: a Swedish nationwide family studyBMJ open201554e007310
- Kurzius-SpencerMSherrillDLHolbergCJMartinezFDLebowitzMDFamilial correlation in the decline of forced expiratory volume in one secondAm J Respir Crit Care Med200116471261126511673220
- LuBHeQCorrelation of pulmonary functions of COPD patients to those of their first-degree childrenChin Med J2003116799199512890369
- GivelberRJCouropmitreeNNGottliebDJSegregation analysis of pulmonary function among families in the Framingham StudyAm J Respir Crit Care Med19981575144514519603122
- MiravitllesMSorianoJBGarcia-RioFPrevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activitiesThorax2009641086387319553233
- ManninoDMHomaDMAkinbamiLJFordESReddSCChronic obstructive pulmonary disease surveillance – United States, 1971–2000Respir Care2002761011841199
- HardinMSilvermanEKChronic obstructive pulmonary disease genetics: a review of the past and a look into the futureJ COPD F2014113346
- MadisonRMittmanCAfifiAZelmanRRisk factors for obstructive lung diseaseAm Rev Respir Dis198112421491536973302
- UptonMNSmithGDMcConnachieAHartCLWattGCMaternal and personal cigarette smoking synergize to increase airflow limitation in adultsAm J Respir Crit Care Med2004169447948714630616
- HaydenLPHobbsBDCohenRTChildhood pneumonia increases risk for chronic obstructive pulmonary disease: the COPDGene studyRespir Res201516111526392057
- LøkkeALangePScharlingHFabriciusPVestboJDeveloping COPD: a 25 year follow up study of the general populationThorax2006611193593917071833
- DeJongSRVeltmanRHThe effectiveness of a CNS-led community-based COPD Screening and Intervention ProgramClin Nurse Spec2004182727915164668
- SorianoJBZielinskiJPriceDScreening for and early detection of chronic obstructive pulmonary diseaseLancet2009374969172173219716965
- Garcia-AymerichJSerraIGómezFPPhysical activity and clinical and functional status in COPDChest20091361627019255291
- VinckenWVan NoordJGreefhorstAImproved health outcomes in patients with COPD during 1 yr’s treatment with tiotropiumEur Respir J200219220921611871363
- FishmanAPOne hundred years of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2005171994194815849329
- NgMFreemanMKFlemingTDSmoking prevalence and cigarette consumption in 187 countries, 1980–2012JAMA2014311218319224399557