Abstract
Objective
Arformoterol is the (R,R)-enantiomer of formoterol. Preclinical studies suggest that it is a stronger bronchodilator than the racemic (R,R/S,S)-formoterol; however, its potential clinical advantages have not been demonstrated. This study compared the length of stay (LOS), 30-day readmission rates, and doses of rescue medication administered in hospitalized patients with COPD who were treated with nebulized arformoterol or nebulized formoterol.
Methods
This retrospective analysis utilized data from Premier, Inc. (Charlotte, NC, USA), the largest nationwide hospital-based administrative database. COPD patients ≥40 years of age were included if they were hospitalized between January 2011 and July 2014, had no asthma diagnoses, and were treated with nebulized arformoterol or nebulized formoterol. LOS was measured from the day the patients initiated the study medication (index day). Rescue medications were defined as short-acting bronchodilators used from the index day onward. Multivariate statistical models included a random effect for hospital and controlled for patient demographics, hospital characteristics, admission characteristics, prior hospitalizations, comorbidities, pre-index service use, and pre-index medication use.
Results
A total of 7,876 patients received arformoterol, and 3,612 patients received nebulized formoterol. There was no significant difference in 30-day all-cause (arformoterol =11.9%, formoterol =12.1%, odds ratio [OR] =0.981, P=0.82) or COPD-related hospital readmission rates (arformoterol =8.0%, formoterol =8.0%, OR =1.002, P=0.98) after adjusting for covariates. The adjusted mean LOS was significantly shorter for arformoterol-treated vs formoterol-treated patients (4.6 vs 4.9 days, P=0.039), and arformoterol-treated patients used significantly fewer doses of rescue medications vs formoterol-treated patients (5.9 vs 6.6 doses, P=0.006).
Conclusion
During inpatient stays, treating with arformoterol instead of nebulized formoterol may lead to shorter LOS and lower rescue medication use.
Introduction
COPD is the third leading cause of death in the US, and over 10.5 million individuals in the US have been estimated to have COPD.Citation1,Citation2 Patients with this progressive disease have persistent symptoms of limited airflow and experience atypical inflammatory reactions to respiratory irritants.Citation3 As the disease progresses, patients experience more frequent acute exacerbations that require a change in medication, as well as more severe exacerbations that result in hospitalization.Citation4 Exacerbations lead to further progression of COPD and are the main cause of morbidity and mortality in COPD.Citation5,Citation6 In the US, the direct costs of COPD have been estimated at $29.5 billion per year.Citation7 Patients with exacerbations incur substantially higher treatment costs, particularly when the exacerbations are severe and require hospitalization, compared with those without any exacerbations.Citation8
When hospitalized for acute exacerbations, patients are usually treated with short-acting beta-agonists (SABAs), antibiotics, and systemic corticosteroids.Citation9–Citation11 In addition, nebulized treatment appears to be the preferred drug delivery method over hand-held inhalers during inpatient stays.Citation10,Citation12 The delivered dose has been shown to be influenced by the type of nebulizer employed.Citation13 Although one study found no difference between delivering long-acting beta-agonists (LABAs) by either metered-dose inhalers or nebulizers,Citation14 nebulized treatment has been recommended for certain patient populationsCitation12 and is the most likely route for hospitalized patients.Citation10 Based on clinical evidence, initiation of maintenance treatment with long-acting bronchodilators is recommended for hospitalized patients after stabilization and prior to discharge from the hospital.Citation3 Not surprisingly, treatment with long-acting bronchodilators in the outpatient setting has been found to reduce future hospital admissions.Citation15 When patients with COPD are effectively treated, their symptoms can be minimized and disease progression can be slowed.Citation16
The LABAs, arformoterol and formoterol, have both been proven to be efficacious and are available as nebulized treatment agents.Citation17,Citation18 Arformoterol is the (R,R)-enantiomer of formoterol, and appears to have more potent bronchodilator properties than racemic (R,R/S,S)-formoterol.Citation19,Citation20 However, despite the availability of approved effective nebulized treatments, there is limited comparative evidence between these agents. Results from a clinical trial reported no significant differences between nebulized arformoterol and dry powder inhaler-delivered formoterol when examining traditional efficacy outcomes such as change in forced expiratory volume in 1 second (FEV1), symptoms, functional outcomes, and rescue medication use.Citation21 The clinical trial was conducted in controlled settings among clinically stable COPD patients in the community – those with unstable respiratory illness or a recent respiratory infection were excluded. This study may not accurately represent patients treated in a real-world setting. Additionally, there is no study to date that has compared the outcomes of arformoterol vs formoterol among patients hospitalized for COPD symptoms, particularly on length of stay (LOS), a common measure of performance and determinant of costs.Citation22 The objective of this study was to compare the LOS, 30-day readmission rates (all-cause and COPD-related), and number of doses of rescue medications among inpatients with COPD who were treated with nebulized arformoterol or nebulized formoterol.
Methods
Study design and database
This retrospective cohort study used administrative data from Premier, Inc. (Charlotte, NC, USA), the largest hospital-based, service-level database in the US. The Premier database contains detailed service information from over 500 hospitals for more than 50 million inpatient discharges since the year 2000. The analysis was restricted to hospitalizations between January 1, 2010 and January 31, 2015. This was a retrospective analysis of de-identified data, as designated by the Health Insurance Portability and Accountability Act,Citation23 and therefore did not require institutional review board approval.
Inclusion/exclusion criteria
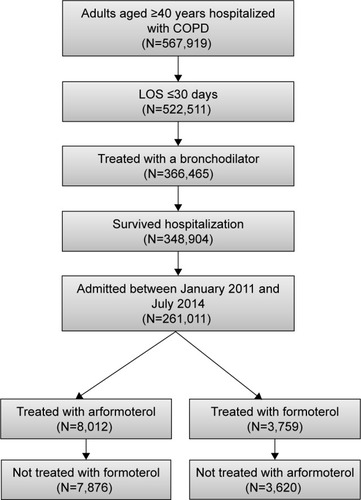
Patients were included if they had a primary COPD discharge diagnosis (ICD-9-CM 491.xx [chronic bronchitis], 492. xx [emphysema], or 496.xx [chronic airway obstruction, not elsewhere classified]) or a secondary COPD discharge diagnosis with a primary respiratory diagnosis (ICD-9-CM 460.xx–519.xx), were at least 40 years of age, were admitted to the hospital between January 1, 2011 and July 31, 2014, had an admission with an LOS ≤30 days, and were treated with either arformoterol or formoterol (but not both) during the hospital stay. Patients were excluded if they had an asthma diagnosis (ICD-9-CM 493.xx) or died during the hospitalization. shows the patient flow through the inclusion and exclusion criteria.
Definitions
The first hospitalization for a patient who met all inclusion and exclusion criteria stated above was designated as the index hospitalization. The index day was defined as the day that the patient initiated the index drug (either arformoterol or formoterol) during the index hospitalization. The baseline period was the 12-month period preceding the admission month for the index hospitalization. The follow-up period was the 6-month period following the discharge month for the index hospitalization.
The primary outcome variable was the LOS, which was defined as the number of days from the index day (ie, the day of drug initiation) to the discharge day. Because the data truncated dates to month and year only, 30-day all-cause readmission was defined as any readmission to the same hospital during the current or next month. Consistent with the patient selection criteria, 30-day COPD-related readmissions were defined as any readmission in the current or next calendar month with a primary COPD diagnosis or a secondary COPD diagnosis with a primary respiratory diagnosis. Doses of rescue medication were the number of times a SABA, a short-acting muscarinic agonist (SAMA), or a combination product including a SABA and a SAMA was given during the hospitalization. Doses of rescue medication were counted beginning with the index day.
The definitions of outcome variables were varied in sensitivity analyses to confirm the robustness of the results. LOS was also modeled as the time from admission to discharge rather than index day to discharge. In addition to examining any 30-day readmission, the count of readmissions, both all-cause and COPD-related, during the 30 days and 6 months following discharge was also examined.
Multiple background characteristics were used to statistically adjust for any potential differences between the arformoterol and formoterol cohorts. These included patient demographics, hospital characteristics, admission characteristics, prior hospitalizations, and inpatient respiratory services that were used prior to or on the index day (). In addition, several comorbid conditions were examined including those that have been linked to differential outcomes in COPD,Citation24,Citation25 the broader Elixhauser comorbidities,Citation26 and several potentially relevant comorbidities for respiratory conditions ().
Table 1 Baseline characteristics
Table 2 Comorbidities
Statistical methods
For all the outcome variables, multivariate generalized linear mixed models (GLIMMIX models) that included a random intercept for hospital were used. All models included all background variables defined in and as covariates. For dichotomous variables (ie, readmission in 30 days), the GLIMMIX models with a binomial distribution and a logit link were used. For count variables (number of rescue doses, LOS, and number of hospitalizations in the 6-month follow-up), the GLIMMIX models with a negative binomial distribution and a log link were used. All results reported are the least square means, which are adjusted for background characteristics. Sensitivity analyses of all outcomes adjusting for concomitant medication use anytime during hospitalization (instead of pre-index period only) were performed. The two-tailed alpha was set at P=0.05. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Cost estimates
To estimate cost implications for differences in LOS, external information was utilized. The Healthcare Cost and Utilization Project reported that the average LOS of all hospitalizations with primary diagnoses of COPD in 2008 was 4.8 days with a reported mean cost per stay of $7,500.Citation27 Dividing the average cost by LOS, the cost per day was estimated to be $1,562.50 (7,500/4.8). This cost-per-day value was multiplied by the average difference in LOS.
Results
Patient selection
The database contained information on 567,919 patients aged ≥40 years who met the COPD diagnostic criteria (including no asthma diagnoses) and who had a hospital admission. The patient flow through selection criteria can be seen in . The final cohorts consisted of 11,496 patients from 271 unique hospitals: 7,876 (68.5%) treated with arformoterol and 3,620 (31.5%) treated with formoterol.
Patient baseline characteristics
The baseline characteristics of the patients treated with arformoterol and formoterol can be seen in . With the large sample size, there were several statistically significant differences between the arformoterol and formoterol cohorts on patient demographics, admission characteristics, prior hospitalization, and pre-index service use, but the magnitude of the differences was generally small (<5% difference). However, some differences in hospital characteristics (region, urban setting [66.1% vs 86.3%], and size based on number of beds) and prior medication use of arformoterol and formoterol cohorts (long-acting muscarinic agonist: 26.8% vs 19.6% and inhaled corticosteroids: 61.4% vs 73.4%, respectively) were larger. There was no difference in the number of prior hospitalizations or All Patients Refined Diagnosis Related Groups severity score between the two cohorts. While both cohorts had similar Charlson Comorbidity Index scores, there were some significant differences in specific comorbidities ().
Outcomes
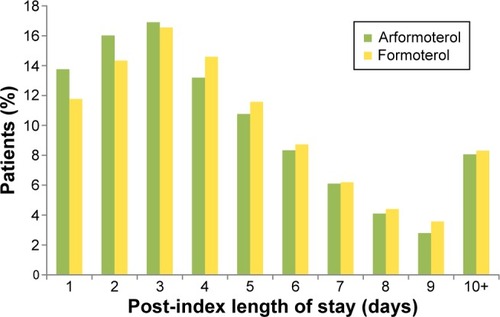
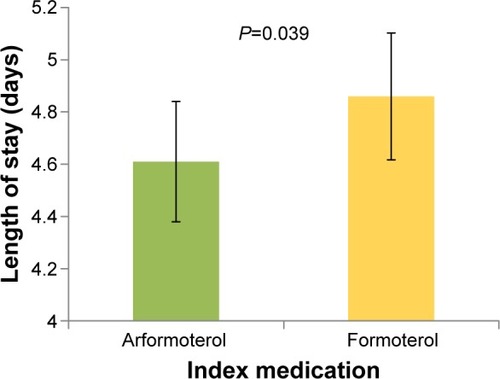
shows the LOS by index medication. After adjusting for baseline characteristics, the adjusted mean post-index LOS was significantly shorter for arformoterol-treated vs formoterol-treated patients (4.6 vs 4.9 days, P=0.039). As seen in , a higher percentage of patients who were treated with arformoterol were discharged within 4 days of index medication initiation compared to patients treated with formoterol (46.7% vs 42.7%). The LOS difference was the same after adjusting for concomitant medication use anytime during hospitalization (instead of pre-index), and the difference was marginally significant (4.7 vs 4.9 days, P=0.08). Multiplying the 0.3 difference in LOS from the primary LOS comparison by the average cost per day of $1,562.50 yielded an estimated cost difference of $469 per patient.
Figure 2 Length of stay following arformoterol or formoterol initiation.
Notes: Error bars represent 95% confidence intervals. Results were similar, but became marginally significant (P=0.06), when the full length of stay for the hospitalization was used instead of length of stay from the start of the index medication.
After adjusting for baseline characteristics, there was no significant difference in 30-day all-cause readmission rates (11.9% arformoterol vs 12.1% formoterol, odds ratio [OR] =0.981, P=0.82). There was also no significant difference in 30-day COPD-related readmission rates after adjusting for covariates (8.0% arformoterol vs 8.0% formoterol, OR =1.002, P=0.98). These results were confirmed by sensitivity analyses that showed no significant difference between arformoterol and formoterol on the number of readmissions (0.34 for arformoterol and 0.35 for formoterol, P=0.44) and number of COPD-related readmissions (0.20 for arformoterol and 0.21 for formoterol, P=0.34) during the 6-month follow-up period.
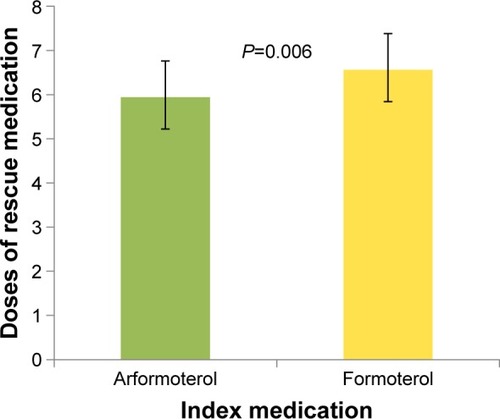
gives the doses of rescue medications used for each index treatment. Patients treated with arformoterol used significantly fewer doses of rescue medications after the index day than patients treated with formoterol (5.9 vs 6.6 doses, P=0.006). This difference remained similar and statistically significant after replacing pre-index by any-time concomitant medication use in the regression model (5.8 vs 6.3 doses, P=0.035).
Discussion
Nebulized arformoterol 15 µg and formoterol 20 µg inhalation solutions are two nebulized long-acting bronchodilator treatments that are commonly used in hospitals for the management of COPD. A comparative analysis of the real-world outcomes of these two drugs in hospitalized COPD patients has not been done before. This analysis examined if the superior bronchodilator properties of arformoterol over formoterol translated into real-world benefits in health care resource utilization related to hospital LOS, use of short-acting agents, and a reduction in hospital readmissions for patients admitted for an acute exacerbation of COPD. The data source used for this analysis is a very large hospital claims database not restricted by any provider or payer type, and is nationally representative of hospitalized COPD patients.Citation28–Citation31 The analysis reported in this paper showed that patients treated with nebulized arformoterol may have shorter LOS as compared to patients treated with nebulized formoterol. Arformoterol-treated inpatients also used fewer rescue medications, a result that was robust as revealed by further sensitivity analysis. A preclinical study reported that the (R,R-)enantiomer (ie, arformoterol) inhibited both histamine and antigen-induced bronchoconstriction with a greater potency than racemic formoterol.Citation19,Citation20,Citation32 While preclinical studies have found that arformoterol acts as a more potent bronchodilator than racemic formoterol, to our knowledge, this is the first study to identify the potential advantage in a clinical population with COPD.
In addition, a recent retrospective cohort study using commercial health care insurance claims reported lower hospitalization costs for arformoterol-treated patients with COPD who were hospitalized compared to formoterol patients who were hospitalized.Citation15 Assuming that a shorter LOS corresponds with lower hospital costs, these findings are consistent with the current study. While none of these previously published studies are directly comparable to the current research, the findings are consistent.
A randomized controlled trial in patients with COPDCitation21 compared outcomes over a 6-month period for patients treated with formoterol 12 µg delivered via a dry powder inhaler (n=147) to that of patients treated with arformoterol 15 µg (n=149) or arformoterol 25 µg (n=147) delivered via a nebulizer. In this well-controlled clinical trial, the arformoterol- and formoterol-treated patients had similar improvements in FEV1, symptoms, functional outcomes, and rescue medication use. However, the study reported significantly higher rate of event-defined exacerbations for the arformoterol-treated patients.Citation21 While the Hanania et al studyCitation21 was randomized and had better internal validity to establish inferences about clinical and patient-reported outcomes, the study was not adequately powered to make meaningful inferences about exacerbation events relative to the previously published retrospective analyses of large claims databases. A comparative randomized clinical trial with a much larger sample size may have helped detect differences, if any, with greater confidence.
In the administrative database analysis, measures of potential adverse events of the medications were not available. In the pivotal trials of arformoterol, the incidence of adverse events or COPD exacerbations was not different from that of placebo. Long-term safety studies of arformoterol had also shown that the medication was very well tolerated in COPD.Citation33,Citation34 Although head-to-head comparison with nebulized formoterol was not available, the 6-month efficacy and safety study that compared nebulized arformoterol 15 µg with formoterol 12 µg delivered via a dry powder inhaler found that the incidence of adverse events was similar between these products.Citation21
While absolute differences found in this study in LOS and doses of rescue medications were not large, the differences could be meaningful at the population level, considering that the expenditures associated with hospitalization represent more than 70% of all COPD-related medical care costs.Citation35 The difference of 0.3 days in LOS found in this study implied a potential cost savings of $469 per each inpatient treated with arformoterol instead of formoterol. For a hospital with 500 COPD discharges in a year, this would be a cost savings of $234,500 (500×$469) per year.
Limitations
The data used in this analysis were collected for administrative and not research purposes. Administrative data allow for observation without intervention of usual clinical care, but the diagnoses are less accurate than in clinical research. Although multivariate regression models were used to adjust for many background differences, unmeasured differences between arformoterol- and formoterol-treated patients could have confounded the results. Thirty-day readmissions were defined based on a readmission in the current or next calendar month because the data only included month and year (not day) of admission. After exiting the hospital, there were no differences between the two medication cohorts in readmission rates, but information about the treatments patients received outside the hospital were not available in the database. After hospital discharge, patients may have switched treatments, which could have potentially obscured differences in readmission rates between patients treated with formoterol or arformoterol.
Conclusion
This study found that inpatients with COPD treated with nebulized arformoterol had a shorter LOS and used fewer doses of rescue medications than patients treated with nebulized formoterol, which may lead to cost savings. Both arformoterol and formoterol are effective nebulized LABAs for COPD treatment, but there is some preclinical evidence that arformoterol may have a more potent bronchodilator effect. While arformoterol was associated with a shorter LOS and fewer doses of rescue medications in this study, 30-day readmission rates were similar for patients treated with arformoterol and formoterol. Large-scale, randomized, comparative-effectiveness studies are needed to confirm these findings.
Acknowledgments
This study was funded by Sunovion Pharmaceuticals Inc.
Disclosure
Vaidyanathan Ganapathy is a full-time employee of Sunovion Pharmaceuticals Inc. Michael D Stensland is a full-time employee and the sole owner of Agile Outcomes Research, Inc., a firm that was hired by Sunovion Pharmaceuticals Inc. to complete this research. The authors report no other conflict of interest in this work.
References
- ManninoDMCOPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneityChest20021215 Suppl121S126S12010839
- ManninoDMHomaDMAkinbamiLJFordESReddSCChronic obstructive pulmonary disease surveillance – United States, 1971–2000MMWR Surveill Summ2002516116
- Global Initiative for Chronic Obstructive Lung DiseaseGOLD 2017 Global Strategy for the Diagnosis, Management and Prevention of COPD2017 Available from: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/Accessed December 15, 2016
- KesslerRFallerMFourgautGMennecierBWeitzenblumEPredictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med199915911581649872834
- Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic SocietyAm J Respir Crit Care Med19951525 Pt 2S77S1217582322
- HurstJRWedzichaJAChronic obstructive pulmonary disease: the clinical management of an acute exacerbationPostgrad Med J20048094749750515356350
- National Heart Lung and Blood InstituteMorbidity and Mortality: 2009 Chart Book on Cardiovascular, Lung, and Blood Diseases2009 Available from: https://ecopmc.files.wordpress.com/2012/04/2009_chartbook.pdfAccessed September 12, 2016
- PasqualeMKSunSXSongFHartnettHJStemkowskiSAImpact of exacerbations on health care cost and resource utilization in chronic obstructive pulmonary disease patients with chronic bronchitis from a predominantly Medicare populationInt J Chron Obstruct Pulmon Dis2012775776423152680
- LindenauerPKPekowPGaoSCrawfordASGutierrezBBenjaminEMQuality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary diseaseAnn Intern Med20061441289490316785478
- AminABolluVKStenslandMDNetzerLGanapathyVTreatment patterns for patients hospitalized with acute exacerbations of chronic obstructive pulmonary diseaseAm J Health Syst Pharm2017
- ChowLParulekarADHananiaNAHospital management of acute exacerbations of chronic obstructive pulmonary diseaseJ Hosp Med201510532833925820201
- DhandRDolovichMChippsBMyersTRRestrepoRFarrarJRThe role of nebulized therapy in the management of COPD: evidence and recommendationsCOPD201291587222292598
- ButtiniFRossiIDi CuiaMCombinations of colistin solutions and nebulisers for lung infection management in cystic fibrosis patientsInt J Pharm20165021–224224826854429
- TurnerMOPatelAGinsburgSFitzGeraldJMBronchodilator delivery in acute airflow obstruction. A meta-analysisArch Intern Med199715715173617449250235
- ChenYJMakinCBolluVKNavaieMCelliBRExacerbations, health services utilization, and costs in commercially-insured COPD patients treated with nebulized long-acting β2-agonistsJ Med Econ2016191112026357881
- Rodríguez-RoisinRThe airway pathophysiology of COPD: implications for treatmentCOPD20052225326217136952
- AalbersRAyresJBackerVFormoterol in patients with chronic obstructive pulmonary disease: a randomized, controlled, 3-month trialEur Respir J200219593694312030736
- BaumgartnerRAHananiaNACalhounWJSahnSASciarappaKHanrahanJPNebulized arformoterol in patients with COPD: a 12-week, multicenter, randomized, double-blind, double-dummy, placebo- and active-controlled trialClin Ther200729226127817472819
- KingPRole of arformoterol in the management of COPDInt J Chron Obstruct Pulmon Dis20083338539118990965
- HandleyDASenanayakeCHDutczakWBiological actions of formoterol isomersPulm Pharmacol Ther200215213514512090787
- HananiaNADonohueJFNelsonHThe safety and efficacy of arformoterol and formoterol in COPDCOPD201071173120214460
- ThomasJWGuireKEHorvatGGIs patient length of stay related to quality of care?Hosp Health Serv Adm199742448950710174462
- United States CongressHealth Insurance Portability and Accountability Act of 19961996 Available from: http://www.gpo.gov/fdsys/pkg/PLAW-104publ191/html/PLAW-104publ191.htmAccessed September 10, 2016
- PutchaNHanMKMartinezCHthe COPDGene® InvestigatorsComorbidities of COPD have a major impact on clinical outcomes, particularly in African AmericansChronic Obstruct Pulm Dis201411105114
- PutchaNPuhanMADrummondMBA simplified score to quantify comorbidity in COPDPLoS One2014912e11443825514500
- QuanHSundararajanVHalfonPCoding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative dataMed Care200543111130113916224307
- WierLMElixhauserAPfuntnerAAuDHOverview of hospitalizations among patients with COPD, 20082011 Available from: http://www.ncbi.nlm.nih.gov/books/NBK53969/?report=printableAccessed September 10, 2016
- BolluVErnstFRKarafilidisJRajagopalanKRobinsonSBBramanSSHospital readmissions following initiation of nebulized arfor-moterol tartrate or nebulized short-acting beta-agonists among inpatients treated for COPDInt J Chron Obstruct Pulmon Dis2013863163924353413
- LindenauerPKShiehMSPekowPSStefanMSUse and outcomes associated with long-acting bronchodilators among patients hospitalized for chronic obstructive pulmonary diseaseAnn Am Thorac Soc20141181186119425167078
- SniderJTJenaABLinthicumMTEffect of hospital use of oral nutritional supplementation on length of stay, hospital cost, and 30-day readmissions among Medicare patients with COPDChest201514761477148425357165
- Premier, IncPremier Research Services creates insights through data2017 Available from: https://www.premierinc.com/transforming-healthcare/healthcare-performance-improvement/premier-research-services/Accessed February 7, 2017
- MhannaMJKoesterJFCohnRCEffects of (r,r)- and (r,r/s,s)-formoterol on airway relaxation and contraction in an experimental rat modelCurr Ther Res Clin Exp200768424926124683215
- DonohueJFHananiaNAMakeBOne-year safety and efficacy study of arformoterol tartrate in patients with moderate to severe COPDChest201414661531154225451347
- DonohueJFHananiaNASciarappaKAArformoterol and salmeterol in the treatment of chronic obstructive pulmonary disease: a one year evaluation of safety and toleranceTher Adv Respir Dis200822374819124357
- SullivanSDRamseySDLeeTAThe economic burden of COPDChest20001172 Suppl5S9S10673466