Abstract
Background
Platelets may actively participate in inflammation in COPD. Platelet distribution width (PDW), a measure of platelets’ volume heterogeneity, may increase in platelets’ activation. We hypothesized that PDW may be a marker of hypercoagulation, which plays a significant role in conditions associated with worse survival of patients with COPD, eg, acute myocardial infarction and other forms of ischemic heart disease.
Methods
Retrospective analysis of 79 patients. Variables were compared after grouping patients according to the upper normal limit of PDW, using Welch’s t-tests or Mann–Whitney U, and chi-square tests. Survival in the two groups was compared using the Kaplan–Meier method and Cox proportional hazards regression.
Results
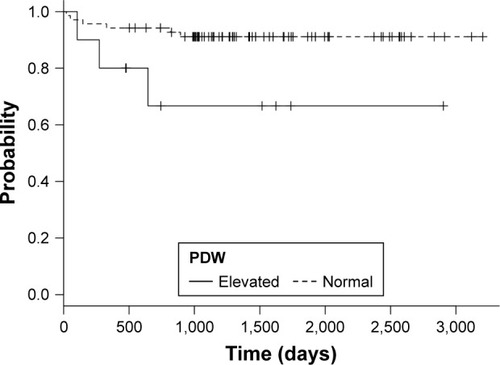
Ten patients presented values of PDW above 16 fL, which was the upper limit of normality for our laboratory. Compared to patients with normal PDW, they had lower forced expiratory flow between 25% and 75% of vital capacity (FEF 25–75) – 35% of reference value vs 57% (P=0.003) and peak expiratory flow – 39% vs 54% (P<0.001). The median survival of patients with elevated PDW was 743 days compared to those with normal PDW (1,305 days) (P=0.025). The adjusted HR was 4.59 (95% CI: 1.1, 19.19; P=0.04).
Conclusion
Our analysis indicates that elevated PDW is associated with reduced survival of patients with COPD. If our data are to be confirmed, PDW may be used as an inexpensive and repeatable prognostic tool in COPD.
Keywords:
Background
Increasing evidence suggests that the pathophysiology of COPD is characterized by the interaction between several factors, including local and systemic inflammation.Citation1,Citation2 IL-6 plays a central role in the inflammatory process underlying COPD, with increased concentrations reported in plasma, sputum, and exhaled breath of patients with COPD, especially during exacerbations.Citation3–Citation7 IL-6 stimulates the synthesis of acute phase proteins, but also affects platelet count by stimulating the differentiation of megakaryocytes into platelets and possibly by stimulating thrombopoietin production.Citation8–Citation12
It has been shown that platelets actively participate in inflammation in several conditions.Citation13 Upon activation, platelets release chemokines from their α-granules such as MIF, CXCL4, CXCL7 or CXCL5 which play a role in inflammatory processes.Citation14 CXCL5 is a chemokine of potential importance in regulation of lung inflammation, by modulating neutrophil transepithelial and transendothelial migration and accumulation in the airspace.Citation15 Furthermore, platelets regulate MMPs’ activity which significantly contributes to inflammation. Platelets regulate MMPs both directly by secretion of the proteases and their inhibitors, and indirectly by influencing the behavior of other cells such as neutrophils and monocytes.Citation16 Platelet-derived CD40L, which is shed from the platelet surfaces by MMPs, induces MT-1-MMP, MMP-1, MMP-2, and MMP-9 on endothelial cells.Citation17,Citation18 There is evidence proving the role of these MMPs in the pathophysiology of COPD.Citation19–Citation23
The routinely available indices which describe platelets’ morphology and function are the platelet count (PLT), the platelet-to-lymphocyte ratio (PLR), the mean platelet volume (MPV), and the platelet distribution width (PDW). Thrombocytopenia was associated with poor outcomes in acute exacerbations of COPD.Citation24 On the other hand, thrombocytosis may be associated with increased short- and long-term mortality after exacerbations.Citation25 PLR describes the correlation between changes in levels of platelets and lymphocytes. PLR has been reported to play a significant role in cardiovascular diseases,Citation26,Citation27 diabetic ketoacidosis,Citation28 and numerous neoplasms.Citation8,Citation12,Citation29–Citation40 MPV reflects changes in either the level of platelet stimulation or the rate of platelet production.Citation41 It has been assessed as an inflammatory marker in several diseases.Citation42–Citation44 COPD patients, during acute exacerbation and in stable phase, have lower MPV compared with healthy controls, and the MPV increases once patients have recovered from exacerbation.Citation45 PDW is an index of platelets’ volume heterogeneityCitation46 and may increase in platelets’ activation.Citation47 The role of PDW has been assessed in several conditions, such as acute gangrenous appendicitis, carotid artery stenosis, coronary artery disease, angina pectoris, idiopathic pulmonary hypertension, ovarian torsion, and preeclampsia.Citation48–Citation56 PDW can serve as useful prognostic factor for long-term mortality in patients with acute myocardial infarction and was found to be an independent risk factors for cardiovascular mortality.Citation57 Because hypercoagulation may play a significant role in conditions associated with worse survival of patients with COPD,Citation58,Citation59 we hypothesized that PDW may be associated with survival in these patients. The objective of this study was to evaluate the mortality rate of patients with COPD with and without an abnormal PDW.
Methods
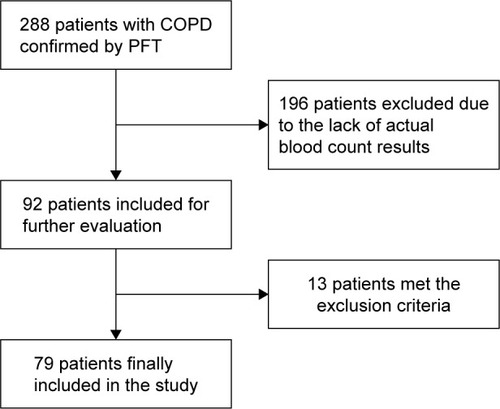
From the electronic archive of the Campus Bio-Medico Hospital in Rome, Italy, between March 2006 and December 2014, we identified 288 patients with post-bronchodilator forced expiratory volume in 1 second to forced vital capacity ratio (FEV1/FVC) <0.7 and for whom information on vital status could be obtained from the regional death registry. Subsequently, we selected patients with a blood cell count performed within 2 weeks of the index spirometry (N: 92).
We excluded patients with conditions that, based on the available evidence, may affect PDW: acute exacerbation of COPD, any active acute inflammation, connective tissue disorder, diabetic ketoacidosis, recent history of myocardial infarction, end-stage renal disease, history of any active malignancy, and hematological system diseases, or blood transfusion in the last 2 months.
Demographic data, medical history, and routine laboratory measurements, including white blood count, neutrophils, lymphocytes, PLT, MPV, and PDW, were collected from the digital database of medical records of Campus Bio-Medico di Roma.
Pulmonary function tests (PFTs) including vital capacity (VC), FVC, FEV1, FEV1/FVC ratio, forced expiratory flow between 25% and 75% of VC (FEF 25–75), peak expiratory flow (PEF), total lung capacity (TLC), and residual volume, were collected from the digital database of medical records of Campus Bio-Medico di Roma. PFTs were performed with the water-bell volume spirometry device between March 2006 and December 2012 by the same technician. All lung function parameters were measured according to the American Thoracic Society/European Respiratory Society guidelines.Citation60–Citation63
Continuous data are presented as the mean with 95% CI, except survival that was presented as median. Variables were compared after grouping patients according to the laboratory upper limit of PDW, using t-test for normally distributed continuous data, and Mann–Whitney U test for not normally distributed continuous data. Categorical variables were compared using chi-square test with Yates’ continuity correction.
Survival analysis was performed after grouping patients according to the laboratory normal limit for PDW values. Kaplan–Meier analysis and log-rank test were used to compare survival curves. Multivariable analyses were performed using Cox proportional hazards regression to adjust for age, comorbidities, and FEV1.
Data were analyzed using R software for MacOS (R Core Team [2016]. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria).
The protocol of the study was approved by the Ethics Committee of the Campus Bio-Medico University.
Results
Seventy-nine patients met the inclusion criteria. The flow chart of sample selection is reported in . Most patients were GOLD stage II (49.4%), ex-smokers (62.5%), and had comorbidities (90%). Mean age of the study population was 73.6 (95% CI: 72.32, 74.95). The overall male-to-female ratio was 1.93:1. The means of baseline values of the study population are presented in .
Table 1 Baseline values of the study population
Figure 1 Flow chart of patient selection for inclusion in the study.
Ten patients presented values of PDW above 16 fL, which was the upper limit of normality for our laboratory. There were no differences in age and gender distribution between the groups. Participants with higher PDW showed significantly lower FEF 25–75 and PEF values compared to those with normal PDW – both in absolute values, and expressed as percent of reference value ().
Table 2 Functional and demographic parameters of the study population according to upper limit of PDW values
Patients with high PDW had worse survival compared to patients with normal PDW, with a median survival time of 743 days compared to 1,305 days of patients with normal PDW (). Log rank test showed significant differences between groups (chi-square =4.9; P=0.025). Abnormal PDW values were associated with an RR of death of 3.18 (95% CI 0.6, 7.79; P=0.07). The HR for unadjusted Cox regression was 4.34 (95% CI: 1.072, 17.6; P=0.04) and HR after adjustment for gender, age, comorbidities, and FEV1% was 4.59 (95% CI: 1.1, 19.19; P=0.04).
Discussion
In our study, we found that elevated PDW was associated with increased risk for mortality in COPD patients. This result was confirmed after adjustment for age, comorbidities, and FEV1%, suggesting that in our sample PDW was an independent risk factor for mortality.
Activation of platelets causes morphological changes, including pseudopodia formation. Pseudopodium formation enhances platelet–surface interactions (adhesion) and platelet–platelet interaction (cohesion).Citation64 Progressively activated platelets with pseudopodia formation have heterogeneous size, and PDW may increase.Citation47 The association of PDW with survival may be hypothetically linked with hypercoagulation, which plays a significant role in conditions associated with survival in COPD, eg, acute myocardial infarction and other forms of ischemic heart disease.Citation58,Citation59 According to Wang et al, elevated PDW is a risk factor for pulmonary embolism and may be observed in COPD patients with this condition.Citation65
Our results are in line with other evidence which shows that PDW may be a marker of COPD exacerbations,Citation66 while patients with stable COPD do not seem to have higher PDW compared to controls.Citation67 Furthermore, the use of antiplatelet drugs was associated with improved survival in oxygen-dependent COPD patients.Citation68
While we excluded patients with diseases with an obvious impact on PDW or inflammation, in our sample, comorbidities were common, eg, prevalence of diabetes mellitus was 23%. Makhlouf et al showed that COPD patients with diabetes mellitus had significantly higher PDW compared to healthy controls, but there was no difference in PDW between COPD patients with and without diabetes mellitus.Citation69
We found significantly lower values of FEF 25–75 and PEF among patients with elevated PDW. This is a novel finding in a population of patients with COPD. Indeed, FEF 25–75 reflects the expiratory flows in peripheral airways, whereas PEF is strictly dependent upon the strength of expiratory muscles. Thus, these findings, though preliminary in nature, suggest that higher PDW, as a marker of inflammatory and hypercoagulable status, is linked to lung metabolism/inflammation and sarcopenia. Unfortunately, we lacked additional information, such as breath pattern analysis and direct measurement of respiratory muscle strength, to test this hypothesis. However, we think that it is unlikely that PDW per se has any direct effect on pulmonary function. The associations that we have found are most likely due to platelet morphology acting as a marker for some other biological process, such as inflammation. Due to the retrospective character of our study, causal relationship cannot be verified, and our results must also be interpreted in light of the fact that both of these parameters have limited clinical value in COPD, while we found no difference in spirometric parameters known to have important prognostic significance (FEV1, FVC). Measurement of FEF 25–75 does not contribute to clinical decision making.Citation70 PEF is recorded in the first tenth of a second of forced expiration, while FEV1 continues to record forced expiration for a further 0.9 s, therefore FEV1 records what happens to expired air after peak flow is reached. It is in this component of forced expiration that the changes characteristic of COPD are observed.Citation71
There are some limitations of our study that need to be taken into account. Our sample size is relatively small and we could not fully investigate the relationship between PFT and PDW. Furthermore, our study is a retrospective analysis and we have to be aware of a risk of selection bias.
Conclusion
Our pilot analysis shows that PDW is associated with survival in patients with COPD. As it can be simply and rapidly measured from routine blood examination, should our results be confirmed in larger samples, it may prove to be a widely available, inexpensive, and repeatable prognostic marker. Furthermore, it might contribute to the characterization of the phenotypic variability of COPD.
Author contributions
AJB – study conception, design, and coordination, acquisition of data, analysis, and interpretation of data, statistical analysis, drafting of manuscript; CP – study conception, design, and coordination, acquisition of data, analysis and interpretation of data, statistical analysis, drafting of manuscript; WJP – contributed to the design of the study, analysis, and interpretation of data, critical revision; RAI – study design and coordination, analysis and interpretation of data, critical revision, drafting of manuscript. All authors edited and approved the final version of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- BarnesPJThe cytokine network in chronic obstructive pulmonary diseaseAm J Respir Cell Mol Biol200941663163819717810
- AgustiAGCOPD, a multicomponent disease: implications for managementRespir Med200599667068215878483
- BhowmikASeemungalTASapsfordRJWedzichaJARelation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbationsThorax200055211412010639527
- BucchioniEKharitonovSAAllegraLBarnesPJHigh levels of interleukin-6 in the exhaled breath condensate of patients with COPDRespir Med200397121299130214682411
- GessnerCScheibeRWötzelMExhaled breath condensate cytokine patterns in chronic obstructive pulmonary diseaseRespir Med200599101229124016140223
- Emami ArdestaniMZaerinORole of serum interleukin 6, albumin and C-reactive protein in COPD PatientsTanaffos201514213414026528368
- YasudaNGotohKMinatoguchiSAn increase of soluble Fas, an inhibitor of apoptosis, associated with progression of COPDRespir Med19989289939999893764
- TempletonAJAceOMcNamaraMGPrognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysisCancer Epidemiol Biomark Prev201423712041212
- OhsugiYRecent advances in immunopathophysiology of interleukin-6: an innovative therapeutic drug, tocilizumab (recombinant humanized anti-human interleukin-6 receptor antibody), unveils the mysterious etiology of immune-mediated inflammatory diseasesBiol Pharm Bull200730112001200617978466
- ImaiTKoikeKKuboTInterleukin-6 supports human megakaryocytic proliferation and differentiation in vitroBlood1991788196919741912578
- LippitzBECytokine patterns in patients with cancer: a systematic reviewLancet Oncol2013146e218e22823639322
- KlingerMHJelkmannWRole of blood platelets in infection and inflammationJ Interferon Cytokine Res200222991392212396713
- NordingHMSeizerPLangerHFPlatelets in inflammation and atherogenesisFront Immunol201569825798138
- KarshovskaEWeberCvon HundelshausenPPlatelet chemokines in health and diseaseThromb Haemost2013110589490223783401
- MeiJLiuYDaiNCXCL5 regulates chemokine scavenging and pulmonary host defense to bacterial infectionImmunity201033110611720643340
- SeizerPMayAEPlatelets and matrix metalloproteinasesThromb Haemost2013110590390923864155
- RahmanMRollerJZhangSMetalloproteinases regulate CD40L shedding from platelets and pulmonary recruitment of neutrophils in abdominal sepsisInflamm Res201261657157922349180
- MayAEKälschTMassbergSHerouyYSchmidtRGawazMEngagement of glycoprotein IIb/IIIa (alpha(IIb)beta3) on platelets upregulates CD40L and triggers CD40L-dependent matrix degradation by endothelial cellsCirculation2002106162111211712379582
- Skjøt-ArkilHClausenRENguyenQHMeasurement of MMP-9 and -12 degraded elastin (ELM) provides unique information on lung tissue degradationBMC Pulm Med2012123422818364
- IlumetsHRytiläPDemedtsIMatrix metalloproteinases -8, -9 and -12 in smokers and patients with stage 0 COPDInt J Chron Obstruct Pulmon Dis20072336937918229576
- BrajerBBatura-GabryelHNowickaAKuznar-KaminskaBSzczepanikAConcentration of matrix metalloproteinase-9 in serum of patients with chronic obstructive pulmonary disease and a degree of airway obstruction and disease progressionJ Physiol Pharmacol200859Suppl 6145152
- KwiatkowskaSNowetaKZiebaMNowakDBialasiewiczPEnhanced exhalation of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in patients with COPD exacerbation: a prospective studyRespiration201284323124122832426
- NavratilovaZZatloukalJKriegovaEKolekVPetrekMSimultaneous up-regulation of matrix metalloproteinases 1, 2, 3, 7, 8, 9 and tissue inhibitors of metalloproteinases 1, 4 in serum of patients with chronic obstructive pulmonary diseaseRespirology20121761006101222591289
- Rahimi-RadMHSoltaniSRabieepourMRahimiradSThrombocytopenia as a marker of outcome in patients with acute exacerbation of chronic obstructive pulmonary diseasePneumonol Alergol Pol201583534835126378995
- HarrisonMTShortPWilliamsonPASinganayagamAChalmersJDSchembriSThrombocytosis is associated with increased short and long term mortality after exacerbation of chronic obstructive pulmonary disease: a role for antiplatelet therapy?Thorax201469760961524743560
- AzabBShahNAkermanMMcGinnJTJrValue of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarctionJ Thromb Thrombolysis201234332633422466812
- SunbulMGerinFDurmusENeutrophil to lymphocyte and platelet to lymphocyte ratio in patients with dipper versus non-dipper hypertensionClin Exp Hypertens201436421722123786430
- LiuWYLinSGWangLRPlatelet-to-lymphocyte ratio: a novel prognostic factor for prediction of 90-day outcomes in critically ill patients with diabetic ketoacidosisMedicine (Baltimore)2016954e259626825908
- AsherVLeeJInnamaaABaliAPreoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancerClin Transl Oncol201113749950321775277
- BhattiIPeacockOLloydGLarvinMHallRIPreoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratioAm J Surg2010200219720320122680
- CarruthersRThoLMBrownJKakumanuSMcCartneyEMcDonaldACSystemic inflammatory response is a predictor of outcome in patients undergoing preoperative chemoradiation for locally advanced rectal cancerColorectal Dis20121410e701e70722731833
- DuttaSCrumleyABFullartonGMHorganPGMcMillanDCComparison of the prognostic value of tumour and patient related factors in patients undergoing potentially curative resection of gastric cancerAm J Surg2012204329429922444831
- DuttaSCrumleyABFullartonGMHorganPGMcMillanDCComparison of the prognostic value of tumour- and patient-related factors in patients undergoing potentially curative resection of oesophageal cancerWorld J Surg20113581861186621538187
- FoxPHudsonMBrownCMarkers of systemic inflammation predict survival in patients with advanced renal cell cancerBr J Cancer2013109114715323778526
- RaungkaewmaneeSTangjitgamolSManusirivithayaSSrijaipracharoenSThavaramaraTPlatelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancerJ Gynecol Oncol201223426527323094130
- SmithRABosonnetLRaratyMPreoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinomaAm J Surg2009197446647218639229
- WangDSLuoHYQiuMZComparison of the prognostic values of various inflammation based factors in patients with pancreatic cancerMed Oncol20122953092310022476808
- WangDSRenCQiuMZComparison of the prognostic value of various preoperative inflammation-based factors in patients with stage III gastric cancerTumour Biol201233374975622198641
- LiXChenZHXingYFPlatelet-to-lymphocyte ratio acts as a prognostic factor for patients with advanced hepatocellular carcinomaTumour Biol20153642263226925409616
- TianXZengFWuDPlatelet-to-lymphocyte ratio: a prognostic factor for patients with advanced hepatocellular carcinoma?Tumour Biol20153674935493626025113
- BancroftAJAbelEWMclarenMBelchJJMean platelet volume is a useful parameter: a reproducible routine method using a modified Coulter thrombocytometerPlatelets200011737938711132104
- GasparyanAYAyvazyanLMikhailidisDPKitasGDMean platelet volume: a link between thrombosis and inflammation?Curr Pharm Des2011171475821247392
- KimDSLeeJKimSHKimSMLeeMGMean platelet volume is elevated in patients with psoriasis vulgarisYonsei Med J201556371271825837177
- KisacikBTufanAKalyoncuUMean platelet volume (MPV) as an inflammatory marker in ankylosing spondylitis and rheumatoid arthritisJoint Bone Spine200875329129418403245
- WangRTLiJYCaoZGLiYMean platelet volume is decreased during an acute exacerbation of chronic obstructive pulmonary diseaseRespirology20131881244124823786593
- BessmanJDWilliamsLJGilmerPRJrPlatelet size in health and hematologic diseaseAm J Clin Pathol19827821501536954850
- BaeMHLeeJHYangDHParkHSChoYChaeSCWhite blood cell, hemoglobin and platelet distribution width as short-term prognostic markers in patients with acute myocardial infarctionJ Korean Med Sci201429451952624753699
- FanZPanJZhangYMean platelet volume and platelet distribution width as markers in the diagnosis of acute gangrenous appendicitisDis Markers2015201554201326688600
- AydoganAAkkucukSAricaSThe analysis of mean platelet volume and platelet distribution width levels in appendicitisIndian J Surg201577Suppl 2495500
- KokluEYukselIOArslanSPredictors of symptom development in intermediate carotid artery stenosis: mean platelet volume and platelet distribution widthAngiology201667762262926514416
- YilmazMCimilliGSaritemurMDiagnostic accuracy of neutrophil/lymphocyte ratio, red cell distribution width and platelet distribution width in ovarian torsionJ Obstet Gynaecol201636221822226467739
- KaratekeAKurtRKBaloğluARelation of platelet distribution width (PDW) and platelet crit (PCT) to preeclampsiaGinekol Pol201586537237526117976
- ZhengYGYangTXiongCMPlatelet distribution width and mean platelet volume in idiopathic pulmonary arterial hypertensionHeart Lung Circ201524656657225573235
- OzyurtluFYavuzVCetinNAcetHAyhanEIsikTThe association between coronary slow flow and platelet distribution width among patients with stable angina pectorisPostępy Kardiol Interwencyjnej201410316116525489301
- AdamGKocakEÖzkanAEvaluation of platelet distribution width and mean platelet volume in patients with carotid artery stenosisAngiology201566437537825313243
- BeklerAOzkanMTTenekeciogluEIncreased platelet distribution width is associated with severity of coronary artery disease in patients with acute coronary syndromeAngiology201566763864325112777
- RechcińskiTJasińskaAForyśJPrognostic value of platelet indices after acute myocardial infarction treated with percutaneous coronary interventionCardiol J201320549149824469872
- HansellALWalkJASorianoJBWhat do chronic obstructive pulmonary disease patients die from? A multiple cause coding analysisEur Respir J200322580981414621089
- ZielinskiJMacNeeWWedzichaJCauses of death in patients with COPD and chronic respiratory failureMonaldi Arch Chest Dis199752143479151520
- MillerMRHankinsonJBrusascoVStandardisation of spirometryEur Respir J200526231933816055882
- MillerMRCrapoRHankinsonJGeneral considerations for lung function testingEur Respir J200526115316115994402
- WangerJClausenJLCoatesAStandardisation of the measurement of lung volumesEur Respir J200526351152216135736
- MacintyreNCrapoROViegiGStandardisation of the single-breath determination of carbon monoxide uptake in the lungEur Respir J200526472073516204605
- BickRodger LMuranoGenesioPhysiology of hemostasisDisorders of Thrombosis and Hemostasis: Clinical and Laboratory PracticeLippincott Williams & Wilkins20026
- WangMZhangJJiQEvaluation of platelet distribution width in chronic obstructive pulmonary disease patients with pulmonary embolismBiomark Med20161058759626567584
- KaradenizGAktoğuSErerOFPredictive value of platelet-to-lymphocyte ratio in exacerbation of chronic obstructive pulmonary diseaseBiomark Med201610770171027339097
- SteiropoulosPPapanasNNenaEMean platelet volume and platelet distribution width in patients with chronic obstructive pulmonary disease: the role of comorbiditiesAngiology201364753553923052724
- EkströmMPHermanssonABStrömKEEffects of cardiovascular drugs on mortality in severe chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2013187771572023328521
- MakhloufHASadekSHNafadyAAPlatelet function in diabetic and nondiabetic patients with chronic obstructive pulmonary disease: a case control studyClin Respir J201610.1111/crj.12477
- QuanjerPWeinerDJPrettoJJBrazzaleDJBorosPWMeasurement of FEF25%–75% and FEF75% does not contribute to clinical decision makingEur Respir J20144341051105824072211
- WhitePSpirometry and peak expiratory flow in the primary care management of COPDPrim Care Respir J20041315816701628