Abstract
Many patients suffering from asthma or COPD have overlapping features of both diseases. However, a phenotypical approach for evaluating asthma–COPD overlap syndrome (ACOS) has not been established. In this report, we examined the phenotypes in patients with ACOS. Patients diagnosed with ACOS between 2011 and 2015 were identified and classified into four phenotype groups. Group A was composed of patients who smoked <10 pack years and had blood eosinophil counts ≥300. Group B was composed of patients who smoked <10 pack years and had blood eosinophil counts <300. Group C was composed of patients who smoked ≥10 pack years and had blood eosinophil counts ≥300. Group D was composed of patients who smoked <10 pack years and had blood eosinophil counts <300. Clinical characteristics were analyzed and compared among groups. Comparisons were made among 103 ACOS patients. Patients in group D were oldest, while patients in group A were youngest. There were relatively more female patients in groups A and B; the majority of patients in groups C and D were male. The degree of airflow obstruction was most severe in group C. The rate of being free of severe exacerbation was significantly lower in group C than in the other groups. In this study, each ACOS phenotype showed different characteristics. The proportion of patients free of severe exacerbation differed significantly among groups. At this time, further studies on the phenotypes of ACOS are required.
Keywords:
Introduction
Asthma and COPD are common diseases characterized by the presence of airway obstruction and have traditionally been considered different diseases. However, many patients with asthma or COPD have overlapping features of both diseases. Recently, the Global Initiative for Asthma (GINA) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) committees accepted the concept of asthma–COPD overlap syndrome (ACOS), described as follows:
ACOS is characterized by persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD. ACOS is therefore identified by the features that it shares with both asthma and COPD.Citation1,Citation2
Asthma and COPD are heterogeneous disorders with many phenotypes. Thus, ACOS may show a spectrum of phenotypes, which is particularly true when the diagnostic criteria of ACOS are broad. Different diagnostic criteria (broad and narrow) for ACOS have been proposed by various groups.Citation1–Citation6 Depending on the various definitions, the prevalence of ACOS also varies.Citation7 One broad definition of ACOS has been proposed by Gibson and Simpson.Citation3 Patients with fixed airflow obstruction with bronchodilator reversibility (BDR) or bronchial hyperresponsiveness (BHR) can be considered to have ACOS.
Studies on the classification and clinical features of ACOS have been steadily increasing. However, a phenotypical approach for the classification of ACOS is not yet available. Some experts discussed the heterogeneity of ACOS in previous review articles.Citation5,Citation8,Citation9 However, few studies have explored the phenotype of ACOS. RheeCitation5 previously proposed four phenotypes of ACOS classified mainly based on eosinophilic inflammation and smoking history. Each phenotype has a different underlying pathophysiology and requires different medications. In this study, we applied this ACOS phenotype in clinical practice, examined the prevalence of each phenotype, and determined whether there are different clinical characteristics between these disorders.
Methods
Study population
We identified all outpatients diagnosed with COPD at Seoul St Mary’s Hospital (1,356 bed tertiary referral hospital) between May 2011 and May 2015. Among them, we selected patients who were managed by a pulmonary specialist. We retrospectively reviewed the medical records during the study period and applied the definition of ACOS from a previous study by Gibson and Simpson.Citation3 We utilized this definition because it is much simpler and clearer than GINA/GOLD ACOS definition. We enrolled patients with symptoms of increased variability of airflow and incompletely reversible airflow obstruction. Patients with ACOS were eligible if their post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio was <0.7 with a positive BDR (>200 mL and >12% increase in FEV1) and/or positive BHR (positive methacholine or mannitol provocation test). Patients with lung cancer or cystic fibrosis were excluded in this study.
This study was approved by the Institutional Review Board of Seoul St Mary’s Hospital. The requirement for written informed consent from each patient was waived due to the retrospective nature of the study.
Clinical evaluation
Clinical data, including demographic data, smoking history, and laboratory tests such as eosinophil counts, COPD assessment test (CAT), pulmonary function test (PFT), frequency of severe exacerbation, and use of medication, were collected by retrospective review of medical records. For data regarding exacerbation, only severe exacerbation was recorded due to the potential for underdetection of mild or moderate exacerbation based on chart review. Severe exacerbation was defined as a worsening of any respiratory symptoms or increased dyspnea, which required an emergency room (ER) visit or hospitalization with prescription of systemic corticosteroid and/or antibiotics. History of medication use included inhaled corticosteroid (ICS) with long-acting beta agonist (LABA), long-acting muscarinic antagonist (LAMA), LABA, phosphodiesterase 4 inhibitor (PDE4I), and leukotriene receptor antagonist (LTRA).
Classification of the ACOS phenotype
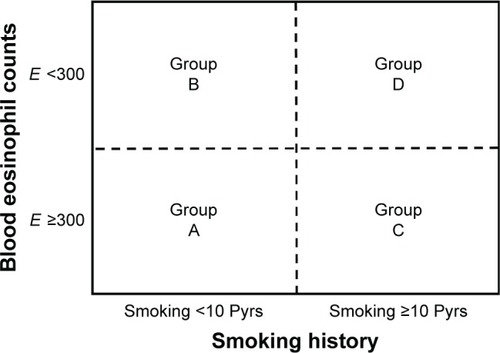
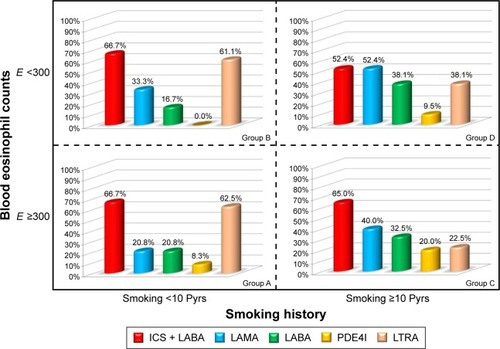
The enrolled patients were divided into four phenotype groups according to blood eosinophil counts and smoking history. The classification was based on Rhee’s previous articleCitation5 and modified in a simple way. ACOS phenotype A group was composed of patients who smoked <10 pack years and had blood eosinophil counts ≥300. Group B was composed of patients who smoked <10 pack years and had blood eosinophil counts <300. Group C was composed of patients who smoked ≥10 pack years and had blood eosinophil counts ≥300. Group D was composed of patients who smoked <10 pack years and had blood eosinophil counts <300 (). Patients were classified as high blood eosinophil group if blood eosinophil count was ≥300 even once among multiple measurements. We analyzed the characteristics and compared differences among the four groups.
Figure 1 Four phenotype groups of ACOS.
Abbreviations: ACOS, asthma–COPD overlap syndrome; E, blood eosinophil count; Pyrs, pack years.
PFT
PFTs were performed for all patients by experienced technicians. PFT was performed following the American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines in a licensed laboratory. Bronchodilator test was performed 15 minutes after the administration of 400 μg of salbutamol. Positive test for methacholine provocation was defined as provocative concentrations of methacholine required to decrease FEV1 (PC20) by 20% (≤16 mg/mL). Positive test for mannitol provocation was defined as PD15 (≤635 mg). Medications that can affect BDR or BHR were stopped before the test according to ATS guideline. Baseline PFT data were used in this study.
Statistical analysis
Categorical data were analyzed using χ2 or Fisher’s exact test. For normally distributed data, Student’s t-test or one-way analysis of variance was utilized for between-group comparisons. For nonnormally distributed data, we used the Mann–Whitney U-test or the Kruskal–Wallis test for between-group comparisons. The proportion of patients free of severe exacerbation was compared using the log-rank test for the four groups. The proportion of patients free of severe exacerbation was also compared between ICS (± LABA) users and nonusers. All statistical analyses were performed using the SPSS statistical package (version 18.0; SPSS Inc, Chicago, IL, USA); P-values <0.05 were considered statistically significant.
Results
Baseline characteristics
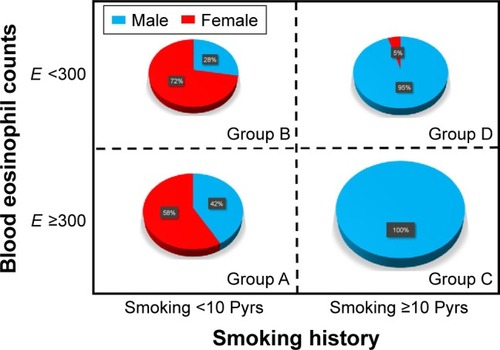
A total of 1,017 COPD patients were identified during the study period. Of these, 120 (11.8%) patients showed fixed airflow obstruction with BDR or BHR. Due to a lack of laboratory tests or smoking history, 17 patients were excluded. In total, 103 patients were included in the study (). shows the number of patients classified into each group. The mean age (range: 35–94 years) in each group differed significantly (). Patients in group D were oldest, while group A patients were youngest. The percentage of males also differed significantly (, P<0.001). There were relatively more female patients in groups A and B; the majority of patients in groups C and D were male. There were no significant differences in body mass index (BMI) among these groups. Post-bronchodilator FVC (%) or FEV1 (%) did not differ significantly among the four groups. However, FEV1/FVC (%) differed significantly (P=0.003) and was highest in group A and lowest in group C. There were no significant differences in CAT scores among the four groups.
Table 1 Characteristics of the ACOS phenotype groups
Figure 2 Flow diagram for subject enrollment.
Notes: Group A: patients who smoked <10 Pyrs and had blood eosinophil counts ≥300; group B: patients who smoked <10 Pyrs and had blood eosinophil counts <300; group C: patients who smoked ≥10 Pyrs and had blood eosinophil counts ≥300; and group D: patients who smoked ≥10 Pyrs and had blood eosinophil counts <300.
Abbreviations: BDR, bronchodilator response; BHR, bronchial hyperresponsiveness.
Figure 3 Distribution of patients into four phenotype groups of ACOS.
Notes: The size of the circle represents the number of patients in each group. Group A: patients who smoked <10 Pyrs and had blood eosinophil counts ≥300; group B: patients who smoked <10 Pyrs and had blood eosinophil counts <300; group C: patients who smoked <10 Pyrs and had blood eosinophil counts ≥300; and group D: patients who smoked ≥10 Pyrs and had blood eosinophil counts <300.
Abbreviations: ACOS, asthma–COPD overlap syndrome; E, blood eosinophil count; Pyrs, pack years.
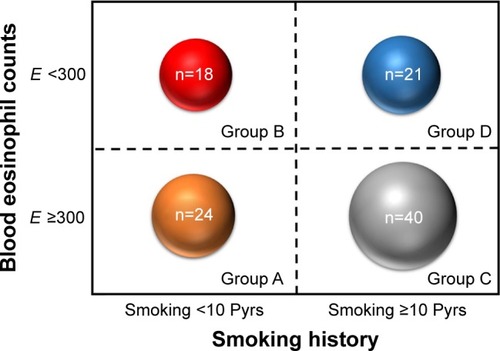
Figure 4 Gender distribution according to four phenotype groups of ACOS.
Abbreviations: ACOS, asthma -COPD overlap syndrome; E, blood eosinophil count; Pyrs, pack years.
Frequency of medication use
There was a different pattern of medication prescription among the four groups (). Overall, ICS + LABA was most frequently prescribed. For LAMA, the prescription rate was highest in group D followed by groups C, B, and A. In contrast, the LTRA prescription rate was highest in group A followed by groups B, D, and C. LABA and PDE4I were more frequently prescribed in groups C/D than in groups A/B.
Figure 5 Pattern of medication prescription among four phenotype groups of ACOS.
Abbreviations: ACOS, asthma -COPD overlap syndrome; E, blood eosinophil count; ICS, inhaled corticosteroid; LABA, long-acting beta agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; PDE4I, phosphodiesterase 4 inhibitor.
Severe exacerbation
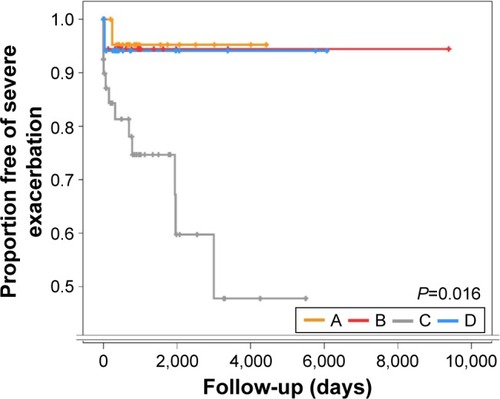
The mean follow-up period of enrolled patients was 1,229.8±152.2 days (mean ± standard error of the mean). The proportion of patients free of severe exacerbation was analyzed using log-rank tests among the four groups (). There were significant differences in the proportion of patients free of severe exacerbation (P=0.016). The survival rate for patients free of exacerbation was lower in group C than in other groups.
Figure 6 The proportion of patients free of severe exacerbation.
Among 103 patients, ICS was prescribed to two patients and ICS + LABA was prescribed to 65 patients. The proportion of patients free of severe exacerbation was compared between ICS group (patients with ICS or ICS + LABA) and non-ICS group. There was no significant difference between the two groups (P=0.663). Among 40 group C patients, ICS was prescribed to zero patient and ICS + LABA was prescribed to 14 patients. The proportion of patients free of severe exacerbation was compared between ICS and non-ICS groups among group C patients. There was also no significant difference between the two groups (P=0.383).
Discussion
After the GINA and GOLD committees accepted and published the concept of ACOS,Citation1,Citation2 several articles have been published regarding ACOS.Citation5,Citation6,Citation8–Citation13 However, the definition of ACOS remains unclear. The GINA and GOLD committees provided a table to help clinicians diagnose ACOS. However, this table is not clinically practical because it requires extensive time to complete. Moreover, some questions are vague and the answer can be very subjective. Even for the same patient, the answer can be changed according to the clinician who checks the table. Thus, few ACOS studiesCitation14 have utilized this table for the definition of ACOS. In contrast, Gibson and SimpsonCitation3 proposed a definition of ACOS based on spirometry results. Compared to the GINA/GOLD definition, this definition is much simpler and very clear. It is also simple to apply in clinical practice. For these reasons, there have been many ACOS studiesCitation11,Citation15,Citation16 using this definition. However, compared to the definition by GINA and GOLD, this definition is considered broad. Thus, a relatively large number of patients can be classified as ACOS according to this definition. It is expected that patients with predominant features of asthma or COPD are likely to be considered as ACOS according to this definition.
The purpose of this study was to examine how many patients are enrolled by this definition and determine whether patients can be classified into four different phenotypes. Interestingly, among 1,017 COPD patients, a relatively small percentage (11.8%) of patients showed positive BDR or BHR. In addition, COPD patients with BDR or BHR could be classified into four different phenotypes. Although phenotype C was dominant (38.8%), several patients were classified into other phenotypes (A: 23.3%, B: 17.4%, and D: 20.4%). This result suggests that heterogeneity exists even in patients with ACOS.
This is the first study to show the heterogeneity of ACOS. Several previous studies have explored the heterogeneity of asthmaCitation17–Citation19 or COPD.Citation20–Citation29 However, few studies have explored the heterogeneity of ACOS. The concept of heterogeneity in ACOS has been suggested in previous review articles by experts.Citation5,Citation8,Citation9 However, few studies have supported the concept of heterogeneity in ACOS. In this study, we used COPD patients managed by a pulmonary specialist in a tertiary referral hospital. We then classified patients into four categories according to relatively simple criteria – blood eosinophils and smoking history. There are different characteristics among the four groups. Moreover, the prognosis (exacerbation) differed significantly among the four groups. These results are valuable in that we demonstrated heterogeneity in ACOS patients.
Phenotypic classification based on the underlying disease mechanism is important for the appropriate management of individual patients. Eosinophilic inflammation and a history of smoking are important components in the classification of ACOS. According to these factors, RheeCitation5 proposed four phenotypes of ACOS and specific treatment options for each phenotype. Phenotype A is an allergic asthma-predominant phenotype. Phenotype B is characterized by features of severe noneosinophilic asthma. Phenotype C is very compatible with ACOS in that these patients have features of both asthma and COPD. Phenotype D is relatively pure COPD with reversibility. In this study, we investigated baseline characteristics and clinical features of each phenotype in real-world patients with ACOS. There are a relatively large number of patients in group C; however, the number of patients was relatively evenly distributed among groups. This study validated that the previously proposed phenotypes of ACOS by Rhee are clinically applicable and meaningful.
One interesting result of this study is the different pattern of medication use in each group. All enrolled patients were managed by a pulmonary specialist in tertiary referral hospitals. Because these patients were considered as ACOS, ICS + LABA was prescribed most commonly across the four phenotypes. However, LAMA, LABA, and PDE4I, which are mainly COPD medications, were more frequently prescribed in COPD-predominant phenotypes (C and D). In contrast, LTRA, which is mainly an asthma medication, was more frequently prescribed in asthma-predominant phenotypes (A and B). This phenomenon also supports the usefulness of ACOS classification by Rhee. The medication prescription in this study was determined by an experienced pulmonary specialist. They can classify phenotypes of ACOS patients and prescribe appropriate medication in a personalized manner. However, for general practitioners (GPs), it can be difficult to classify ACOS patients into subtypes. Thus, simple criteria to classify ACOS will be helpful for GPs. Based on our results, patients are well-characterized by two simple clinically relevant criteria – blood eosinophil count and smoking history. Thus, GPs can easily diagnose ACOS patients (fixed airflow obstruction with BDR or BHR) and subsequently classify these patients into four phenotypes based on blood eosinophil count and smoking history. Finally, GPs can choose a specific medication according to the phenotype. This allows GPs to treat ACOS patients in a personalized manner.
Preventing exacerbations in patients with ACOS is one of the unmet needs in the pharmacological treatment, similar to asthma and COPD. In this study, the differences that exist in severe exacerbation among the four phenotype groups is one of the important outcomes. When classified into the four phenotypes, severe exacerbation is most frequent in phenotype C. Based on these results, pulmonary function is not the reason for the differences in the frequency of acute exacerbation. This is because the frequency of acute exacerbation in phenotype C was worst while its pulmonary function was not. This suggests that different phenotypes exist in ACOS, and each phenotype has a different prognosis. Actually, it is well known that the prognosis of ACOS was poorer than that of COPD alone.Citation10,Citation30 They have more symptomsCitation25 and exacerbate more frequently.Citation31 The poor prognosis of phenotype C in this study is in agreement with previous reports because phenotype C is very compatible with ACOS. The ACOS definition based only on BDR or BHR is a broad definition. In contrast, including other criteria such as blood eosinophil count and history of smoking results in a narrow definition. RheeCitation5 proposed a broad and narrow definition of ACOS in a previous review article. This narrow definition, which is compatible with phenotype C, is similar to other definitions proposed by Spanish colleagues.Citation4 Thus, it is understandable that patients with phenotype C show more exacerbations.
There are some limitations in this study. 1) The sample size may not be sufficiently large to clarify the clinical features of each phenotype group. In addition, this was a single-center study. However, there has been no prior classification of ACOS phenotypes or studies on clinical patients. The results of this study should be validated in a large-scale cohort study. Whether two simple criteria – blood eosinophil count 300 and 10 pack years smoking history – used in this study will be useful should be validated in future study. Moreover, whether different prognoses of four groups will be replicated or not is also important. 2) It is likely that there was bias in this study due to its retrospective nature. The history of exacerbation may not be correct. Due to this limitation, only severe exacerbation was analyzed in this study; mild or moderate exacerbation was not considered. By reviewing the charts, it was difficult to detect mild or moderate exacerbation.
Conclusion
In this study, we classified ACOS patients into four phenotypes. Each phenotype showed different characteristics. The proportion of patients free of severe exacerbation differed significantly among groups. However, further studies on the phenotype of ACOS are required.
Disclosure
CKR received consulting/lecture fees from MSD, AstraZeneca, Novartis, GSK, Takeda, Mundipharma, Sandoz, Boehringer-Ingelheim, and Teva-Handok. The other authors report no conflicts of interest in this work.
References
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease2016 Available from: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/Accessed October 15, 2016
- Global Initiative for AsthmaGlobal Strategy for Asthma Management and Prevention2015 Available from: http://ginasthma.org/wp-content/uploads/2016/01/GINA_Report_2015_Aug11-1.pdfAccessed October 15, 2016
- GibsonPGSimpsonJLThe overlap syndrome of asthma and COPD: what are its features and how important is it?Thorax200964872873519638566
- Soler-CatalunaJJCosioBIzquierdoJLConsensus document on the overlap phenotype COPD-asthma in COPDArch Bronconeumol201248933133722341911
- RheeCKPhenotype of asthma–chronic obstructive pulmonary disease overlap syndromeKorean J Intern Med201530444344926161009
- SinDDMiravitllesMManninoDMWhat is asthma–COPD overlap syndrome? Towards a consensus definition from a round table discussionEur Respir J201648366467327338195
- WurstKEKelly-ReifKBushnellGAPascoeSBarnesNUnderstanding asthma–chronic obstructive pulmonary disease overlap syndromeRespir Med201611011126525374
- BatemanEDReddelHKvan Zyl-SmitRNAgustiAThe asthma–COPD overlap syndrome: towards a revised taxonomy of chronic airways diseases?Lancet Respir Med20153971972826255108
- ReddelHKTreatment of overlapping asthma–chronic obstructive pulmonary disease: can guidelines contribute in an evidence-free zone?J Allergy Clin Immunol2015136354655226343938
- RheeCKYoonHKYooKHMedical utilization and cost in patients with overlap syndrome of chronic obstructive pulmonary disease and asthmaCOPD201411216317024111662
- LeeHYKangJYYoonHKClinical characteristics of asthma combined with COPD featureYonsei Med J201455498098624954327
- HardinMChoMMcDonaldMLThe clinical and genetic features of COPD-asthma overlap syndromeEur Respir J201444234135024876173
- BarrechegurenMRoman-RodriguezMMiravitllesMIs a previous diagnosis of asthma a reliable criterion for asthma–COPD overlap syndrome in a patient with COPD?Int J Chron Obstruct Pulmon Dis2015101745175226366067
- GaoYZhaiXLiKAsthma COPD overlap syndrome on CT densitometry: a distinct phenotype from COPDCOPD201613447147626742511
- LeeSYParkHYKimEKCombination therapy of inhaled steroids and long-acting beta2-agonists in asthma–COPD overlap syndromeInt J Chron Obstruct Pulmon Dis2016112797280327877033
- MenezesAMMontes de OcaMPerez-PadillaRIncreased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthmaChest2014145229730424114498
- WenzelSEAsthma phenotypes: the evolution from clinical to molecular approachesNat Med201218571672522561835
- SklootGSAsthma phenotypes and endotypes: a personalized approach to treatmentCurr Opin Pulm Med20162213926574717
- LotvallJAkdisCABacharierLBAsthma endotypes: a new approach to classification of disease entities within the asthma syndromeJ Allergy Clin Immunol2011127235536021281866
- AgustiABelEThomasMTreatable traits: toward precision medicine of chronic airway diseasesEur Respir J201647241041926828055
- AgustiAThe path to personalised medicine in COPDThorax201469985786424781218
- AgustiAPhenotypes and disease characterization in chronic obstructive pulmonary disease. Toward the extinction of phenotypes?Ann Am Thorac Soc201310supplS125S13024313762
- AgustiAEdwardsLDRennardSIPersistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotypePLoS One201275e3748322624038
- de OcaMMHalbertRJLopezMVThe chronic bronchitis phenotype in subjects with and without COPD: the PLATINO studyEur Respir J2012401283622282547
- Izquierdo-AlonsoJLRodriguez-GonzalezmoroJMde Lucas-RamosPPrevalence and characteristics of three clinical phenotypes of chronic obstructive pulmonary disease (COPD)Respir Med2013107572473123419828
- JamiesonDBMatsuiECBelliAEffects of allergic phenotype on respiratory symptoms and exacerbations in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2013188218719223668455
- KimVHanMKVanceGBThe chronic bronchitic phenotype of COPD: an analysis of the COPDGene StudyChest2011140362663321474571
- MiravitllesMSoler-CatalunaJJCalleMSorianoJBTreatment of COPD by clinical phenotypes: putting old evidence into clinical practiceEur Respir J20134161252125623060631
- HurstJRVestboJAnzuetoASusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- NielsenMBarnesCBUlrikCSClinical characteristics of the asthma–COPD overlap syndrome – a systematic reviewInt J Chron Obstruct Pulmon Dis2015101443145426251584
- de MarcoRPesceGMarconAThe coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general populationPLoS One201385e6298523675448
