Abstract
Background
Although vitamin D is well known for its function in calcium homeostasis and bone mineralization, several studies have shown positive effects on muscle strength and physical function. In addition, vitamin D has been associated with pulmonary function and the incidence of airway infections. As vitamin D deficiency is highly prevalent in chronic obstructive pulmonary disease (COPD) patients, supplementation might have a beneficial effect in these patients.
Objective
To assess the effect of vitamin D supplementation on respiratory muscle strength and physical performance in vitamin D-deficient COPD patients. Secondary outcomes are pulmonary function, handgrip strength, exacerbation rate, and quality of life.
Methods
We performed a randomized, double-blind, placebo-controlled pilot trial. Participants were randomly allocated to receive 1,200 IU vitamin D3 per day (n=24) or placebo (n=26) during 6 months. Study visits were conducted at baseline, and at 3 and 6 months after randomization. During the visits, blood was collected, respiratory muscle strength was measured (maximum inspiratory and expiratory pressure), physical performance and 6-minute walking tests were performed, and handgrip strength and pulmonary function were assessed. In addition, participants kept a diary card in which they registered respiratory symptoms.
Results
At baseline, the mean (standard deviation [SD]) serum 25-hydroxyvitamin D (25(OH)D) concentration (nmol/L) was 42.3 (15.2) in the vitamin D group and 40.6 (17.0) in the placebo group. Participants with vitamin D supplementation had a larger increase in serum 25(OH)D compared to the placebo group after 6 months (mean difference (SD): +52.8 (29.8) vs +12.3 (25.1), P<0.001). Primary outcomes, respiratory muscle strength and physical performance, did not differ between the groups after 6 months. In addition, no differences were found in the 6-minute walking test results, handgrip strength, pulmonary function, exacerbation rate, or quality of life.
Conclusion
Vitamin D supplementation did not affect (respiratory) muscle strength or physical performance in this pilot trial in vitamin D-deficient COPD patients.
Background
Vitamin D deficiency in patients with chronic obstructive pulmonary disease (COPD) is highly prevalent and associated with disease severity.Citation1,Citation2 Although vitamin D is classically known for its function in calcium and bone metabolism, studies in the past decades have suggested a far broader range of physiological effects of vitamin D, including effects on muscle function and the immune system, linked to the presence of vitamin D receptors (VDR) on the cells of these tissues.Citation3 These effects might have clinical implications for patients with COPD.Citation4
Impaired muscle function and poor physical performance form a major part of the disease burden in patients with COPD.Citation5 Quadriceps and handgrip weakness as well as poorer score in the 6-minute walking test are predictors of increased mortality risk in COPD patients.Citation6–Citation8 In the general population, several meta-analyses of randomized clinical trials have shown positive effects of vitamin D supplementation on muscle strength and physical performance.Citation9–Citation11 This effect was larger in participants with severe vitamin D deficiency at baseline.Citation11,Citation12 Observational studies on the relationships between vitamin D status and muscle function in COPD patients are controversial, although a polymorphism in the VDR gene has been shown to affect quadriceps muscle strength.Citation5,Citation13,Citation14 Only two intervention studies have been carried out in COPD patients without vitamin D deficiency selection. These studies were too small to perform post hoc analyses in deficient groups. Therefore, there is a need to specifically study COPD patients with vitamin D deficiency.
Besides muscle strength and physical function, some observational studies have shown that serum 25-hydroxyvitamin D (25(OH)D) concentrations are positively associated with pulmonary function.Citation15,Citation16 One hypothesis for this relationship is that vitamin D might affect pulmonary function through beneficial effects on muscle strength:Citation5 worse muscle function might lead to impaired respiratory muscle strength and thus poor pulmonary function.
These findings have led us to the question of whether vitamin D supplementation might influence disease course through an effect on muscle function in COPD patients. Vitamin D might be an attractive treatment option, as it is easy, safe, and inexpensive. With this study, we aim to assess the effects of vitamin D supplementation on respiratory muscle strength and physical performance specifically in vitamin D-deficient COPD patients. In addition, we assess its effects on pulmonary function, hand grip strength, exacerbation rate, and quality of life.
Methods
Study design and participants
We performed a randomized placebo-controlled trial in two centers. Participants were recruited from the departments of pulmonary disease of a university medical center (VU University Medical Center) and a large teaching hospital (Northwest Hospital group) in the period 2012–2014. Patients with COPD who met the inclusion criteria were asked to participate in the study (). If patients agreed to participate, their serum 25(OH)D concentration was assessed. Patients who had a vitamin D deficiency (25(OH)D <50 nmol/L) were included in the study. Study visits took place at baseline and after 3 and 6 months. All participants provided written informed consent. The study was approved by the local medical ethics committee of the VU University Medical Center and Northwest Hospital group (NL36386.029). The study was registered in the Netherlands Trial Register (NTR2827).
Table 1 Eligibility criteria
Randomization and masking
Participants were allocated by computer-generated block randomization (blocks of four patients) to receive either vitamin D or placebo with stratification by gender and institution. Pharmacists of the VU University Medical Center, who were independent from the clinical study team, performed the allocation. After the last participant completed the study, masking continued until all analyses were performed.
Intervention
Participants received either 1,200 IU colecalciferol or a matching placebo during 6 months according to randomization. They were allowed to use vitamin D supplementation to a maximum of 400 IU/day, as advised by the Dutch Health Council for adults aged <70 years. During the study, the participants were advised to ensure a dietary calcium intake of at least 1,000 mg per day. If this was not feasible, they received calcium supplementation to ensure a total calcium intake of 1,000 mg/day, as advised by the Dutch Health Council.
Outcomes
Primary outcomes
Primary outcomes were respiratory muscle strength and physical performance. Respiratory muscle strength was assessed by measuring maximal inspiratory and expiratory pressure according to American Thoracic Society (ATS) and European Respiratory Society (ERS) guidelines.Citation17 Physical performance score was assessed by the tandem test, chair stands test, 3-m walking test, and 6-minute walking test.Citation18,Citation19 The tandem test is a measure which estimates balance. The chair stands test largely represents quadriceps muscle strength as well as balance and the 3-meter walking test depicts a combination of gait performance and balance. The 6-minute walking test measures general functional exercise capacity.
Secondary outcomes
Secondary outcomes in this study were forced expiratory volume in 1 second and forced vital capacity measured by spirometry according to ATS/ERS guidelines,Citation20 peak expiratory flow determined using the Mini-Wright peak flow meter, handgrip muscle strength measured using a dynamometer,Citation21 and scores on physical activity measured by LASA Physical Activity Questionnaire (LAPAQ).Citation22 LAPAQ is a questionnaire covering several areas of activities. Scores are presented as activity in minutes per day and can be separated into sports and non-sports activities. In addition, participants were asked to maintain a diary card during the whole study period, in which they registered the occurrence of exacerbations and changes in medication. An exacerbation was defined as the presence, for at least 2 consecutive days, of an increase in any two major symptoms (dyspnea, sputum purulence, sputum amount) or an increase in one major and one minor symptom (wheeze, sore throat, cough, symptoms of a common cold).Citation23 Finally, quality of life was assessed using the Short Form Health Survey (SF-12)Citation24 and EuroQol (EQ-5D) questionnaires.Citation25 SF-12 can be subdivided into a physical and mental component summary and is scored within the 0–100 range, with a higher score indicating better quality of life. The EQ-5D score ranges from 0 to 1 with 1 indicating perfect health status.
25(OH)D
Blood samples were drawn from all participants at baseline and after 6 months. Samples were centrifuged and stored at −80°C until determination. After the study was completed, 25(OH)D was assessed in all samples by isotope dilution-solid phase extraction liquid chromatography-tandem mass spectrometry at the Endocrine Laboratory of the VU University Medical Center as described before.Citation26 The limit of quantitation was 4.0 nmol/L, intra-assay coefficient of variation (CV) was <6%, and inter-assay CV was <8% for concentrations between 25 and 180 nmol/L. 25(OH)D2 and 25(OH)D3 were measured separately.
Statistical analyses
At the start of this study, no trials on the effects of vitamin D supplementation in patients with COPD had been performed. Therefore, there were no data available on which we could base a sample size calculation. Because of the pilot nature of the study, we decided to include 50 participants.
All analyses were performed using the intention-to-treat analysis. In addition, sensitivity analyses including only severe vitamin D-deficient participants (<25 nmol/L) were performed to assess potential effects in this group. Finally, we also performed a subgroup analysis excluding noncom-pliant participants and participants who had used vitamin D supplementation.
Data are presented as mean ± standard deviation (sd), median (interquartile range) if data were not normally distributed or as number (%). We tested for differences between the placebo and vitamin D group at baseline and for differences between the groups in changes after 6 months. We used an unpaired t-test to test for normally distributed continuous variables, Mann–Whitney test for skewed continuous variables, and Pearson χ2 tests for categorical variables. All analyses were performed using IBM SPSS Statistics for Windows, version 22 (IBM Corporation, Armonk, NY, USA).
Results
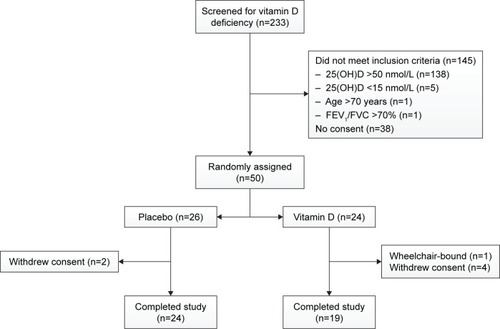
We screened 233 patients with COPD for vitamin D deficiency (). Of these patients, 145 did not meet the inclusion criteria and 38 did not want to participate in the study. Eventually, 50 participants were randomized to receive either placebo (n=26) or vitamin D supplementation (n=24). After randomization, one participant was excluded from the study, because he was wheelchair-bound and could not comply with all the study requirements. During follow-up, six participants withdrew consent.
Figure 1 Flowchart study.
shows the baseline characteristics for both subgroups. Mean serum 25(OH)D levels were deficient in both groups (mean serum 25(OH)D in nmol/L (SD): 42.3 (15.2) and 40.6 (17.0) in the vitamin D and placebo groups, respectively). The placebo group scored slightly better in the 3-meter walking test. No other differences between the groups were detected.
Table 2 Baseline characteristics
In , the differences after 6 months are shown for both groups. The vitamin D group showed a significant increase in serum 25(OH)D levels compared to the placebo group (mean difference (SD): +52.8 (29.8) vs +12.3 (25.1), P<0.001). We did not find any differences between the changes in pulmonary muscle strength and physical performance scores after 6 months. In addition, no differences were found in changes of the secondary outcomes (pulmonary function, handgrip strength, exacerbation rate, or quality of life). Sensitivity analyses in severe vitamin D-deficient participants (n=6) and subgroup analyses excluding noncom-pliant participants (n=9) also did not show any differences (data not shown). A subgroup analysis excluding participants with self-administered vitamin D supplementation also did not show any differences (Table S1).
Table 3 Differences (Δ) after 6 months
No differences were found between the vitamin D group and placebo group after 3 months (Table S2).
Discussion
This pilot study aimed to assess the effects of vitamin D supplementation on respiratory muscle strength and physical performance in patients with COPD. To the best of our knowledge, this is the first study that has been performed in COPD patients with a vitamin D deficiency. We did not find any effect of vitamin D supplementation on respiratory muscle strength or physical performance. In addition, we did not find any effects on the secondary outcomes – pulmonary function, hand grip strength, exacerbation rate, and quality of life.
While several observational studies point toward a positive relationship between vitamin D and physical performance in the general population, the number of randomized clinical trials to study a potential causal effect remains very limited. Two trials have assessed the effect of vitamin D supplementation on physical performance in patients with COPD. The first study was a post hoc subgroup analysis of a larger trial assessing the effects of vitamin D supplementation to reduce exacerbations. In this subgroup study, the effects of vitamin D were studied during a rehabilitation program.Citation27 Participants (n=50) received a monthly dose of 100,000 IU vitamin D during 3 months. Vitamin D supplementation did not influence physical performance as compared to placebo, although it was related to a slight increase in inspiratory muscle strength. In the second study, participants (n=36) received a daily dose of 2,000 IU vitamin D during 6 weeks. Vitamin D supplementation also did not affect physical performance score in this study.Citation28 These findings are in line with our results. Both previous studies did not select patients with vitamin D deficiency at baseline.
Trials assessing the effects of vitamin D supplementation in the general population show conflicting results. One meta-analysis showed no effects of vitamin D supplementation on muscle strength in the general adult population, except in participants with 25(OH)D concentrations <25 nmol/L at baseline.Citation12 Another meta-analysis, including only studies in participants aged ≥60 years, did show an effect on muscle strength and balance.Citation9 This might suggest that a potential effect is only relevant in older and vitamin D-deficient participants. This was indeed confirmed in a meta-analysis by Beaudart et alCitation11 which showed a larger effect of vitamin D supplementation on global muscle strength in participants with baseline 25(OH)D levels <30 nmol/L and participants aged >65 years. A more recent meta-analysis in participants aged ≥65 years, however, did not show an effect of vitamin D supplementation on muscle strength.Citation29 A subgroup analysis of participants with serum 25(OH)D <25 nmol/L in our study did not show any effect. This group, however, was very small (n=6), because we excluded patients with severe vitamin D deficiency (<15 nmol/L), due to ethical considerations. We also excluded participants aged >70 years, because the Dutch Health Council recommends vitamin D supplementation in this population. This has led to a lower mean age of our study population compared to other trials.
Two other trials assessing the effects of vitamin D supplementation in COPD have been reported since the start of our study.Citation30,Citation31 The primary outcome of both studies was exacerbation rate. Both studies did not show an effect of vitamin D supplementation in the total study population, but did find an effect in their subgroup analysis of participants with a (severe) vitamin D deficiency. In our study, we assessed exacerbation rate as a secondary outcome. However, we did not find any differences between the supplementation group and the placebo group. This might be explained by the differences in inclusion criteria. In both the previous studies, participants were included if they had had ≥1 exacerbations in the past year. In our study, however, having an exacerbation in the preceding year was not an inclusion criterion. This might have led to the selection of COPD patients with a milder disease course. As stated earlier, we excluded patients with severe vitamin D deficiency (<15 nmol/L) in our study, which might have affected our outcomes, as a potential effect of vitamin D supplementation is expected to be stronger in patients with lower vitamin D levels.
A major limitation of our study is the small sample size. As this was considered a pilot trial, we only included 50 participants. This number was chosen as no previous trials were performed on which sample size could be calculated. However, we did not observe any differences which might have reached statistical significance in a larger study population.
Another limitation is the relatively low dose used in our study compared to previous studies. Because the threshold of vitamin D levels for extra-skeletal effects is currently not known, our dose might have been too low to provide a clinical effect. In addition, we used a daily dosing regimen, which leads to more stable levels, but can decrease participants’ compliance. However, participants in the supplementation group showed a significant increase in serum 25(OH)D levels, reaching a mean level of 96 nmol/L after 6 months, which is considered sufficient and comparable to levels reached in other studies. A subgroup analysis excluding noncompliant participants did also not affect our results.
To conclude, this trial did not show an effect of vitamin D supplementation on respiratory muscle strength and physical performance. It has shown several factors that can be addressed in future studies. The daily dosing regimen of 1,200 IU might be too low for a clinical effect in patients with COPD. In addition, our study population might have been too good to show a potential effect. Since the start of our study, two other trials assessing the effects of vitamin D supplementation in COPD have been reported.Citation30,Citation31 These trials did show an effect of vitamin D supplementation on exacerbation rate in subgroups of deficient participants. Therefore, we are currently performing an additional large multicenter trial on the effects of vitamin D supplementation in COPD patients with a vitamin D deficiency (ClinicalTrials.gov, ID number NCT2122627).Citation32 Primary outcome in this study is exacerbation rate, but muscle strength and physical performance are secondary outcomes. In this trial, we selected patients that have had an exacerbation in the previous year. In addition, we have increased the supplementation dose to 16,800 IU a week and extended the follow-up to 1 year. This trial is still ongoing and expected to be completed in 2018.
Author contributions
All authors have made substantial contributions to the conception and design of this study. All were equally involved in drafting the manuscript or revising it critically and have given final approval of the version to be published.
Supplementary materials
Table S1 Differences (Δ) after 6 months with exclusion of participants using vitamin D supplements
Table S2 Differences (Δ) after 3 months
Disclosure
The authors report no conflicts of interest in this work.
References
- JanssensWBouillonRClaesBVitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding geneThorax201065321522019996341
- PerssonLJPAanerudMHiemstraPSHardieJABakkePSEaganTMLChronic obstructive pulmonary disease is associated with low levels of vitamin DPLoS One201276e3893422737223
- HolickMFChenTCVitamin D deficiency: a worldwide problem with health consequencesAm J Clin Nutr20088741080S1086S18400738
- JanssensWLehouckACarremansCBouillonRMathieuCDecramerMVitamin D beyond bones in chronic obstructive pulmonary disease: time to actAm J Respir Crit Care Med2009179863063619164701
- JacksonASShrikrishnaDKellyJLVitamin D and skeletal muscle strength and endurance in COPDEur Respir J201341230931622556020
- SwallowEBReyesDHopkinsonNSQuadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary diseaseThorax200762211512017090575
- BurtinCTer RietGPuhanMAHandgrip weakness and mortality risk in COPD: a multicentre analysisThorax2016711868726514408
- CoteCGPinto-PlataVKasprzykKDordellyLJCelliBRThe 6-min walk distance, peak oxygen uptake, and mortality in COPDChest200713261778178517925409
- MuirSWMontero-OdassoMEffect of vitamin D supplementation on muscle strength, gait and balance in older adults: a systematic review and meta-analysisJ Am Geriatr Soc201159122291230022188076
- Bischoff-FerrariHADawson-HughesBStaehelinHBFall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trialsBMJ2009339b369219797342
- BeaudartCBuckinxFRabendaVThe effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trialsJ Clin Endocrinol Metab201499114336434525033068
- StocktonKAMengersenKParatzJDKandiahDBennellKLEffect of vitamin D supplementation on muscle strength: a systematic review and meta-analysisOsteoporos Int201122385987120924748
- RommeEAPMRuttenEPASmeenkFWJMSpruitMAMenheerePPCAWoutersEFMVitamin D status is associated with bone mineral density and functional exercise capacity in patients with chronic obstructive pulmonary diseaseAnn Med2013451919622462562
- HopkinsonNSLiKWKehoeAVitamin D receptor genotypes influence quadriceps strength in chronic obstructive pulmonary diseaseAm J Clin Nutr200887238539018258629
- BlackPNScraggRRelationship between serum 25-hydroxyvitamin D and pulmonary function in the Third National Health and Nutrition Examination SurveyChest200512863792379816354847
- van SchoorNMde JonghRTDanielsJMAHeymansMWDeegDJHLipsPPeak expiratory flow rate shows a gender-specific association with vitamin D deficiencyJ Clin Endocrinol Metab20129762164217122472566
- EvansJAWhitelawWAThe assessment of maximal respiratory mouth pressures in adultsRespir Care200954101348135919796415
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function LaboratoriesATS statement: guidelines for the six-minute walk testAm J Respir Crit Care Med2002166111111712091180
- GuralnikJMSimonsickEMFerrucciLA short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admissionJ Gerontol1994492M85M948126356
- MillerMRHankinsonJBrusascoVStandardisation of spirometryEur Respir J200526231933816055882
- VisserMDeegDJHLipsPLow vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study AmsterdamJ Clin Endocrinol Metab200388125766577214671166
- StelVSSmitJHPluijmSMFVisserMDeegDJHLipsPComparison of the LASA Physical Activity Questionnaire with a 7-day diary and pedometerJ Clin Epidemiol200457325225815066685
- AnthonisenNRManfredaJWarrenCPHershfieldESHardingGKNelsonNAAntibiotic therapy in exacerbations of chronic obstructive pulmonary diseaseAnn Intern Med198710621962043492164
- WareJJKosinskiMKellerSDA 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validityMed Care19963432202338628042
- GusiNOlivaresPRajendramRThe EQ-5D health related quality of life questionnairePreedyVWatsonRHandbook of Disease Burdens and Quality of Life Measures1New YorkSpringer-Verlag200987100
- DirksNFVesperHWvan HerwaardenAEVarious calibration procedures result in optimal standardization of routinely used 25(OH)D ID-LC-MS/MS methodsClin Chim Acta2016462495427570062
- HornikxMVan RemoortelHLehouckAVitamin D supplementation during rehabilitation in COPD: a secondary analysis of a randomized trialRespir Res2012138423006613
- BjerkSMEdgingtonBDRectorTSKunisakiKMSupplemental vitamin D and physical performance in COPD: a pilot randomized trialInt J Chron Obstruct Pulmon Dis201389710423430315
- Rosendahl-RiiseHSpielauURanhoffAGudbrandsenODierkesJVitamin D supplementation and its influence on muscle strength and mobility in community-dwelling older persons: a systematic review and meta-analysisJ Hum Nutr Diet201630131527460044
- LehouckAMathieuCCarremansCHigh doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trialAnn Intern Med2012156210511422250141
- MartineauAJamesWHooperRVitamin D supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trialLancet Respir Med20153212013025476069
- RafiqRAlevaFESchrumpfJAPrevention of exacerbations in patients with COPD and vitamin D deficiency through vitamin D supplementation (PRECOVID): a study protocolBMC Pulm Med20151510626399451
