Abstract
COPD is recognized as having a series of comorbidities potentially related to common inflammatory processes. Periodontitis is one of the most common human inflammatory diseases and has previously been associated with COPD in numerous observational studies. As periodontitis and COPD are both chronic, progressive conditions characterized by neutrophilic inflammation with subsequent proteolytic destruction of connective tissue, it has been proposed that they share common pathophysiological processes. The mechanisms proposed to link COPD and periodontitis include mechanical aspiration of oral contents into the respiratory tree, overspill of locally produced inflammatory mediators into the systemic circulation or oral or lung-derived bacteremia activating an acute-phase response and also reactive oxygen species (ROS) and cytokine release by systemic neutrophils at distant sites. Studies of systemic neutrophils in COPD and chronic periodontitis describe altered cellular functions that would predispose to inflammation and tissue destruction both in the lung and in the mouth, again potentially connecting these conditions. However, COPD and periodontitis also share risk factors such as age, chronic tobacco smoke exposure, and social deprivation that are not always considered in observational and interventional studies. Furthermore, studies reporting associations have often utilized differing definitions of both COPD and periodontitis. This article reviews the current available evidence supporting the hypothesis that COPD and inflammatory periodontal disease (periodontitis) could be pathologically associated, including a review of shared inflammatory mechanisms. It highlights the potential limitations of previous studies, in particular, the lack of uniformly applied case definitions for both COPD and periodontitis and poor recognition of shared risk factors. Understanding associations between these conditions may inform why patients with COPD suffer such a burden of comorbid illness and new therapeutic strategies for both the diseases. However, further research is needed to clarify factors that may be directly causal as opposed to confounding relationships.
Introduction
COPD is defined as a chronic, progressive disease characterized by airflow obstruction due to an enhanced chronic inflammatory response within the airways.Citation1,Citation2 COPD is an important cause of morbidity and mortality and is now recognized as having a series of comorbidities hypothesized to be related to a common systemic or organ-specific inflammatory process. This concept is reflected in the updated Global initiative for chronic Obstructive Lung Disease (GOLD) classification systems for COPD. GOLD now recognizes the significance of extra-pulmonary effects of COPD and that comorbidities contribute to the overall severity of disease and as such should be actively sought and treated.Citation3 An observational study found that >97% of a COPD cohort had at least one other comorbidity, with >50% having at least 4.Citation4 This impacts the health care costs of COPD patients with multiple comorbidities that are 4.7 times higher than those with no comorbidity.Citation5
Respiratory comorbidities associated with COPD include bronchiectasis,Citation6 asthma, pulmonary fibrosis,Citation7 and lung cancer,Citation7 but extra thoracic chronic inflammatory disease also co-occurs.Citation8 Cardiovascular diseases are the most common,Citation9 including hypertension,Citation7 venous thromboembolism, ischemic heart disease, arrhythmias,Citation7,Citation10 and heart failure.Citation7,Citation11 This high prevalence may, in part, reflect the presence of common risk factors including age, smoking, and sedentary lifestyle, but even when such factors are accounted for, there remains increased risk, supporting linked pathogenic drivers of disease. Cerebrovascular disease,Citation9,Citation10,Citation12 cognitive impairment,Citation13 diabetes,Citation9,Citation14 skeletal muscle dysfunction,Citation15 low bone mineral density and osteoporosisCitation11,Citation16 also frequently accompany COPD and support the hypothesis that the pathophysiology underlying these conditions relates to systemic inflammation and hypoxia.Citation11,Citation13 Recently, there has been increasing interest in the association between several chronic diseases including COPD and the chronic inflammatory oral disease, periodontitis, although the underlying nature of the association is unclear.
Periodontitis
Teeth are attached to the surrounding and supporting alveolar bone by periodontal ligament fibers that run from the bone into the cementum on the entire root surface of teeth. Periodontitis is a chronic, bacteria-initiated inflammatory disease, affecting ~50% of adults, with 11.2% suffering from severe periodontitis.Citation17 Sites with periodontitis exhibit clinical signs of gingival inflammation and loss of connective tissue attachment. Plaque biofilm accumulation above and below the gingival margin leads to a dysbiosis (a microbial imbalance or maladapted inflammatory response to bacteria), which in turn drives a dysfunctional host-immune-inflammatory response, connective tissue destruction, and the formation of periodontal pockets.Citation18 The active periodontal lesion is neutrophil and B-cell dominant and associated with systemic inflammation, probably arising due to repeated bacteremias of periodontal origin activating an acute-phase response.Citation19 This subsequently triggers priming and activation of peripheral blood neutrophils with an exaggerated release of reactive oxygen species (ROS) and pro-inflammatory cytokines.Citation20 Finally, the local destructive inflammatory processes lead to loss of alveolar bone and subsequent tooth loss.Citation18
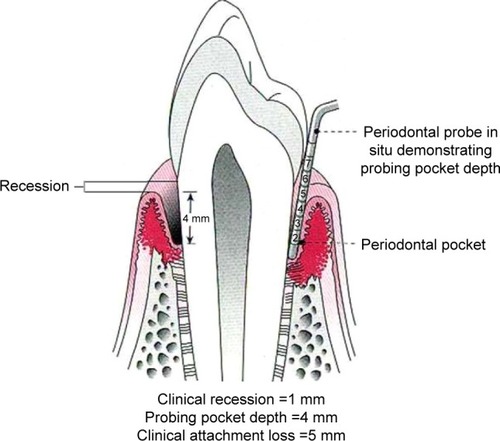
Generally, a diagnosis of periodontitis is based on the combination of periodontal and gingival inflammation, determined as the presence of sub-gingival bleeding on probing (BOP), the number and depth of periodontal pockets, the amount of clinical attachment loss (CAL), and presence of radiologically evaluated alveolar bone loss.Citation21–Citation23 Probing pocket depth (PPD) and CAL are measured by using manual or controlled force periodontal probes. The PPD is the distance from the gingival margin to the base of the gingival sulcus (or periodontal pocket). CAL is the distance from the cemento–enamel junction to the base of the sulcus or periodontal pocket.Citation21 summarizes these parameters.
Figure 1 Probing pocket depth (PPD – distance in millimeter from gingival margin to base of pocket) and clinical attachment loss (CAL – distance in millimeter from cement–enamel junction to base of pocket).
Periodontitis has also been linked to a number of other chronic systemic diseases as well as to COPD. These include diabetes mellitus,Citation24,Citation25 rheumatoid arthritis,Citation25,Citation26 and cardiovascular diseases. The increased prevalence of cardiovascular disease,Citation27,Citation28 hypertension,Citation25 and stroke in periodontal diseaseCitation25,Citation29 has been attributed to a common susceptibility hypothesis, that of direct bacterial invasion of blood vessels, and/or a systemic inflammatory response leading to increased circulating cytokines that damage vascular endothelium.Citation30 Importantly, treating periodontitis in patients with common comorbidities has shown to be an effective way of reducing both financial costs and admissions for cardiovascular disease and diabetes mellitus,Citation31 lending credence to this hypothesis. Thus, both periodontitis and COPD occur with a similar spectrum of comorbid disease. This needs recognition and documentation when reporting associations between periodontitis and COPD as it likely reflects a subgroup of patients with a predisposition or the presence of common etiological factors for this spectrum of diseases.
In keeping with this, there are important similarities in both the mechanisms underpinning periodontitis and COPD and the risk factors for developing these conditions that are worthy of exploration. Both periodontitis and COPD are chronic and progressive conditions characterized by neutrophilic inflammation with subsequent proteolytic destruction of connective tissues.Citation32–Citation34 Of note, not all patients with common risk factors (chronic cigarette smoke inhalation for both condition and poor dental hygiene for periodontitis) develop disease, for example, only 20% of smokers go on to develop significant COPD,Citation35 supporting the concept of susceptibility. They also share several other common risk factors including age and poor socioeconomic status.Citation33,Citation36
As both the conditions result in tissue destruction by neutrophilic inflammation, it has been suggested that they share common pathophysiological processes. If true, this would have important implications for the diagnosis and potentially even the treatment of these conditions, as diagnosing and controlling one with an appropriate intervention may reduce severity and/or progression in the other.
Mechanisms
There are several mechanisms that may potentially link respiratory disease with periodontitis.
Aspiration of potentially pathogenic bacteria
The first is mechanical; the aspiration of oral contents including microbial pathogens into the respiratory tract with airway inflammation and remodeling (caused by COPD) supporting adherence, immune evasion, colonization, and subsequent pulmonary infections. Micro-aspiration is common even in healthy patients and frequently occurs during sleep. Studies have shown that typical volumes aspirated are of a magnitude likely to contain bacterial pathogens.Citation37 In healthy patients, there are defense mechanisms to ensure that, despite aspiration, the distal airway and lung parenchyma remain protected.Citation38 This clearance is mediated by both mechanical and immunological mechanisms and includes cough and mucociliary clearance of pathogens and phagocytosis of microorganisms by local immune cells.Citation37 However, in patients with underlying chronic health problems, aspiration of oral secretions containing bacterial pathogens may not always be cleared.Citation39,Citation40 In these cases, the periodontium could act as a repository of bacteria, enhancing respiratory tract colonization and inflammation. For COPD patients, this is important as recurrent infections causing exacerbations are known to amplify the decline in lung functionCitation41 but current evidence to support the link between COPD and periodontitis remains, at best, indirect.
Oral colonization by respiratory pathogens, fostered by poor oral hygiene and periodontal diseases, seems to be associated with nosocomial pneumonia in some studies. A recent meta-analysis identified 11 observational studies of intubated patients where periodontal disease was assessed with respiratory infections as an outcome.Citation42 Approximately half of these studies reported an association between dental status and ventilator-associated pneumonia, with one studyCitation43 reporting that the pathogen thought to cause pneumonia had first colonized the dental plaque biofilm. However, these studies were conducted in extremely unwell patients who were at significant risk of pneumonia and some did not include a control population. Studies have associated pneumonia with poor dental hygiene and periodontitis in both the community and the outpatient setting but most do not provide information about potentially confounding factors (comorbidities, smoking status, etc) or information about microbial pathogens. There are five intervention trialsCitation44–Citation48 where a mixed population of patients received treatments to lower oropharyngeal bacterial load, and a meta-analysis suggested that oral hygiene and antibiotic intervention significantly reduced the risk of developing pneumonia in these populations, supporting a causal link between oral pathogens and lower respiratory tract infections.Citation42 However, once again, the majority of studies were conducted in an intensive care setting, and it is unclear whether the findings are generalizable.
Approximately 10 bacterial species have been identified as putative pathogens in inflammatory periodontal diseases. These are mainly gram-negative rods and include Aggregatibacter actinomycetemcomitans in aggressive periodontitis and Porphyromonas gingivalis, Treponema denticola, and Tanerella forsythia in chronic periodontitis.Citation49 However, these are not commonly associated with lower respiratory infections in the general population or in COPD, and there are no studies in COPD patients that have directly associated oral pathogens with COPD exacerbations.
Periodontitis as an inflammatory reservoir
Alternative hypotheses linking the two conditions suggest bacterial aspiration is not required to initiate shared pathology. The entry of periodontal bacteria into the vasculature during eating and tooth brushing can trigger an acute-phase response in the systemic circulation as evidenced by elevated serum levels of inflammatory mediators.Citation21 In keeping with this hypothesis, periodontitis is now recognized as a significant and independent risk factor for atherogenic cardiovascular disease, and the mechanisms seem to involve periodontal pathogens disseminating throughout the vasculature.Citation27 Moreover, peripheral blood neutrophils from periodontitis patients exhibit hyper-reactivity to bacterial stimuli and hyperactivity (in absence of a stimulus) with respect to ROS and pro-inflammatory cytokine release.Citation50–Citation54 Alternatively, the local inflammatory response to periodontitis could lead to an “overspill” of periodontal cytokines into the systemic circulation, with subsequent distant inflammatory sequelae. Evidence to support this is weakCitation55 although this may just reflect the rapid distribution and binding of pro-inflammatory cytokines to receptors as argued for COPD.Citation56 Nevertheless pro-inflammatory cytokine networks have been found to be activated in periodontitis tissuesCitation57 and many of the same cytokines have been found to be raised in bloodCitation58–Citation60 and lung secretions from patients with COPDCitation61–Citation65 at least supporting a common inflammatory process.
If present, any shared mechanisms are likely to impact on both the diseases, with periodontal inflammation and infection impacting on lung pathology and inflammatory lung disease amplifying periodontal disease risk. These mechanisms are summarized in .
Figure 2 Shared inflammatory mechanisms that might facilitate disease progression in both COPD and periodontitis.
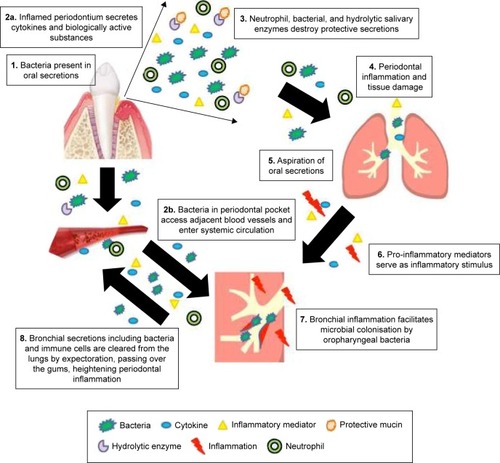
The most compelling evidence for a common mechanism causing both periodontitis and COPD is that of the neutrophilic inflammation and neutrophil-mediated tissue damage that characterizes both the diseases.
Neutrophilic inflammation in COPD
Neutrophils have a key role in inflammatory processes, and there is compelling evidence to support their role in the pathogenesis of COPD. Neutrophils have been implicated in the development and progression of all of the pulmonary features of COPD in animal and cell studies, including emphysema through the release of destructive mediators such as neutrophil elastase and matrix metalloproteinases (MMPs)Citation66 and chronic bronchitis through mucous gland hyperplasia.Citation68 Pulmonary neutrophilic inflammation is a feature of cigarette smoking, but importantly, in patients with COPD it is sustained even following smoking cessation,Citation68,Citation69 though not in smokers without airflow obstruction.
As well as being increased in number in bronchial tissue and airway secretions, there is evidence to suggest that neutrophils in COPD patients are also dysfunctional. Circulating neutrophils produce more ROS than those from healthy nonsmokers or smokers with normal lung function.Citation70 The cells also have enhanced, rapid, and tortuous chemotactic responses to inflammation and evidence of increased degranulation and tissue destruction both in vitro and systemically.Citation71,Citation72
The level of neutrophilic inflammation in airways correlates with disease severity and progression in COPD whether measured by lung physiology, radiologically, or health status.Citation63,Citation73–Citation76 These data support the concept that activated neutrophils play an important role in lung injury and emphysema through neutrophilic release of inflammatory cytokines, destructive proteases such as neutrophil elastase, proteinase 3, MMP-8, and MMP-9Citation77–Citation79 all of which are capable of degrading components of the extracellular matrix, promoting inflammatory responses in the surrounding tissueCitation80 and increasing the generation of ROS.Citation70 Cigarette smoking further enhances recruitment of these activated neutrophils with more subsequent release of tissue destructive proteinases,Citation81 supporting the faster physiological decline seen in COPD patients who continue to smoke.
Neutrophils in periodontitis
Periodontitis is also characterized by a neutrophilic infiltrate.Citation82 A review by HajishengallisCitation83 highlighted the increased prevalence of periodontitis in conditions characterized by abnormal neutrophil function. These include defective neutrophil production as seen in autoimmune neutropenia or chemotherapy-induced neutropenia, defective neutrophil release from the bone marrow as seen in WHIM (warts, hypogammaglobulinemia, infections, and myelokathexis), inherited leukocyte adhesion deficiency, excessive neutrophil activation, deficient clearance of neutrophils as in autoimmune inflammatory diseases including systemic lupus erythematosus and the Papillon–Lefevre syndrome (neutrophil cathepsin C deficiency).
There is evidence of both hyper-reactive and hyperactive neutrophil functions in periodontitis. Systemic neutrophil chemotaxis studies in patients with chronic periodontitis showed reduced speed (movement in any direction), velocity (speed of movement toward the chemoattractant), and accuracy (accuracy of movement toward the chemoattractant).Citation84 This behavior improved after treatment of periodontitis,Citation85 suggesting a causal link between periodontitis and the systemic cell function. A similar pattern of neutrophil migration has been described in COPD where cells displayed poor migratory accuracy but with random movement in all directions at increased speed.Citation72 Recent publications have also described compromised neutrophil bacterial phagocytosis in periodontitisCitation86 (studies in COPD are less clear); enhanced neutrophil-derived ROSCitation87,Citation88 and increased NET formation in both COPD and periodontitis,Citation89,Citation90 all of which could contribute to the sustained inflammation that characterizes both the conditions.Citation34,Citation53 The cause for such injurious cell behavior is unclear, and it is unknown whether neutrophil dysfunction precedes or is a consequence of disease. However, these impaired neutrophil functions may mean that the host is less able to deal efficiently with bacteria either in the periodontium or in the lung. Furthermore, as neutrophils release both proteinasesCitation91,Citation92 and ROS while migrating through complex tissues,Citation93 it is possible that the tortuous abnormal migratory pathways lead to increased by-stander tissue damage, thereby perpetuating the inflammatory response leading to excess tissue damage seen as alveolar bone loss in the mouth and emphysema and COPD in the lung. This concept has been supported in COPD where there is systemic evidence of increased proteinase activity,Citation94 but remains unconfirmed in periodontitis.
COPD and periodontitis
There is evidence of a clinical association between periodontitis and COPD including a meta-analysis of 14 observational studies.Citation36 The overall sample size represented 3,988 COPD patients and 22,871 controls. The authors reported a pooled odds ratio (OR) for having COPD in populations with periodontitis as 2.08 (95% confidence interval [CI] 1.48–2.91; P<0.001).
Alternatively, data from a large longitudinal COPD cohort study found that patients had a significantly increased cumulative incidence of periodontitis with adjusted hazard ratio (HR) of 1.20 (95% CI 1.15–1.25) rising to 3.17 (95% CI 2.81–3.57) in the subgroup that had required hospitalization for COPD.Citation33 The risk of developing periodontitis increased proportionately with the annual number of emergency room visits and admissions for COPD.Citation33 A further in-patient study supported these findings, noting significantly increased markers of periodontitis in a hospitalized COPD group compared with an in-patient control group (probing depth [PD] 2.57±0.85 vs 1.76±0.7, P<0.0001; and clinical attachment level [CAL] 3.16±0.53 vs 2.12±0.92, P<0.0001).Citation95
A recent, large cross-sectional study found that after adjustment for the confounders of age, smoking, socioeconomic status, and dental habits, moderate periodontitis was still associated with COPD in male patients (relative risk =1.38, 95% CI 1.12–2.05), but not female patients, and the relative risk of severe periodontitis in the male group was 1.23 (95% CI 1.06–1.56).Citation96 Prasanna also found significantly higher periodontal indices in COPD patients in comparison with non-COPD controls that persisted after correction for age.Citation40 A Chinese case–control study of 306 dentate COPD patients and 328 dentate healthy controls found the COPD cohort had fewer remaining teeth and a greater percentage of the remaining teeth had CAL ≥4 mm.Citation97 In addition, an Indian observational study of 150 dentate patients also found patients with COPD had significantly higher mean CAL measurements.Citation98
Using a different measure of periodontitis, Leuckfeld et al assessed orthopantomograms in a retrospective cross-sectional study of patients evaluated for lung transplant. They found a significant difference in radiographically determined periodontitis, with 44% of COPD patients affected compared with 7% of non-COPD patients. The results also remained significantly different after adjustment for age, gender, and smoking.Citation99
Several studies have also found associations between indices of periodontitis and lung function measurements. An observational study of 102 dentate COPD patients found significantly worse indices of periodontitis after adjusting for age and smoking.Citation100 The same study confirmed a significant correlation between several adverse periodontal outcomes; CAL, PD, and gingival index (GI) and forced expiratory volume in 1 s (FEV1) (r=−0.63, −0.60, and −0.44, respectively; P<0.0001). A large German prospective cohort study of 1,268 people found that pulmonary function parameters were significantly associated with CAL measurements after adjustment for confounding factors.Citation101
However, other studies have failed to find such associations. Bergstrom et alCitation102 found that smoking rather than COPD was the major risk factor for periodontal pocket depth (OR 24.2; 95% CI 2.0–286.8). One analysis of the NHANES III survey found no relationship between the two diseases, although subgroup analysis found an association in current smokers aged ≥30 years with CAL ≥4 mm.Citation103 Finally, a cross-sectional study of 392 dentate patients with COPD was also unable to find significant differences between periodontal measurements and exacerbation rates.Citation104 However, the lack of consistency in findings also reflects the lack of consistency in case definitions, data collection, and analysis (eg, case definitions vs continuous outcome variables such as PPD, CAL, etc).
Potential limitations of these studies
The data summarized above remains controversial as there are several potential limitations in the studies that have assessed associations between COPD and periodontitis.
First, many studies have not accounted for common risk factors that might contribute to the presence of both periodontitis and COPD. For example, the longitudinal study by Shen et al failed to correct for smoking status, dental habits, or socioeconomic status,Citation33 and no adjustment was made for socioeconomic factors in the radiographic study by Leuckfeld et al.Citation99 Where appropriate allowance had occurred, the OR for having COPD in the populations with periodontitis (OR 1.78; 95% CI 1.23–2.58) was lower than initially reported.Citation36
Second, the manner in which the diseases are diagnosed in the epidemiological studies has a significant impact on the results of prevalence and associations. For epidemiological studies, a case definition needs to be easily and accurately measurableCitation21 and a uniform definition is essential to allow calculations and comparisons of relative risks to be made across studies.Citation105
Most studies investigating associations between COPD and periodontitis base the diagnosis of COPD on post- bronchodilator spirometry and GOLD staging.Citation95,Citation96,Citation100,Citation102,Citation103,Citation106 However, some authors used pre-bronchodilator spirometry,Citation107 which could lead to an overestimation of the prevalence of COPD, others report lung function measurements but not whether pre- or post-bronchodilation,Citation40,Citation98 and one study failed to mention how COPD was confirmed.Citation99 A German prospective study used body plethysmography to determine lung function,Citation101 whereas Shen et al used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes that do not provide details of how the diagnosis was established.Citation33
Third, there is also great variation in how authors define periodontitis. Savage et al conducted a systematic review of 3,472 papers analyzing 104 of these in detail and found that only 15 studies gave a definition of periodontitis.Citation23 This is a continuing problem in the literature studying COPD and periodontitis. Published studies have used various periodontal measurement parameters for analysis but do not always give a specific definition for the disease,Citation40,Citation95,Citation100,Citation104,Citation107 which likely reflects the lack of a uniformly accepted definition of periodontitis. When defined, measurements to determine the presence or severity of periodontitis vary and include one or a combination of the PPD, CAL, bleeding index (BI), gingival index (GI), plaque index (PI), or oral hygiene index (OHI), reflecting the lack of an agreed GOLD standard. Wang et al measured PPD, CAL, and BI, but in their statistical analysis report, the percentage of sites with PD of ≥4 and 5 mm and CAL ≥3 and 4 mm,Citation97 since these values are regarded as representing active periodontitis (PPD >4 mm). Deo et al measured PPD, CAL, and OHI but reported only on CAL and OHI.Citation98 Other studies have favored the use of PD and CAL in combination.Citation103,Citation106 However, Hyman et al only found significant associations with CAL alone.Citation103 This may reflect the fact that PPD used in isolation accounts only for current disease status, whereas CAL represents the cumulative periodontal tissue damage with age.Citation102
The studies by Chung et al and Kim et al used the crude screening index called the World Health Organization community periodontal index to screen for periodontitis in large populations, defining the disease as present with a score of 3 (>3.5 mm pocket) or 4 (>5.5 mm pocket).Citation96,Citation108 Mean pocket depth of ≥4 mm was arbitrarily chosen as a cut-off point to define periodontitis in the Bergstrom et al’s study,Citation102 since this value represents clinical pathology, and pocket depths of missing teeth were set as the values of neighboring tooth sites. Interestingly, when analyzed separately, recession was more prevalent in smokers, suggesting measurement of pocket depth alone may underestimate disease severity in smoking groups.Citation102
Two further studies employed very different methods to define periodontitis. Leuckfeld et al used mean marginal bone level ≥4 mm on orthopantomograms,Citation99 and Shen et al used ICD-9-CM codes.Citation33 Although radiographs allow assessment of cumulative effects on alveolar bone loss, they do not allow assessment of current disease activity, they are not always available, and radiation exposure for epidemiological purposes is unlikely to be acceptable.Citation22
In younger populations, PPD and CAL correlate well, but as people age, gingival recession and therefore measures of attachment loss become a normal part of the ageing process. In addition, increased PPD may arise as a false-positive clinical finding. This can occur when gingival tissues become swollen due to inflammation (in the absence of periodontitis), thus moving the gingival margin upwards (false pocketing). Periodontitis can progress without significant pockets if gingival recession is also present, as frequently found in smokers.Citation23 The use of CAL alone may also overestimate periodontitis because attachment loss can occur with non-inflammatory gingival recession, such as that induced by trauma from over tooth brushing.Citation21
During the 5th European Workshop in Periodontology, a two threshold level criterion was proposed to diagnose periodontitis. This was the presence of proximal attachment loss of ≥3 mm in ≥2% of nonadjacent teeth to diagnose incipient cases and the presence of proximal attachment loss of ≥5 mm in ≥30% of teeth to determine severe disease. However, the authors of this proposal also stated that a single-index measurement of periodontitis does not uniformly reflect the disease and that these criteria are designed for use in studies determining risk factors for periodontitis and not the prevalence of periodontitis.Citation105
In an attempt to produce a standard case definition for periodontitis, the Division of Oral Health at the Centers for Disease Control and Prevention (CDC) alongside the American Academy of Periodontology appointed a working group in 2003Citation21 to define severe and moderate periodontitis. Severe periodontitis required involvement of two or more interproximal sites with CAL ≥6 mm and one or more interproximal sites with PD ≥5 mm. Moderate periodontitis required involvement of two or more interproximal sites with CAL ≥4 mm or ≥2 interproximal sites with PD ≥5 mm.Citation21 Since the development of these definitions, Eke et al as part of the group at the CDC have added a further definition classifying mild periodontitis as ≥2 interproximal sites with attachment loss ≥3 mm and ≥2 interproximal sites with PD ≥4 mm, or one with PD ≥5 mm.Citation109 These authors applied their criteria to a group of 456 dentate individuals and found that the addition of mild cases increased the total prevalence of periodontitis from 22.3% to 29.4%. They also applied the criteria established by the 5th European Workshop and found the European criteria gave a greater prevalence of 42% for incipient cases, compared with 24.6% for the CDC criteria, although the prevalence was the same for severe periodontitis (4.8%).Citation109
The current American classification system was developed in 1999 at the International Workshop for a Classification of Periodontal Diseases and Conditions. This led to the addition of a subcategory for gingival conditions, recognition of subtypes of periodontitis, and importantly the clarification of periodontitis as the manifestation of systemic disease.Citation110 In 2014, a task force developed a clinical interpretation of this classification, addressing the concept of attachment levels in the diagnosis of periodontitis. This recognized the importance of attachment levels but also difficulties in accurate measurement and interpretation especially in those with non-inflammatory gum recession. Clarification was also made regarding localized versus generalized periodontitis, and these criteria are now widely adopted in the USA.Citation111
Importantly, these variations in definition of periodontal disease will influence prevalence rates and hence ORs of both COPD in periodontitis and vice versa.
Socioeconomic status
Both COPD and periodontitis share the common risk factors of poor socioeconomic status and smoking,Citation33,Citation36 and it is possible that the link seen between the two conditions also reflects poor oral health in combination with other factors such as smoking, poor nutrition, poor self-care behaviors, and exposure to other environmental factors such as pollutants.
Kim et al found COPD patients with and without periodontitis were significantly older, less well educated, took less exercise, had lower income, smoked more, and had a greater alcohol intake.Citation108 Chung et al also noted that the COPD patients were generally less well educated, had a lower income, smoked more, and drank more alcohol.Citation96 The COPD patients in Prasanna’s study were also noted to belong to a lower income group, were more illiterate, and were of a lower socioeconomic status regardless of periodontal status.Citation40 Holtfreter et al found that patients with higher indices of periodontitis were less well educated and more often current smokers and had higher levels of systemic inflammation and worse lung function.Citation101 All these studies indicate significant confounders that likely impact on results published to date even though Bhavsar et al did not find any differences in socioeconomic status or educational qualifications between their COPD cohort and control groups.Citation95
Dental habits
It seems intuitive that poor dental hygiene would also increase the prevalence of periodontal diseases and may reflect education and socioeconomic status. Thus, it is important that future studies assessing specific comorbid associations between COPD and periodontitis also include the potential confounding effect of dental habits.
In support of this, Bhavsar et al found patients in the COPD group, as a whole, had significantly reduced brushing frequency compared with a non-COPD group (independent of periodontal status).Citation95 Similarly, Liu et al found that a higher proportion of patients with frequent COPD exacerbations brushed their teeth less than once per day and had higher PI scores after adjusting for other confounding factors.Citation104 Wang et al also found that their COPD group generally had lower tooth brushing times (P<0.0001) and oral health knowledge scores (P<0.0001) and were also less likely to use dental floss and have regular dental visits than controls.Citation97 All these studies indicate that lifestyle factors are also likely to be a crucial confounder when seeking associations and/or a common pathophysiological process.
Conclusion
The available evidence still provides some support for the hypothesis that COPD and periodontitis could be causally linked; raising the possibility that treatment of one could influence the severity and progression of the other. There are similarities in disease mechanisms (that of dysfunctional neutrophil behaviors, sustained neutrophilic inflammation, and connective tissue loss) that suggest a shared pathophysiology and support epidemiological evidence of association. However, some studies conducted to date have significant limitations that need to be overcome in order to provide a clear picture. First, the presence and severity of periodontitis must be defined using robust criteria, and second, the diagnosis of COPD must be confirmed according to international standards; this will require close collaboration between dentists and physicians. Third, known relevant confounding factors must be recorded and taken into account, and this will require a significant sample size to allow appropriate statistical modeling. Finally, the inflammatory profiles (systemically or locally) in groups of patients with COPD and periodontitis need to be compared with that of matched patients with COPD and no periodontitis. Such studies could then be used to design appropriately powered interventional trials to determine whether treating periodontitis is of benefit to COPD-related lung disease.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
SH is funded by an FP7 EU grant, and RAS is the principal investigator (PI). ES is funded by the Medical Research Council. Both SH and ES are supported by the National Institute for Health Research (NIHR) Wellcome Birmingham Clinical Research Facility.
Disclosure
The authors report no conflicts of interest in this work.
References
- SapeyEBayleyDAhmadANewboldPSnellNStockleyRAInter-relationships between inflammatory markers in stable COPD patients with bronchitis; the intra and inter patient variabilityThorax20086349349918057097
- StockleyRANeutrophils and the pathogenesis of COPDChest2002121151S155S12010844
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease2015 Available from: http://goldcopd.org/Accessed January 14, 2017
- VanfleterenLESpruitMAGroenenMClusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2013187772873523392440
- MapelDWHurleyJSFrostFJPetersenHVPicchiMACoultasDBHealth care utilization in chronic obstructive pulmonary disease: a case-control study in a health maintenance organizationArch Intern Med2000160172653265810999980
- DuQJinJLiuXSunYBronchiectasis as a comorbidity of chronic obstructive pulmonary disease: a systematic review and meta-analysisPLoS One2016113e015053226978269
- HillasGPerlikosFTsiligianniITzanakisNManaging comorbidities in COPDInt J Chron Obstruct Pulmon Dis2015109510925609943
- LiXKongLLiFAssociation between psoriasis and chronic obstructive pulmonary disease: a systematic review and meta-analysisPLoS One20151012e014522126700640
- FearyJRRodriguesLCSmithCJHubbardRBGibsonJEPrevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary careThorax2010651195696220871122
- SchneiderCBothnerUJickSSMeierCRChronic obstructive pulmonary disease and the risk of cardiovascular diseasesEur J Epidemiol201025425326020191376
- PatelARHurstJRExtrapulmonary comorbidities in chronic obstructive pulmonary disease: state of the artExpert Rev Respir Med20115564766221955235
- TruelsenTPrescottELangePSchnohrPBoysenGLung function and risk of fatal and non-fatal stroke. The Copenhagen City Heart StudyInt J Epidemiol200130114515111171876
- DoddJWGetovSVJonesPWCognitive function in COPDEur Respir J201035491392220356988
- ManninoDMThornDSwensenAHolguinFPrevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPDEur Respir J200832496296918579551
- SeymourJMSpruitMAHopkinsonNSThe prevalence of quadriceps weakness in COPD and the relationship with disease severityEur Respir J2010361818819897554
- ChenSJLiaoWCHuangKHChronic obstructive pulmonary disease and allied conditions is a strong independent risk factor for osteoporosis and pathologic fractures: a population-based cohort studyQJM2015108863364025614611
- KassebaumNJBernabeEDahiyaMBhandariBMurrayCJMarcenesWGlobal burden of severe periodontitis in 1990–2010: a systematic review and meta-regressionJ Dent Res201493111045105325261053
- MeyleJChappleIMolecular aspects of the pathogenesis of periodontitisPeriodontol 2000201569171726252398
- CekiciAKantarciAHasturkHVan DykeTEInflammatory and immune pathways in the pathogenesis of periodontal diseasePeriodontol 20002014641578024320956
- LingMRChappleILMatthewsJBPeripheral blood neutrophil cytokine hyper-reactivity in chronic periodontitisInnate Immun201521771472526055820
- PageRCEkePICase definitions for use in population-based surveillance of periodontitisJ Periodontol2007787 Suppl1387139917608611
- LeroyREatonKASavageAMethodological issues in epidemiological studies of periodontitis – how can it be improved?BMC Oral Health201010820409298
- SavageAEatonKAMolesDRNeedlemanIA systematic review of definitions of periodontitis and methods that have been used to identify this diseaseJ Clin Periodontol200936645846719508246
- WangTFJenIAChouCLeiYPEffects of periodontal therapy on metabolic control in patients with type 2 diabetes mellitus and periodontal disease: a meta-analysisMedicine (Baltimore)20149328e29225526470
- ChrysanthakopoulosNAChrysanthakopoulosPAAssociation between indices of clinically-defined periodontitis and self-reported history of systemic medical conditionsJ Investig Clin Dent2016712736
- ScherJUBretzWAAbramsonSBPeriodontal disease and subgingival microbiota as contributors for rheumatoid arthritis pathogenesis: modifiable risk factors?Curr Opin Rheumatol201426442442924807405
- DietrichTSharmaPWalterCWestonPBeckJThe epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular diseaseJ Periodontol2013844 SupplS70S8423631585
- WidenCHolmerHColemanMSystemic inflammatory impact of periodontitis on acute coronary syndromeJ Clin Periodontol20164371371926935585
- SfyroerasGSRoussasNSaleptsisVGArgyriouCGiannoukasADAssociation between periodontal disease and strokeJ Vasc Surg20125541178118422244863
- SeymourGJFordPJCullinanMPLeishmanSYamazakiKRelationship between periodontal infections and systemic diseaseClin Microbiol Infect200713Suppl 431017716290
- JeffcoatMKJeffcoatRLGladowskiPABramsonJBBlumJJImpact of periodontal therapy on general health: evidence from insurance data for five systemic conditionsAm J Prev Med201447216617424953519
- FokkemaSJTimmermanMFvan der WeijdenFAWolffeGNRenggliHHA possible association of alpha1-antitrypsin deficiency with the periodontal condition in adultsJ Clin Periodontol19982586176239722265
- ShenTCChangPYLinCLRisk of periodontal diseases in patients with chronic obstructive pulmonary disease: a nationwide population-based cohort studyMedicine (Baltimore)20159446e204726579813
- StockleyJAWaltonGMSapeyEAberrant neutrophil functions in stable chronic obstructive pulmonary disease: the neutrophil as an immunotherapeutic targetInt Immunopharmacol20131741211121723994347
- TashkinDPClarkVACoulsonAHThe UCLA population studies of chronic obstructive respiratory disease. VIII. Effects of smoking cessation on lung function; a prospective study of a free-living populationAm Rev Respir Dis19841307077156497153
- ZengXTTuMLLiuDYZhengDZhangJLengWPeriodontal disease and risk of chronic obstructive pulmonary disease: a meta-analysis of observational studiesPLoS One2012710e4650823094025
- GleesonKEggliDFMaxwellSLQuantitative aspiration during sleep in normal subjectsChest19971115126612729149581
- BansalMKhatriMTanejaVPotential role of periodontal infection in respiratory diseases – a reviewJ Med Life20136324424824155782
- AzarpazhoohALeakeJLSystematic review of the association between respiratory diseases and oral healthJ Periodontol20067791465148216945022
- PrasannaSJCausal relationship between periodontitis and chronic obstructive pulmonary diseaseJ Indian Soc Periodontol201115435936522368360
- DonaldsonGCSeemungalTABhowmikAWedzichaJARelationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax2002571084785212324669
- ScannapiecoFABushRBPajuSAssociations between periodontal disease and risk for nosocomial bacterial pneumonia and chronic obstructive pulmonary disease. A systematic reviewAnn Periodontol200381546914971248
- FourrierFDuboisDPronnierPEffect of gingival and dental plaque antiseptic decontamination on nosocomial infections acquired in the intensive care unit: a double-blind placebo-controlled multicenter studyCrit Care Med20053381728173516096449
- FourrierFCau-PottierEBoutignyHRoussel-DelvallezMJourdainMChopinCEffects of dental plaque antiseptic decontamination on bacterial colonization and nosocomial infections in critically ill patientsIntensive Care Med20002691239124711089748
- GenuitTBochicchioGNapolitanoLMMcCarterRJRoghmanMCProphylactic chlorhexidine oral rinse decreases ventilator-associated pneumonia in surgical ICU patientsSurg Infect (Larchmt)20012151812594876
- BergmansDCBontenMJGaillardCAPrevention of ventilator-associated pneumonia by oral decontamination: a prospective, randomized, double-blind, placebo-controlled studyAm J Respir Crit Care Med2001164338238811500337
- YoneyamaTHashimotoKFukudaHOral hygiene reduces respiratory infections in elderly bed-bound nursing home patientsArch Gerontol Geriatr1996221111915374188
- YoneyamaTYoshidaMOhruiTOral care reduces pneumonia in older patients in nursing homesJ Am Geriatr Soc200250343043311943036
- HaffajeeADSocranskySSMicrobial etiological agents of destructive periodontal diseasesPeriodontol 200019945781119673164
- GeivelisMTurnerDWPedersonEDLambertsBLMeasurements of interleukin-6 in gingival crevicular fluid from adults with destructive periodontal diseaseJ Periodontol199364109809838277408
- ShimadaYKomatsuYIkezawa-SuzukiITaiHSugitaNYoshieHThe effect of periodontal treatment on serum leptin, interleukin-6, and C-reactive proteinJ Periodontol20108181118112320370420
- MatthewsJBWrightHJRobertsALing-MountfordNCooperPRChappleILNeutrophil hyper-responsiveness in periodontitisJ Dent Res200786871872217652198
- WrightHJMatthewsJBChappleILLing-MountfordNCooperPRPeriodontitis associates with a type 1 IFN signature in peripheral blood neutrophilsJ Immunol200818185775578418832737
- DiasIHMatthewsJBChappleILWrightHJDunstonCRGriffithsHRActivation of the neutrophil respiratory burst by plasma from periodontitis patients is mediated by pro-inflammatory cytokinesJ Clin Periodontol201138117
- LiXKolltveitKMTronstadLOlsenISystemic diseases caused by oral infectionClin Microbiol Rev200013454755811023956
- SindenNJStockleyRASystemic inflammation and comorbidity in COPD: a result of ‘overspill’ of inflammatory mediators from the lungs? Review of the evidenceThorax2010651093093620627907
- PreshawPMTaylorJJHow has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis?J Clin Periodontol201138Suppl 11608421323705
- ScholsAMBuurmanWAStaal van den BrekelAJDentenerMAWoutersEFEvidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary diseaseThorax19965188198248795671
- EidAAIonescuAANixonLSInflammatory response and body composition in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20011648 Pt 11414141811704588
- KaradagFKarulABCildagOYilmazMOzcanHBiomarkers of systemic inflammation in stable and exacerbation phases of COPDLung2008186640340918807087
- SapeyEBayleyDAhmadANewboldPSnellNStockleyRAInter-relationships between inflammatory markers in patients with stable COPD with bronchitis: intra-patient and inter-patient variabilityThorax200863649349918057097
- HacievliyagilSSGunenHMutluLCKarabulutABTemelIAssociation between cytokines in induced sputum and severity of chronic obstructive pulmonary diseaseRespir Med2006100584685416214322
- HeZChenYChenPWuGCaiSLocal inflammation occurs before systemic inflammation in patients with COPDRespirology201015347848420210891
- KuschnerWGD’AlessandroAWongHBlancPDDose-dependent cigarette smoking-related inflammatory responses in healthy adultsEur Respir J1996910198919948902455
- StoneHMcNabGWoodAMStockleyRASapeyEVariability of sputum inflammatory mediators in COPD and alpha1-antitrypsin deficiencyEur Respir J201240356156922700846
- CaramoriGAdcockIMDi StefanoAChungKFCytokine inhibition in the treatment of COPDInt J Chron Obstruct Pulmon Dis2014939741224812504
- KimVCornwellWDOrosMDurraHCrinerGJRogersTJPlasma chemokine signature correlates with lung goblet cell hyperplasia in smokers with and without chronic obstructive pulmonary diseaseBMC Pulm Med20151511126424214
- LouhelainenNRytilaPHaahtelaTKinnulaVLDjukanovicRPersistence of oxidant and protease burden in the airways after smoking cessationBMC Pulm Med200992519473482
- GambleEGrootendorstDCHattotuwaKAirway mucosal inflammation in COPD is similar in smokers and ex-smokers: a pooled analysisEur Respir J200730346747117504799
- NogueraABatleSMirallesCEnhanced neutrophil response in chronic obstructive pulmonary diseaseThorax200156643243711359957
- BurnettDChambaAHillSLStockleyRANeutrophils from subjects with chronic obstructive lung disease show enhanced chemotaxis and extracellular proteolysisLancet198728567104310462889963
- SapeyEStockleyJAGreenwoodHBehavioral and structural differences in migrating peripheral neutrophils from patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med201118391176118621257786
- StanescuDSannaAVeriterCAirways obstruction, chronic expectoration, and rapid decline of FEV1 in smokers are associated with increased levels of sputum neutrophilsThorax19965132672718779129
- Di StefanoACapelliALusuardiMSeverity of airflow limitation is associated with severity of airway inflammation in smokersAm J Respir Crit Care Med19981584127712859769292
- VernooyJHKucukaycanMJacobsJALocal and systemic inflammation in patients with chronic obstructive pulmonary disease: soluble tumor necrosis factor receptors are increased in sputumAm J Respir Crit Care Med200216691218122412403691
- HigashimotoYIwataTOkadaMSatohHFukudaKTohdaYSerum biomarkers as predictors of lung function decline in chronic obstructive pulmonary diseaseRespir Med200910381231123819249197
- KheradmandFShanMXuCCorryDBAutoimmunity in chronic obstructive pulmonary disease: clinical and experimental evidenceExpert Rev Clin Immunol20128328529222390492
- BoschettoPQuintavalleSZeniEAssociation between markers of emphysema and more severe chronic obstructive pulmonary diseaseThorax200661121037104216769715
- BarnesPJShapiroSDPauwelsRAChronic obstructive pulmonary disease: molecular and cellular mechanismsEur Respir J200322467268814582923
- HoggJCSeniorRMChronic obstructive pulmonary disease – part 2: pathology and biochemistry of emphysemaThorax200257983083412200530
- HoughtonAMEndogenous modifiers of cigarette smoke exposure within the lungProc Am Thorac Soc201292666822550246
- ScottDAKraussJNeutrophils in periodontal inflammationFront Oral Biol201215568322142957
- HajishengallisEHajishengallisGNeutrophil homeostasis and periodontal health in children and adultsJ Dent Res201493323123724097856
- RobertsHMLingMRInsallRImpaired neutrophil directional chemotactic accuracy in chronic periodontitis patientsJ Clin Periodontol2015421111
- KumarRSPrakashSImpaired neutrophil and monocyte chemotaxis in chronic and aggressive periodontitis and effects of periodontal therapyIndian J Dent Res2012231697422842253
- CarneiroVMABezerraACBGuimarÃEsMdCMMuniz-JunqueiraMIDecreased phagocytic function in neutrophils and monocytes from peripheral blood in periodontal diseaseJ Appl Oral Sci201220550350923138734
- SammutDLewisABuddRIncreased reactive oxygen species generation in quiescent and activated peripheral blood neutrophils in chronic obstructive pulmonary diseaseB31 Inflamm COPD2015 A2710 Abstract
- ChappleILCReactive oxygen species and antioxidants in inflammatory diseasesJ Clin Periodontol19972452872969178107
- WhitePCooperPMilwardMChappleIP95 – differential activation of neutrophil extracellular traps by specific periodontal bacteriaFree Rad Biol Med201475Suppl 1S53
- WrightTKGibsonPGSimpsonJLMcDonaldVMWoodLGBainesKJNeutrophil extracellular traps are associated with inflammation in chronic airway diseaseRespirology201621346747526804470
- StockleyRANeutrophils and protease/antiprotease imbalanceAm J Respir Crit Care Med19991605 Pt 2S49S5210556170
- SharafkhanehAHananiaNAKimVPathogenesis of emphysema: from the bench to the bedsideProc Am Thorac Soc20085447547718453358
- BarnesPJMediators of chronic obstructive pulmonary diseasePharmacol Rev200456451554815602009
- CarterRIUngursMJMumfordRAStockleyRAAalpha-Val360: a marker of neutrophil elastase and COPD disease activityEur Respir J2013411313822523359
- BhavsarNVDaveBDBrahmbhattNAParekhRPeriodontal status and oral health behavior in hospitalized patients with chronic obstructive pulmonary diseaseJ Nat Sci Biol Med20156Suppl 1S93S9726604629
- ChungJHHwangHJKimSHKimTHAssociations between periodontitis and chronic obstructive pulmonary disease; the 2010–2012 Korean National Health and Nutrition Examination Survey (KNHANES)J Periodontol201687886487126912338
- WangZZhouXZhangJPeriodontal health, oral health behaviors, and chronic obstructive pulmonary diseaseJ Clin Periodontol200936975075519614723
- DeoVBhongadeMLAnsariSChavanRSPeriodontitis as a potential risk factor for chronic obstructive pulmonary disease: a retrospective studyIndian J Dent Res200920446647020139573
- LeuckfeldIObregon-WhittleMVLundMBGeiranOBjortuftOOlsenISevere chronic obstructive pulmonary disease: association with marginal bone loss in periodontitisRespir Med2008102448849418191392
- PeterKPMuteBRDoiphodeSSBardapurkarSJBorkarMSRajeDVAssociation between periodontal disease and chronic obstructive pulmonary disease: a reality or just a dogma?J Periodontol201384121717172323339345
- HoltfreterBRichterSKocherTPeriodontitis is related to lung volumes and airflow limitation: a cross-sectional studyEur Respir J20134261524153523222882
- BergstromJCederlundKDahlenBDental health in smokers with and without COPDPLoS One201383e5949223544074
- HymanJJReidBCCigarette smoking, periodontal disease: and chronic obstructive pulmonary diseaseJ Periodontol200475191515025211
- LiuZZhangWZhangJOral hygiene, periodontal health and chronic obstructive pulmonary disease exacerbationsJ Clin Periodontol2012391455222092913
- TonettiMSClaffeyNAdvances in the progression of periodontitis and proposal of definitions of a periodontitis case and disease progression for use in risk factor research. Group C consensus report of the 5th European Workshop in PeriodontologyJ Clin Periodontol200532Suppl 621021316128839
- BarrosSPSurukiRLoewyZGBeckJDOffenbacherSA cohort study of the impact of tooth loss and periodontal disease on respiratory events among COPD subjects: modulatory role of systemic biomarkers of inflammationPLoS One201388e6859223950871
- ZhouXHanJLiuZSongYWangZSunZEffects of periodontal treatment on lung function and exacerbation frequency in patients with chronic obstructive pulmonary disease and chronic periodontitis: a 2-year pilot randomized controlled trialJ Clin Periodontol201441656457224593836
- KimSWHanKKimSYParkCKRheeCKYoonHKThe relationship between the number of natural teeth and airflow obstruction: a cross-sectional study using data from the Korean National Health and Nutrition Examination SurveyInt J Chron Obstruct Pulmon Dis2016111321
- EkePIPageRCWeiLThornton-EvansGGencoRJUpdate of the case definitions for population-based surveillance of periodontitisJ Periodontol201283121449145422420873
- ArmitageGCDevelopment of a classification system for periodontal diseases and conditionsAnn Periodontol1999411610863370
- American Academy of Periodontology Task Force Report on the Update to the 1999 Classification of Periodontal Diseases and ConditionsJ Periodontol201586783583826125117
- ScannapiecoFAHoAWPotential associations between chronic respiratory disease and periodontal disease: analysis of National Health and Nutrition Examination Survey IIIJ Periodontol2001721505611210073
- ErikssonSPulmonary emphysema and alpha1-antitrypsin deficiencyActa Med Scand196417519720514124635
- ChappleILCGilbertADWilsonNHFUnderstanding Periodontal Diseases: Assessment and Diagnostic Procedures in Practice: 1 (Quintessentials: Endontics)Quintessence Publishing Co Ltd2002
