Abstract
Background
Severe pulmonary hypertension (PH) resulting from a chronic lung disease (CLD) (severe CLD-PH) requires more aggressive treatment due to its increased mortality compared with mild PH. Therefore, we developed a Doppler echocardiography scoring index (ESI) to predict severe CLD-PH.
Methods
A derivation cohort of 107 patients with CLD who underwent echocardiography was classified into two groups, the normal/mild PH group and the severe PH group, based on the right heart catheterization. Meanwhile, we designed the ESI by multivariate logistic regression to validate the predicted outcomes. The ESI was calculated using the following formula: ESI = ESIRVEDTD + ESIPASP + ESIPAd − ESITAPSE. Additionally, the ESI was weighted by +2 points for right ventricular end-diastolic transverse dimension ≥3.8 cm or pulmonary artery diameter ≥2.7 cm, +3 points for systolic pulmonary artery pressure (PASP) ≥61 mmHg, and −3 points for tricuspid annular plane systolic excursion ≥1.65 cm.
Results
In the derivation cohort, PASP ≥61 mmHg estimated by echocardiography exhibited 80.4% sensitivity and 84.3% specificity with area under receiver-operating characteristic curve of 0.823 (95% CI: 0.797–0.942, P<0.0001). Compared with PASP, ESI ≥1.0 exhibited 91.1% sensitivity and 80.4% specificity, resulting in a net improvement in model performance with a change in the c-statistic from 0.823 to 0.937 and an integrated discrimination improvement of 11.3% (95% CI: 4.5%–18.2%, P=0.001). The ESI was applied to the validation cohort, resulting in 84.2% sensitivity and 81.3% specificity with 82.9% accuracy.
Conclusion
The ESI showed high capacity for predicting severe CLD-PH, further implying the value of noninvasive examinations in clinic.
Introduction
Pulmonary hypertension (PH) is a common complication of a chronic lung disease (CLD) and is associated with increased mortality.Citation1,Citation2 PH often progresses to right heart failure (RHF), with initial compensatory right ventricle (RV) hypertrophy becoming overwhelmed by increasing pulmonary artery pressure (PAP). According to the updated conference consensus,Citation2,Citation3 CLD is classified into three groups: without PH (mean PAP [mPAP] <25 mmHg), with PH (mPAP ≥25 mmHg), and with severe PH (mPAP ≥35 mmHg or 25 mmHg < mPAP <35 mmHg with cardiac index <2.0 L/min/m2 or pulmonary vascular resistance [PVR] >6 Wood units). However, the severe PH group includes only a minority of patients with CLD who are suspected of having extensive general vascular remodeling accompanying the parenchymal disease that develops independently from pulmonary functional impairment and who have a poor prognosis. The detection of severe PH is important because it can provide the prognostic information to warrant more aggressive respiratory support and interventional cardiovascular procedures.
Right heart catheterization (RHC) is the gold standard for the diagnosis of severe PH because this method provides the hemodynamic information that defines this disease. Nevertheless, RHC is not routinely and repeatedly performed at initial diagnosis of PH and follow-up, especially in the People’s Republic of China, where performance of RHC is limited by its invasiveness and high expenses. As a PH-screening tool, systolic PAP (PASP) can be estimated by measuring the peak tricuspid regurgitation velocity (TRV) on echocardiography, which continues to be recommended for early screening and as an assessment tool in patients with pulmonary arterial hypertension (PAH). However, in spite of its widespread use, the accuracy and reproducibility of echocardiography in predicting PASP have recently been questioned.Citation4–Citation9
The recently updated European Society of Cardiology (ESC) and European Respiratory Society guidelines on PH recommend testing for additional PH signs by assessing pulmonary artery diameter (PAd) and RV enlargement in addition to PASP.Citation10 Whether a novel comprehensive echocardiography scoring index (ESI) derived from additional PH signs and PASP could improve the value of echocardiography for predicting severe PH was unknown.
Since patients with CLD accompanied by severe PH have a much poorer prognosis, this population attracts clinicians’ attention, particularly because early and adequate treatment is needed to improve patient prognosis. Furthermore, it remains undetermined whether the assessment of a comprehensive ESI is beneficial for predicting severe PH. In the current study, we aimed to analyze the value of a comprehensive ESI for predicting severe PH in patients with CLD.
Materials and methods
Ethics
This study was conducted in accordance with the amended Declaration of Helsinki. The Local Institutional Ethics Committee of Shanghai Pulmonary Hospital approved the protocol (K08-015C), and written informed consent was obtained from all the patients in the validation cohort.
Study design
The retrospective derivation cohort and the prospective validation cohort were obtained from the Cardio-Pulmonary Circulation Center of Shanghai Pulmonary Hospital, which is the largest referral center for the diagnosis and treatment of PH in Shanghai, People’s Republic of China.Citation11 For the derivation cohort, all consecutive patients with CLD hospitalized between January 2012 and December 2014 who were suspected of PH were included for model derivation. The validation cohort included a similar population recruited between January 2015 and July 2015 for model verification.
Inclusion and exclusion criteria
The patient inclusion criteria were as follows: 1) suspected PH associated with CLD;Citation12 2) diagnosis of CLD confirmed by experienced specialists according to the appropriate guidelines;Citation13,Citation14 and 3) performance of RHC and Doppler echocardiography at a clinically stable stage during optimal medical therapy.
Patients were excluded for the following reasons: 1) diagnosis of other types of PH as per the NICE criteria;Citation12 2) lack of RHC or echocardiography at a clinically stable stage; or 3) comorbidity of pulmonary embolism, severe left heart disease, and so on.
Procedures
A comprehensive set of quantitative echocardiography parameters was measured at rest (Vivid7 Dimension; GE Vingmed Ultrasound AS, Horten, Norway). The protocol and reference limits were in accordance with the current guidelines.Citation15 Right ventricular end-diastolic transverse dimension (RVEDTD), right ventricular end-diastolic longitudinal dimension (RVEDLD), right atrial transverse dimension (RATD), right atrial longitudinal dimension (RALD), and end-systolic-stage eccentricity index (ENDSEI) were measured to indicate the presence or absence of right heart enlargement.Citation15 PASP was measured by TRV with right atrium pressure (RAP) estimated by inferior cava diameter and inspiratory collapse.Citation15 The PAd was also measured.Citation15 RV function was assessed by measuring the tricuspid annular plane systolic excursion (TAPSE).Citation15 Left ventricular ejection fraction was measured using M-mode in the parasternal long-axis view.
RHC was performed as described previously.Citation16 The baseline hemodynamic variables evaluated included mPAP, RAP, pulmonary artery wedge pressure (PAWP), cardiac output (CO), cardiac index, and PVR.
All patients underwent RHC and echocardiography within 7 days at a clinically stable stage. Echocardiography was performed by two cardiologists (RJ and Q-HZ) who were blinded to all patients’ medical history and RHC results. Similarly, the doctors who performed RHC were also blinded to the echocardiography results.
Statistical analysis
Continuous variables are described as the mean ± SD and median (interquartile range) for normally distributed variables and skewed distributed variables, respectively. Categorical variables are expressed as percentages. Pearson correlation coefficients for PASP between RHC and echocardiography (abbreviated as PASPRHC and PASPECHO, respectively) were calculated.
All echocardiography parameters were used to model the probability of having severe PH by means of binary logistic regression.Citation17 A stepwise selection procedure was used to find independent predictors of severe PH with p-to-enter of ≤0.10 and p-to-remove of ≥0.15. Variables assigned based on multiples of their rounded β-coefficients from the refitted model were used to define an ESI. All patients were classified into either the normal/PH group or the severe PH group. The receiver-operating characteristic (ROC) method was used to assess the ability of echocardiography variables and ESI to predict severe PH. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated.
To verify the ESI’s diagnostic ability, we conducted an internal and prospective validation. Landis and KochCitation18 defined kappa values of 0.00–0.20 as slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1.00 as almost perfect agreement. CIs were calculated with the adjusted percentile bootstrap method (n=10,000 replicates).
In all univariate analyses, P≤0.05 was considered statistically significant. All statistical methods were performed using SPSS 21.0 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 5.04 software (GraphPad Software, Inc., San Diego, CA, USA).
Results
Derivation cohort
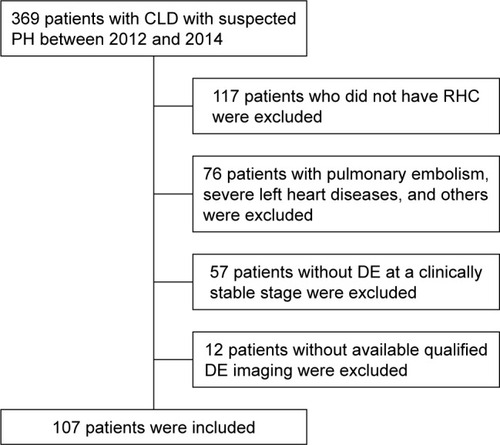
A total of 369 patients with CLD and suspected PH were admitted to our center between January 2012 and December 2014, of whom 107 underwent echocardiography ≤7 days before RHC (mean 3.3±1.3 days before RHC; ). Fifty-six patients with severe PH and 51 patients with normal/PH with a median age of 62.0 (54.0, 67.5) years and consisting of 63.8% men were included. A small proportion of patients had significant comorbidities, such as systemic hypertension (19.0%), diabetes mellitus (3.9%), coronary heart disease (4.8%), arrhythmia (9.5%), and hyperlipidemia (2.9%) (). There were no significant differences in demographic characteristics, diagnostic classification, World Health Organization function class (WHO-FC), comorbidities, or pulmonary function test results between the groups ().
Table 1 Demographic characteristics, pulmonary function test results, hemodynamics, and echocardiography parameters of patients with normal/PH vs severe PH
Figure 1 Flow diagram for the main derivation cohort. Of the 369 patients with CLD and suspected PH who were referred to the Cardio-Pulmonary Circulation Center of Shanghai Pulmonary Hospital within the study period, 107 met the inclusion criteria and were considered in the analysis.
The PASPECHO was measured at a clinically stable stage in 86.9% of all patients, which included 80.4% of the normal/PH group and 92.8% of the severe PH group. There was a moderately strong correlation between PASPECHO and both PASPRHC and mPAPRHC (r=0.665 and r=0.650, both P<0.001). Compared with the normal/PH group, the severe PH group showed significant abnormalities in the variables of echocardiography and RHC (all P<0.001) ().
Multivariate analyses were performed to construct the ESI. Age, RATD, RALD, RVEDLD, and ENDSEI were excluded due to nonsignificant results by multivariate analysis, despite achieving statistical significance by univariate analysis. Stepwise logistic regression analysis revealed four variables that were independently significant: RVEDTD, PASP, PAd, and TAPSE. The formula for ESI was derived from the β-coefficients in the final model (): ESI = ESIRVEDTD + ESIPASP + ESIPAd − ESITAPSE.
Table 2 Logistic regression analysis of echocardiography parameters associated with severe PH
Based on ROC analysis, PASP ≥61 mmHg displayed 80.4% sensitivity and 84.3% specificity with an area under ROC curve (AUC) of 0.823 (95% CI: 0.797–0.942, P<0.0001). The other parameters, except for RVEDTD, did not seem to have good sensitivity and specificity for predicting severe PH (). Compared with PASPECHO, the ESI resulted in a net improvement in model performance, with a change in the c-statistic from 0.823 to 0.937 (95% CI: 0.890–0.984, P<0.001) and an integrated discrimination improvement of 11.3% (95% CI: 4.5%–18.2%, P=0.001) ().
Table 3 The distribution of echocardiography parameters and accuracy for discrimination
Figure 2 The receiver-operator characteristic curve is shown for PASP alone as determined by echocardiography variables and for ESI in predicting severe PH.
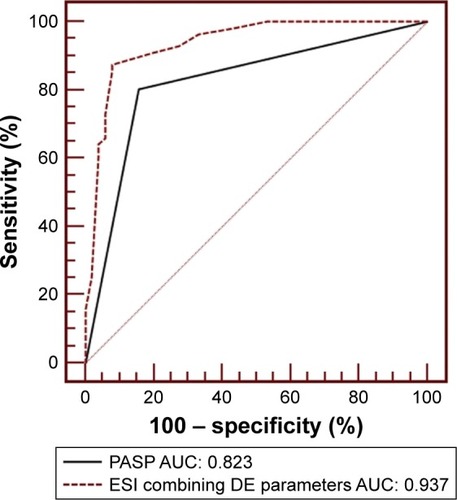
Since severe PH should capture clinicians’ attention due to its poor prognosis, to minimize the chance of overlooking a case of severe PH, we examined several alternate cutoff values of the ESI and determined the cutoff value that maximized sensitivity with the least compromise in specificity (Table S1). Therefore, we chose 1.0 as the optimal cutoff value for the ESI. With ESI ≥1.0 as the definition of a model-predicted case of severe PH, the sensitivity, specificity, PPV, and NPV were 91.1%, 80.4%, 83.6%, and 89.1%, respectively.
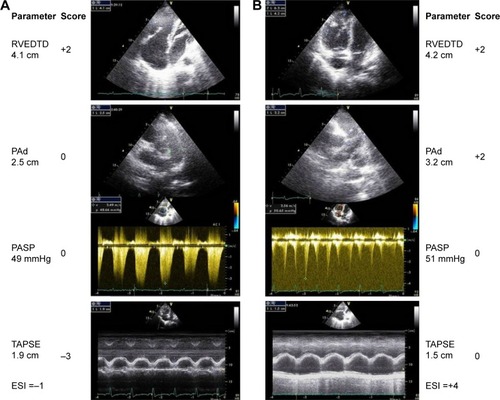
shows representative echocardiography traces from two patients with CLD who were verified as having mild or severe PH on RHC.
Figure 3 Representative echocardiographic images of RVEDTD, PASP, PAd, and tricuspid annular plane systolic excursion as well as the score calculation for two patients. Column A (top to bottom) shows ↑RVEDTD and ↑PASP (score =−1). Invasive hemodynamics: mPAP =30 mmHg, PAWP =11 mmHg, PVR =6.0 Wood units, and cardiac index =3.1 L/min/m2. Column B shows ↑RVEDTD, ↑PASP, ↑PAd, and ↓TAPSE (score =4). Invasive hemodynamics: mPAP =39 mmHg, PAWP =11 mmHg, PVR =6.0 Wood units, and cardiac index =3.35 L/min/m2.
Validation cohort
A total of 127 patients with CLD and suspected PH between Jan 2015 and Jul 2015 were admitted to our center, of whom 16 adult patients had normal/mild PH and 19 had severe PH. Clinical data are presented in Tables S2 and S3. A trend towards lower PASPECHO or PASPRHC and mean RAP was seen in the validation cohort. The ESI was validated in the cohort with 84.2% sensitivity, 81.3% specificity, 84.2% PPV, 81.3% NVP, and 82.9% accuracy. The ESI showed precise and substantial kappa agreement (0.655, 95% CI: 0.370–0.884).
Discussion
We derived and validated a comprehensive ESI by combining additional PH signs and PASPECHO for predicting severe PH in patients with CLD. ESI ≥1.0 displayed satisfactory sensitivity, specificity, PPV, NPV, and accuracy in predicting severe PH and is recommended to be applied in clinical prac-tice due to its noninvasive nature and cost effectiveness.
Despite availability of targeted PH medication for PAH, no targeted medication is approved at the moment for PH in CLD and use of targeted drugs would be off-label. Compared with patients with CLD in the normal/PH group, patients in the severe PH group exhibited poorer prognosis and higher mortality.Citation10,Citation19 Therefore, it is important for clinicians to detect severe PH in patients earlier and to initiate adequate treatment in order to improve patient prognosis. RHC is the gold standard for measuring not only mPAP but also PAWP, cardiac index, CO, and PVR. RHC cannot be replaced by echocardiography. Nevertheless, RHC cannot be performed in every hospital, especially in the People’s Republic of China. Sometimes, patients are not willing to undergo RHC during follow-up because of its invasiveness and cost. However, as a PH-screening tool, PASP can be estimated by measuring the peak TRV on echocardiography, which continues to be recommended for early screening and assessment in patients with idiopathic PAH, chronic thromboembolism-associated PH, or connective tissue disease-associated PAH.Citation20 However, PASP estimation is often inaccurate, especially in patients with CLD, and requires the presence of sufficient tricuspid regurgitation (TR), proper Doppler alignment, and optimal visualization of the regurgitant jet. Moreover, the absence of TR is not sufficient to exclude PH, and the TRV might be underestimated in patients with CLD who have hyperinflation of the lungs and marked respiratory variations in intrathoracic pressure.Citation9 Even if a TR is observed, PAPECHO is often inaccurate and leads to both false-positive and false-negative diagnoses of PH,Citation19 not to mention an inability to determine severe PH. Compared with PASPRHC, PASPECHO was found to be inaccurate in 52% of COPD cases, with a tendency to overestimate PAP.Citation9 Indeed, 48% of patients were misclassified as having PH by echocardiography alone.Citation9,Citation21 In addition, some studies have identified a strong relationship between mPAPECHO and mPAPRHC.Citation22 However, the diagnostic utility of mPAPECHO for specific underlying etiologies, such as emphysema, has been questioned.Citation21 In other studies,Citation9,Citation23 PASPECHO predicted PH in patients with CLD with 76%–85% sensitivity, 17%–38% specificity, 56%–60% PPV, and 44%–60% NPV. Consequently, PASPECHO and mPAPECHO have not yet been adopted as stand-alone tools capable of accurately measuring pulmonary hemodynamics.
At present, this ESC guideline suggests grading the probability of PH based on TRV and additional prespecified echocardiographic variables that are suggestive of PH.Citation10 Additional signs of PH such as RV dilation, eccentricity index, and PAdCitation24 have been reported to discern PAH with high sensitivity. Many years ago, several studies derived and validated equations using additional PH signs to estimate PVR or mPAP as confirmed by RHC in patients with PH.Citation4,Citation5,Citation23 Prior studies have tried but failed to utilize acceleration time in the right ventricular outflow tract (RVOT) to measure PAP or predict PH in patients with CLD.Citation25,Citation26 Opotowsky et al reported that the RVOT velocity or RVOT velocity time integral has been proposed as a PVR prediction modelCitation5,Citation8 for determining the severity of PH with the exception of PH due to CLDCitation24 or for estimating PAP in children with CLD.Citation27 However, the above-stated methods were slightly more cumbersome, and we favor simply measured variables to increase clinical utility.
In our research, we derived a novel comprehensive index by combining RVEDTD, PAd, and TAPSE in addition to PASPECHO. This index has several advantages. First, the ESI’s sensitivity and specificity are substantially higher than PASPECHO, with an increase in the c-statistic from 0.823 to 0.937. Second, the four variables are not cumbersome to measure and are easy to obtain. Third, the ESI is noninvasive, reliable, and easy to integrate into clinical practice, especially during follow-up.
D’Alto et alCitation6 reported that echocardiography allows for accurate measurements of pulmonary circulation but with moderate precision by Bland–Altman analysis, which explains why the procedure is valid for population studies but cannot be used for the diagnosis of PH in individual patients. Because our research objective is to make a cutoff value to differentiate “severe PH” from “normal/PH” rather than replace gold criterion by new parameter, Bland–Altman analysis might not be suitable for our research. We calculated accuracy and precision in accordance with categorical variable and kappa, respectively. In our study, there were no significant biases, and the ESI showed 82.9% accuracy in the validation cohort. However, the ESI had a kappa statistic of 0.655, indicating substantial but not high agreement. Our results are consistent with earlier reports and could help clinicians perform preliminary screening of patients with suspected severe PH.
Although the parameter for the discrimination of severe PH is an mPAP of 35 mmHg by RHC, the corresponding PASPECHO of 61 mmHg derived from the sample seems very high. This is far greater than the calculated PASP of 53 mmHgCitation7 or 54 mmHgCitation28 for an mPAP of 35 mmHg. This indicates that PASPECHO was not sufficient for predicting PH in patients with CLD.
As we know, the severity of PH should not be judged by PAP but rather by clinical outcomes such as WHO-FC, hospitalization, RHF, up-titration of medications, transplantation, or mortality. However, our research was a diagnostic study that validated the capacity of a comprehensive echocardiography index for predicting severe PH in patients with CLD. In the future, our research would be strengthened by determining the predictive nature of the formula in clinical outcomes from the validation cohort. A formula that could predict clinical outcomes in this population would be more useful. We will further consider this problem when we have a large patient cohort that can be followed over time and will develop a formula based on those data.
Study limitations
This study has several limitations that need to be considered when interpreting the results. First, this was a single-center study, which may have patient selection bias because not all the patients with CLD had undergone RHC. However, patients admitted into our center came from all over People’s Republic of China, which may reduce bias. Second, echocardiography and RHC were not conducted simultaneously; therefore, the delay between procedures could be considered a limitation of this study. However, the included patients received similar levels of support and treatment at the times of echocardiography and RHC, and there were no detectable differences in medications or fluid status between the two groups. All patients underwent RHC at a clinically stable stage, manifesting relief of respiratory failure and RHF. The number of days between echocardiography and RHC was also validated as insignificant by logistical regression. Importantly, the strength of the present study is the evaluation of these tests through actual application in clinical practice.
Conclusion
We present a simple clinical tool that helps to predict severe PH in patients with CLD. The integrated ESI combining additional PH signs and PASPECHO improved the accuracy of predicting severe PH based on echocardiography. If patients with CLD have an ESI >1.0, they should receive clinicians’ attention and should undergo RHC because they may have a high risk of severe PH and pulmonary vascular remodeling. Early diagnosis and treatment are crucial in this patient population.
Author contributions
Drs RJ, RZ, and J-ML contributed to the experimental design, study conduct, data analysis, and drafting and revising the manuscript. LW, Q-HZ, and PY contributed to the data collection and revising manuscript. BP, CW, W-HW, and Z-CJ contributed to the experimental design, interpretation of data, and revising the manuscript. All authors had full access to all study data and had final responsibility for the decision to submit for publication. All have reviewed the manuscript and approved the final version for submission. All the authors ensured that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81600032) and the YangFan Program of Shanghai Science and Technology Committee (15YF1409700).
Supplementary materials
Table S1 Sensitivity, specificity, positive predictive values, and negative predictive values of the diagnostic index
Table S2 Comparison of characteristics of patients of derivation cohort vs validation cohort
Table S3 Comparison of hemodynamics and echocardiography parameters of patients of derivation cohort vs validation cohort
Disclosure
Prof Z-CJ serves as a consultant and scientific advisor to Actelion, Bayer Schering, AstraZeneca, Pfizer, and United Therapeutics, in addition to being an investigator in trials sponsored by these companies. The other authors report no conflicts of interest in this work.
References
- MouraniPMSontagMKYounoszaiAIvyDDAbmanSHClinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung diseasePediatrics2008121231732518245423
- SeegerWAdirYBarberàJAPulmonary hypertension in chronic lung diseasesJ Am Coll Cardiol20136225 SupplD109D11624355635
- HoeperMMAndreasSBastianAPulmonary hypertension due to chronic lung disease: updated Recommendations of the Cologne Consensus Conference 2011Int J Cardiol2011154Suppl 1S45S5322221973
- RobinsonBEbeidMA simple echocardiographic method to estimate pulmonary vascular resistanceAm J Cardiol20141132412
- OpotowskyARClairMAfilaloJA simple echocardiographic method to estimate pulmonary vascular resistanceAm J Cardiol2013112687388223735649
- D’AltoMRomeoEArgientoPAccuracy and precision of echocardiography versus right heart catheterization for the assessment of pulmonary hypertensionInt J Cardiol201316844058406223890907
- AduenJFCastelloRDanielsJTAccuracy and precision of three echocardiographic methods for estimating mean pulmonary artery pressureChest2011139234735220651021
- RouleVLabombardaFPellissierAEchocardiographic assessment of pulmonary vascular resistance in pulmonary arterial hypertensionCardiovasc Ultrasound201082120529278
- ArcasoySMChristieJDFerrariVAEchocardiographic assessment of pulmonary hypertension in patients with advanced lung diseaseAm J Respir Crit Care Med2003167573574012480614
- GalièNHumbertMVachieryJL2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT)Eur Heart J20163716711926320113
- ZhangRDaiLZXieWPSurvival of Chinese patients with pulmonary arterial hypertension in the modern treatment eraChest2011140230130921330386
- SimonneauGGatzoulisMAAdatiaIUpdated clinical classification of pulmonary hypertensionJ Am Coll Cardiol20136225 SupplD34D4124355639
- VestboJHurdSSAgustiAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- MeyerKCRaghuGBaughmanRPAmerican Thoracic Society Committee on BAL in Interstitial Lung DiseaseAn official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung diseaseAm J Respir Crit Care Med201218591004101422550210
- RudskiLGLaiWWAfilaloJGuidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of EchocardiographyJ Am Soc Echocardiogr2010237685713 quiz 786–78820620859
- JiangRAiZSJiangXIntravenous fasudil improves in-hospital mortality of patients with right heart failure in severe pulmonary hypertensionHypertens Res201538853954425787034
- ThwaitesGEChauTTStepniewskaKDiagnosis of adult tuberculous meningitis by use of clinical and laboratory featuresLancet200236093421287129212414204
- LandisJRKochGGThe measurement of observer agreement for categorical dataBiometrics1977331159174843571
- BarberàJABlancoIManagement of pulmonary hypertension in patients with chronic lung diseaseCurr Hypertens Rep20151786226115628
- PristeraNMusarraRSchilzRHoitBDThe role of echocardiography in the evaluation of pulmonary arterial hypertensionEchocardiography201633110511626522749
- FisherMRCrinerGJFishmanAPEstimating pulmonary artery pressures by echocardiography in patients with emphysemaEur Respir J200730591492117652313
- BergerMHaimowitzAVan ToshABerdoffRLGoldbergEQuantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasoundJ Am Coll Cardiol1985623593654019921
- ZismanDARossDJBelperioJAPrediction of pulmonary hypertension in idiopathic pulmonary fibrosisRespir Med2007101102153215917604151
- López-CandalesAEdelmanKShape of the right ventricular outflow Doppler envelope and severity of pulmonary hypertensionEur Heart J Cardiovasc Imaging201213430931622087011
- MarchandiseBDe BruyneBDelaunoisLKremerRNoninvasive prediction of pulmonary hypertension in chronic obstructive pulmonary disease by Doppler echocardiographyChest19879133613653816313
- TorbickiASkwarskiKHawrylkiewiczIPasierskiTMiskiewiczZZielinskiJAttempts at measuring pulmonary arterial pressure by means of Doppler echocardiography in patients with chronic lung diseaseEur Respir J1989298568602806512
- NewthCJGowRMRoweRDThe assessment of pulmonary arterial pressures in bronchopulmonary dysplasia by cardiac catheterization and M-mode echocardiographyPediatr Pulmonol19851158624058957
- ChemlaDCastelainVProvencherSHumbertMSimonneauGHervéPEvaluation of various empirical formulas for estimating mean pulmonary artery pressure by using systolic pulmonary artery pressure in adultsChest2009135376076818849403
