Abstract
Purpose
To evaluate the heart rate variability (HRV) indices and heart rate (HR) responses during isometric contraction (IC) and Valsalva maneuver (VM) in COPD patients.
Methods
Twenty-two stable moderate to severe COPD patients were evaluated. R-R intervals were recorded (monitor Polar® S810i) during dominant upper limb IC (2 minutes). Stable signals were analyzed by Kubios HRV® software. Indices of HRV were computed in the time domain (mean HR; square root of the mean squared differences of successive RR intervals [RMSSD] and HRV triangular index [RR tri index]) and in the frequency domain (high frequency [HF]; low frequency [LF] and LF/HF ratio). The HR responses were evaluated at rest, at the peak and at the nadir of the VM (15 seconds). The Valsalva index was also calculated.
Results
During IC: time domain indices (mean HR increased [P=0.001], RMSSD, and RR tri index decreased [P=0.005 and P=0.005, respectively]); frequency domain indices (LF increased [P=0.033] and HF decreased [P=0.002]); associations were found between forced expiratory volume in 1 second (FEV1) vs RMSSD (P=0.04; r=−0.55), FEV1 vs HR (P=0.04; r=−0.48), forced vital capacity (FVC) vs RMSSD (P=0.05; r=−0.62), maximum inspiratory pressure (MIP) vs HF (P=0.02; r=0.68). FEV1 and FVC justified 30% of mean HR. During VM: HR increased (P=0.01); the nadir showed normal bradycardic response; the Valsalva index was =0.7.
Conclusion
COPD patients responded properly to the upper limb IC and to the VM; however, HR recovery during VM was impaired in these patients. The severity of the disease and MIP were associated with increased parasympathetic modulation and higher chronotropic response.
Introduction
COPD is characterized by progressive airflow limitation and is associated with abnormal inflammatory response of the airways and lungs, caused by inhalation of noxious gases and particles, such as those contained in tobacco smoke.Citation1 Besides inducing lung damage, COPD presents multisystemic manifestations and can be associated with comorbidities which greatly affect prognosis.Citation1 The association between these factors can determine structural and functional changes in the respiratory and peripheral skeletal muscles which directly affect cardiac autonomic modulation, possibly increasing risk of mortality in these patients.Citation2,Citation3
The cardiovascular system functioning and the mechanisms regulating the autonomic adjustments have been investigated, both in basal and physical effort conditions by the analysis of heart rate variability (HRV),Citation3 which represents a powerful research tool capable of identifying increased risk of mortalityCitation4–Citation8 and poor prognosisCitation8 in these patients. COPD patients present attenuated response of the HRV index and previous studies have shown increased sympathetic activity, during lower limb aerobic and resistance exercise.Citation2,Citation9–Citation11 In addition, these HRV attenuated responses seem to be related to respiratory muscle weakness.Citation12 Other studies, however, showed that impaired responses during exercise in these patients may be partially explained by bronchoconstriction, hypoxemia, hypercapnia, and systemic inflammation.Citation9,Citation13–Citation16
Isometric contraction (IC) and the Valsalva maneuver (VM) are widely used autonomic tests to assess altered autonomic responses in different populations.Citation11,Citation17 Physiologically, IC causes a marked increase in systolic blood pressure (SBP) and heart rate (HR), due to inhibition of vagal modulation on the sinus node,Citation11,Citation17 which is dependent on the percentage of maximum voluntary contraction. As for the VM, it determines marked hemodynamic changes, with accentuated cardiovascular overload.Citation18,Citation19 However, the studies which investigated the cardiac autonomic responses to these maneuvers in COPD patients are scarce. Stewart et alCitation20 observed the HR responses to the handgrip test and the VM and they concluded that 18% of COPD patients had attenuated autonomic responses consistent with autonomic neuropathy and these were associated with hypoxemia.Citation20 However, in their study, the patients’ cardiac autonomic function was not evaluated by means of HRV analysis.Citation20 It is noteworthy that such maneuvers provide indices which represent powerful predictors of cardiovascular impairment and reliable diagnostic and prognostic markers.Citation21–Citation23
Therefore, the aim of the present study was to evaluate the HRV indices and HR responses during IC and VM in COPD patients. We hypothesized that the severity of COPD would negatively impact on the autonomic responses during IC and VM.
Methods
Study design
We performed a cross-sectional and randomized cross-over study within Santa Cruz Hospital’s Pulmonary Rehabilitation Program (Santa Cruz do Sul, RS, Brazil). All patients were formally invited to participate in the study by the responsible researcher. The study was approved by the Research Ethics Committee of the University of Santa Cruz do Sul, protocol number 128943, and all volunteers signed an informed consent statement prior to participation.
Subjects
After acceptance of the patients, 30 patients with a clinical diagnosis of COPD from a physician; forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio <0.7 and FEV1 <60% of predicted, with good cognitive function and without disease exacerbation during the previous 15 days were included in the study. Patients with musculoskeletal disorders or neurological conditions affecting the locomotor system in such a way that would preclude participation in the protocol, a clinical diagnosis of lung cancer, current alcoholism, complex cardiac arrhythmias, uncontrolled metabolic disease such as diabetes, thyroid changes and systemic arterial hypertension (with or without the use of beta-blockers) or electrocardiogram alterations were excluded from the study (n=8) ().
Measurements
Patients were evaluated in a laboratory at a temperature of 22°C and relative humidity between 50% and 60%. They were instructed to avoid stimulants and alcoholic drinks and not to perform exhausting physical exercise the day before the test; they were also instructed not to smoke or use bronchodilators for 6 hours before the test. Patients underwent a clinical evaluation to record: sex, age, body mass index, smoking history and COPD staging. Afterward, vital signs (SBP, diastolic blood pressure [DBP], respiratory frequency [RF], peripheral oxygen saturation [SpO2]) were measured and the dominant upper limb perceived exertion was estimated using the Borg scale.
In addition, familiarization procedures were performed with the equipment and experimental protocols so that patients could learn how to correctly perform the dominant upper limb IC and the VM before the actual testing. The HRV signal was recorded at rest (5 minutes), during upper limb IC (2 minutes) and during the VM (15 seconds), the order of which was determined by simple randomization by envelope, and during recovery (5 minutes) ().
Pulmonary function
The pulmonary function was assessed using a digital spirometer (Microloop®, MK8, Care Fusion, Hoechberg, Germany), which provided measures of the FVC, the FEV1, and the FEV1/FVC ratio. Spirometry was performed according to the recommendations of the American Thoracic SocietyCitation24 and the results were analyzed according to the values predicted by Pereira et al.Citation25 The classification of severity of airflow limitation in COPD was performed according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations and patients were classified as moderate (GOLD II), severe (GOLD III), or very severe (GOLD IV).Citation1
Respiratory muscle strength (RMS)
The RMS was assessed using a digital manometer (MDI®, MVD300, Porto Alegre, Brazil), which provided measures of the maximum inspiratory pressure (MIP) and the maximum expiratory pressure (MEP). The assessment was performed according to the recommendations for the Brazilian population. MIP and MEP were obtained from residual volume and total lung capacity; during the maneuver, the subjects remained seated, wearing nose clips and with a rigid, plastic flanged mouthpiece in place.Citation26 The values were then compared with those described in literature and expressed as percentage of predicted values.Citation26 The inspiratory muscle weakness was determined by an MIP <60 cmH2O.Citation27
Hand-grip strength (HGS) and IC load determination
The HGS was determined during dominant upper limb IC, using a hand dynamometer (Jamar®, Bolingbrook, IL, USA); three maximum contractions were performed and their average was computed.Citation28 The IC load was determined as 30% of the HGS. The load should be sustained for 2 minutes, preceded by 5 minutes of rest and followed by 5 minutes of recovery. Patients performed dominant upper limb IC while sitting, with shoulder abducted, elbow flexed to 90º, forearm in neutral pronation–supination and wrist extended between 0° and 30°.Citation29
VM
The VM was performed with patients in sitting position. After a deep inspiration their nose was occluded by a nose clip to prevent air leakage. The patients performed a breath-holding maneuver for 15 seconds,Citation30 after which expiration was initiated on the researcher’s command. Facial flushing, jugular stasis, chest movements and rapid HR increase followed by bradycardia were observed to guarantee the correct performance of the VM.Citation30 The test was preceded by 5 minutes of rest and followed by 5 minutes of recovery.
HRV
HR and RR intervals (iR-R) were recorded using a telemetric cardiac monitor (Polar® S810i, Kempele, Finland). An elastic band (Polar T31 transmitter) was placed around the patient’s thorax at the level of the lower third of the sternum, while the patient was in a sitting position; signals were continuously transmitted to the receiving unit by electromagnetic field. Recorded data were then transferred to Kubios HRV® analysis software (version 2.2, Finland) for subsequent analysis.
The signal processing was carried out as follows: at rest (5 minutes), during upper limb IC (90 seconds; the first 30 seconds of the signal were discarded in order to obtain a stable signal portion), and post-exercise recovery (5 minutes). The HRV signal collected during upper limb IC was analyzed in the time domain (the analysis provided mean RR interval [mean RR], RR interval standard deviation [STD RR], mean HR, STD HR, square root of the mean squared differences of successive RR intervals [RMSSD] and the HRV triangular index [RR tri index]) and in the frequency domain (the analysis provided the HRV signal power in the low frequency [LF], indicating sympathetic activity, in the high frequency [HF], indicating parasympathetic activity, and the LF/HF ratio).
As for the VM, the iR-R were analyzed during rest, at the peak of the VM, after cessation of the VM (when the lowest HR value is referred to as nadir), and during recovery. The Valsalva index (VI) was calculated as the ratio between the longest iR-R during recovery and the shortest iR-R during the peak of the VM. The VI is used as an indirect measure of the autonomic nervous system (ANS) integrity, characterized by values above 1.4.Citation31
Statistical analysis
Sample size calculation was performed a priori using Gpower software according to the variable RMSSD; to achieve a statistical power of 80% (β=0.20), with α=0.05, it was calculated that a minimum of 20 patients were needed.Citation11 Data were analyzed using the Sigmaplot® statistical package (version 11.0, Systat Software Inc., San Jose, CA, USA). Data were tested for normality through the Shapiro–Wilk test and presented descriptively as mean and STD (parametric) or as median and minimum and maximum interval (non-parametric). An analysis of variance (ANOVA) analysis for multiple comparisons with Tukey’s post hoc test was performed. A Pearson correlation analysis was performed to investigate the correlations between variables. A linear regression model was used to determine the effect of the clinical variables on the HRV parameters. Residuals were evaluated under the assumptions of normality, constant variance, and independence. P<0.05 was considered significant.
Results
Clinical characteristics of the patients included in the study are shown in . We observed a prevalence of patients of male sex, with obesity, and with severe COPD in stage III and IV (77%). Despite the higher frequency of patients with severe disease, the changes in inspiratory muscle strength were also distributed among the patients studied (50% MIP normal values and 50% inspiratory muscle weakness).
Table 1 COPD patients’ clinical characteristics
shows that the upper limb IC significantly increased the SBP (P=0.002), the DBP (P=0.020), the RF (P=0.007), and the upper limb perceived exertion (P<0.001); as for the time domain index, mean HR increased (P=0.001), while RMSSD and RR tri index decreased (P=0.005 and P=0.005, respectively), suggesting an HR increase and a vagal activity and total HRV decrease. As for the frequency domain index, the LF power increased (P=0.033) and the HF power decreased (P=0.002), indicating a sympathetic modulation increase and a vagal modulation decrease.
Table 2 Clinical and HRV parameters at rest, during IC and during recovery
Moderate to strong correlations were found between inspiratory muscle strength, airway obstruction and lung capacity, and the HRV parameters during upper limb IC (), demonstrating the influence and importance of airflow obstruction and inspiratory muscle weakness over the parasympathetic modulation and HR behavior.
Figure 2 Relationship between respiratory muscle strength and lung function, and heart rate variability index during upper limb isometric contraction.
Abbreviations: RMSSD, square root of the mean squared differences of successive RR intervals; FEV1, forced expiratory volume in 1 second; mean HR, mean heart rate; FVC, forced vital capacity; HF, high frequency; nu, normalized units; MIP, maximum inspiratory pressure; cmH2O, centimeters of water.
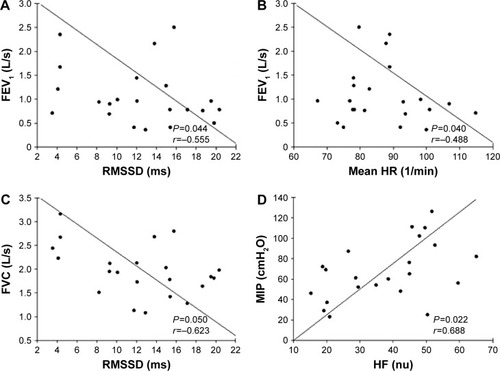
We could observe that the lung function indices (FEV1 and FVC) directly influenced cardiac autonomic modulation during upper limb IC. This result was confirmed by a multiple linear regression, which showed that airway obstruction and lung capacity justified 30% of mean HR during upper limb IC ().
Table 3 Results of multiple linear regression to determine the influence of FEV1 and FVC on mean HR during upper limb isometric contraction
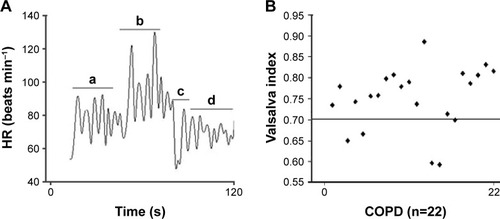
The HR behavior during the VM is shown in . The HR behavior at rest, during and at the peak of the VM, after cessation of the VM (nadir) and during recovery indicates that the COPD patients included in this study presented an HR response pattern similar to that observed in previous, similar studies performed on other populations.Citation19,Citation30 The VI value (=0.7) indicates an integrity loss of the ANS in these patients.
Figure 3 Response of HR during the VM in COPD patients.
Abbreviations: ANS, autonomic nervous system; HR, heart rate; VM, Valsalva maneuver; VI, Valsalva index.
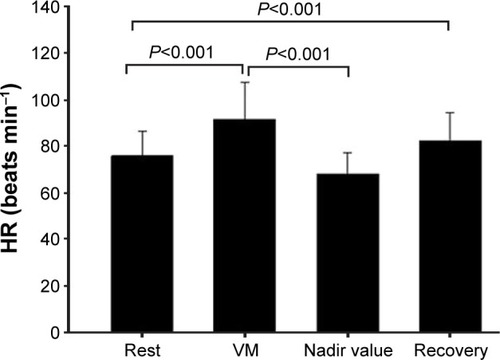
The HR values during the VM are represented in . We could observe a significant HR increase during the VM, with a recovery to baseline values after cessation of the maneuver. The nadir suggests an adequate bradycardic response to the maneuver.
Figure 4 HR values during the VM.
Abbreviations: HR, heart rate; VM, Valsalva maneuver; ANOVA, analysis of variance.
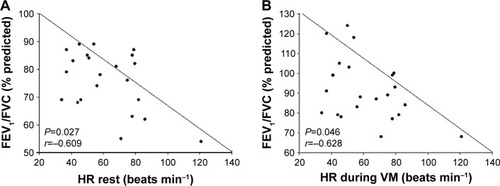
The correlations between FEV1/FVC with rest-HR and VM-HR underscored the direct influence of airway obstruction on cardiac performance in COPD patients ().
Figure 5 Relationship between lung function and HR values during the VM in COPD patients.
Abbreviations: HR, heart rate; VM, Valsalva maneuver; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Discussion
The main findings of the present study can be summarized as follows: 1) COPD patients responded properly to the upper limb IC and to the VM; however, 2) HR recovery from the VM appeared to be impaired; 3) the severity of the disease, quantified by FEV1 and FVC, as well as MIP, were associated with increased parasympathetic modulation; 4) chronotropic responses were associated with bronchoconstriction.
As expected, during upper limb IC, patients presented increased HR, increased sympathetic activation and reduced total HRV; we observed that all indices went back to their normal values during recovery. A previous study showed that 18% of COPD patients presented attenuated autonomic responses, consistent with autonomic neuropathy and associated with hypoxemia.Citation20 However, in that study, the authors did not evaluate cardiac autonomic modulation by computing robust HRV indices, but only by analyzing HR responses,Citation20 different to our study, in which linear and nonlinear HRV indices, widely accepted as important tools to determine cardiac autonomic changes, were studied.Citation32,Citation33
We observed an association between airway obstruction and inspiratory muscle weakness (). These findings were previously reported by other authors in different autonomic tests, as postural change, and during dynamic exercise.Citation2,Citation3,Citation12,Citation13 It has been reported that increased cardiac parasympathetic modulation in COPD patients was directly associated with increased airway obstruction.Citation15 This response may be associated with changes in both lung compliance and lung stretch reflex responses in patients with cardiopulmonary disease.Citation13
A particularly relevant finding of our study is the association between HR chronotropic response during IC and the severity of the disease. These results may be explained by augmented muscle metaboreflex activation that could occur in those patients at a more severe stage of COPD.Citation22 This mechanism, associated with systemic inflammation present in COPD, may contribute to augmented sympathetic responses to exercise.Citation16 Our results confirm the importance of applying the IC maneuver, which has been widely used for risk assessment in cardiovascular disease patients, as a powerful tool to assess the severity of the disease in this population.Citation22
It has been reported that, in cardiac patients and in healthy young people, HR increases at the beginning of the IC, due to increased muscle tension; this phenomenon may be attributed to vagal withdrawal on the sinus node.Citation11,Citation17,Citation34,Citation35 The mechanisms responsible for these responses after muscle fiber activation may have a central role in the activation of central neural circuits and in the determination of cardiovascular adjustments.Citation34–Citation36 In the present study, we observed expected HR responses in COPD patients.
However, the respiratory muscle weakness, suggested by MIP reduction, seems to be associated with cardiac autonomic control impairment.Citation12 Moreover, air trapping and subsequent FEV1 reduction, present in severe COPD patients, as well as accentuated bronchoconstriction, have been shown to be associated with vagal tone attenuation and with cardiac autonomic control alteration.Citation12,Citation13 Therefore, it could be supposed in our study that impaired RMS and reduced FEV1 and FVC could deeply influence the HRV behavior, both at rest and during isometric exercise.
Interestingly, in our study, during the VM, COPD patients presented an expected HR response; however, the HR values could not be fully recovered 5 minutes after cessation of the maneuver. These aspects are particularly relevant since it is well known that the recovery of the HR response represents a powerful prognostic marker for cardiovascular disease.Citation19 The HR response to the VM is mainly influenced by cardiac autonomic control, which is responsible for the rapid increase phase, with vagal withdrawal over the sinus node, as well as for the reduction after cessation of the VM, with vagal tone activation. These adjustments may occur in response to the activation of cardiopulmonary receptors and arterial baroreceptors.Citation17,Citation19,Citation37,Citation38
The present study is relevant as it highlights the direct influence of the airway obstruction on the HR parameters (). The interaction between sympathetic reflex and vagal reflex, probably triggered by the pulmonary stretch receptors, is responsible for the cardiovascular responses during the VM.Citation17,Citation19,Citation37 The peripheral vascular resistance, which increases in response to decreased venous return, caused by increased intrathoracic pressure during the VM, drops immediately after cessation of the maneuver; this promotes an increase in venous return which, through the baroreceptors, stimulates vagal recovery, leading to marked bradycardia.Citation30,Citation37 Taking into account the structural and mechanical pathophysiological changes in the respiratory system of COPD patients, the results reported in literature justify a VI lower than 0.7, confirming an ANS integrity loss in these subjects.
This study has limitations that should be addressed. Given our strict exclusion criteria, and the difficulty in triaging patients with a 95% rate of sinus rhythm, our sample may not be representative for the entire COPD population, as many patients present various types of arrhythmias that compromise the recording and analysis of signals. In accordance with our main objective, we chose to perform a randomized cross-sectional study to minimize inter-individual variability, where the patient represented his or her own control, which justified the absence of a control group.
This study is of great significance for the clinical management of COPD patients; our results about upper limb IC, about the influence of inspiratory muscle weakness and lung function, and about these patients’ responses to the VM, can contribute to the development of novel diagnostic tools; moreover, they may be used to develop modern tools for the evaluation, in the clinical setting, of the effects of pulmonary rehabilitation interventions, such as respiratory muscle training and static and dynamic resistance training.
Conclusion
The COPD patients included in this study responded appropriately to the upper limb IC, with an HR increase, a sympathetic modulation increase, and a parasympathetic modulation decrease. However, the recovery of the HR after the VM was impaired and the VI presented a value below normal range. The severity of the disease and MIP were associated with increased parasympathetic modulation. Finally, higher chronotropic response was associated to bronchoconstriction in these patients.
The main findings of the present study can be summarized as follows: COPD patients responded properly to the upper limb IC and to the VM; HR recovery after VM appeared to be impaired; the severity of the disease was associated with increased parasympathetic modulation; chronotropic responses were associated to bronchoconstriction.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; and agree to be accountable for all aspects of the work.
Acknowledgments
Grants were received from the University of Santa Cruz do Sul – UNISC, Santa Cruz Hospital and DECIT/SCTIE-MS/FAPERGS/CNPq 1264-2551/13-1; CAPES, project no 88881.062123/2014-01; FAPESP, project no 2013/23013-0.
Disclosure
The authors report no conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Lung DiseasePocket Guide to COPD Diagnosis, Management and PreventionGlobal Initiative for Chronic Obstructive Lung Disease2016 Available from: http://www.goldcopd.it/materiale/2016/GOLD_Pocket_2016.pdfAccessed July 27, 2016
- RoqueALValentiVEMassettiTChronic obstructive pulmonary disease and heart rate variability: a literature updateInt Arch Med201474325945125
- NicolinoJRamosDLeiteMRAnalysis of autonomic modulation after an acute session of resistance exercise at different intensities in chronic obstructive pulmonary disease patientsInt J Chron Obstruct Pulmon Dis20151022322925673982
- GunduzHTalayFArincHHeart rate variability and heart rate turbulence in patients with chronic obstructive pulmonary diseaseCardiol J200916655355919950092
- TheofilogiannakosEKAnogeianakiATsekouraPArrhythmo-genesis in patients with stable chronic obstructive pulmonary diseaseJ Cardiovasc Med (Hagerstown)200891899318268428
- MohammedJMeeusMDeromEDa SilvaHCaldersPEvidence for autonomic function and its influencing factors in subjects with COPD: a systematic reviewRespir Care201560121841185126487747
- van GestelAJKohlerMClarenbachCFSympathetic overactivity and cardiovascular disease in patients with chronic obstructive pulmonary disease (COPD)Discov Med2012147935936823272688
- SteinPKNelsonPRottmanJNHeart rate variability reflects severity of COPD in PiZ alpha1-antitrypsin deficiencyChest199811323273339498947
- Borghi-SilvaAArenaRCastelloVAerobic exercise training improves autonomic nervous control in patients with COPDRespir Med2009103101503151019464865
- Machado-VidottiHGMendesRGSimõesRPCastello-SimõesVCataiAMBorghi-SilvaACardiac autonomic responses during upper versus lower limb resistance exercise in healthy elderly menBraz J Phys Ther201418191824675908
- LeitePHMeloRCMelloMFSilvaEDBorghi-SilvaACataiAMHeart rate responses during isometric exercises in patients undergoing a phase III cardiac rehabilitation programRev Bras Fisioter201014538338921180863
- GoulartCLSimonJCSchneidersPBRespiratory muscle strength effect on linear and nonlinear heart rate variability parameters in COPD patientsInt J Chron Obstruct Pulmon Dis2016111671167727555757
- MazzucoAMedeirosWMSperlingMPRelationship between linear and nonlinear dynamics of heart rate and impairment of lung function in COPD patientsInt J Chron Obstruct Pulmon Dis2015101651166126316739
- ChenWLChenGYKuoCDHypoxemia and autonomic nervous dysfunction in patients with chronic obstructive pulmonary diseaseRespir Med200610091547155316488587
- VolterraniMScalviniSMazzueroGDecreased heart rate variability in patients with chronic obstructive pulmonary diseaseChest19941065143214377956396
- ChhabraSKGuptaMRamaswamySDashDJBansalVDeepakKKCardiac sympathetic dominance and systemic inflammation in COPDCOPD201512555255925495489
- FreemanJVDeweyFEHadleyDMMyersJFroelicherVFAutonomic nervous system interaction with the cardiovascular system during exerciseProg Cardiovasc Dis200648534236216627049
- KornerPITonkinAMUtherJBReflex and mechanical circulatory effects of graded Valsalva maneuvre in normal manJ Appl Physiol197640434440931859
- MinatelVTakahashiACPerseguiniNMMaximal expiratory pressure and Valsalva manoeuvre do not produce similar cardiovascular responses in healthy menExp Physiol2016101559961126935142
- StewartAGWaterhouseJCHowardPCardiovascular autonomic nerve function in patients with hypoxaemic chronic obstructive pulmonary diseaseEur Respir J1991410120712141804668
- HumeLIrvingJBKitchinAHReubenSREffects of sustained isometric handgrip on praecordial accelerocardiogram in normal subjects and in patients with heart diseaseBr Heart J19753788738811191449
- GreaneyJLEdwardsDGFadelPJFarquharWBRapid onset pressor and sympathetic responses to static handgrip in older hypertensive adultsJ Hum Hypertens201529740240825471615
- AbramkinDVIavelovISGratsianskiĭNAComparison of various methods of assessment of heart rate variability including simple cardiovascular reflex tests as predictors of sudden cardiac death after myocardial infarctionKardiologiia20044493441 Russian
- GibsonGJWhitelawWSiafakasNTests of overall respiratory function. ATS/ERS statement on respiratory muscle testingAm J Respir Crit Care Med2002166451862412186831
- PereiraCASatoTRodriguesSCNew reference values for forced spirometry in white adults in BrazilJ Bras Pneumol200733439740617982531
- NederJAAndreoniSLerarioMCNeryLEReference values for lung function tests. II. Maximal respiratory pressures and voluntary ventilationBraz J Med Biol Res199932671972710412550
- BeaumontMMialonPLe Ber-MoyCInspiratory muscle training during pulmonary rehabilitation in chronic obstructive pulmonary disease: a randomized trialChron Respir Dis201512430531226170421
- Luna-HerediaEMartin-PenaGRuiz-GalianaJHandgrip dynamometry in healthy adultsClin Nutr200524225025815784486
- CastroCLNóbregaACAraújoCGTestes Autonômicos Cardiovas-culares. Uma Revisão Crítica. Parte I e II. [Autonomic cardiovascular tests: a critical review. I and II]Arq Bras Cardiol1992597583 Portuguese1341153
- MinatelVKarstenMNevesLMBeltrameTBorghi-SilvaACataiAMHeart rate assessment during maximal static expiratory pressure and Valsalva maneuver in healthy young menRev Bras Fisioter201216540641322983214
- O’BrienIAO’HarePCorrallRJHeart rate variability in healthy subjects: effect of age and the derivation of normal ranges for tests of autonomic functionBr Heart J19865543483543964501
- No authors listedHeart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and ElectrophysiologyCirculation1996935104310658598068
- LuczakHLauringWJAn analysis of heart rate variabilityErgonomics197316185974702067
- SilvaEOliveiraLCataiAMFerreira FilhoPBérzinFGallo JúniorLEvaluation of electromyographic activity and heart rate responses to isometric exercise. The role played by muscular mass and typeBraz J Med Biol Res199932111512010347778
- StewartJMMontgomeryLDGloverJLMedowMSChanges in regional blood volume and blood flow during static handgripAm J Physiol Heart Circ Physiol20072921H215H22316936003
- WilliamsonJWFadelPJMitchellJHNew insights into central cardiovascular control during exercise in humans: a central command updateExp Physiol2006911515816239250
- MitchellJHJ.B. Wolffe memorial lecture. Neural control of the circulation during exerciseMed Sci Sports Exerc19902221411542192221
- GalloJRJrMacielBCMarin-NetoJAMartinsLELima-FilhoECMançoJCThe use of isometric exercise as a means of evaluating the parasympathetic contribution to the tachycardia induced by dynamic exercise in normal manPflugers Arch19884121–21281323174376