Abstract
Purpose
Smoking can affect both the phenotypic expression of COPD and factors such as disease severity, quality of life, and comorbidities. Our objective was to evaluate if the impact of active smoking on these factors varies according to the disease phenotype.
Patients and methods
This was a Spanish, observational, cross-sectional, multicenter study of patients with a diagnosis of COPD. Smoking rates were described among four different phenotypes (non-exacerbators, asthma-COPD overlap syndrome [ACOS], exacerbators with emphysema, and exacerbators with chronic bronchitis), and correlated with disease severity (body mass index, obstruction, dyspnea and exacerbations [BODEx] index and dyspnea grade), quality of life according to the COPD assessment test (CAT), and presence of comorbidities, according to phenotypic expression.
Results
In total, 1,610 patients were recruited, of whom 46.70% were classified as non-exacerbators, 14.53% as ACOS, 16.37% as exacerbators with emphysema, and 22.40% as exacerbators with chronic bronchitis. Smokers were predominant in the latter 2 groups (58.91% and 57.67%, respectively, P=0.03). Active smoking was significantly associated with better quality of life and a higher dyspnea grade, although differences were observed depending on clinical phenotype.
Conclusion
Active smoking is more common among exacerbator phenotypes and appears to affect quality of life and dyspnea grade differently, depending on the clinical expression of the disease.
Introduction
COPD is characterized by chronic airflow limitation, which is not fully reversible, generally progressive, and associated with an inflammatory pulmonary response to toxic particles and gases. The systemic impact is severe.Citation1 This disease presents in a wide variety of forms, and the concept that distinct COPD phenotypes can be identified, each with its characteristic clinical, prognostic, and therapeutic impact, is well established.Citation2 In Spain, the GesEPOC guidelines (Spanish COPD guidelines published by the Spanish Society of Pulmonology and Thoracic Surgery, which aim to establish standard recommendations for the management of the disease in Spain) propose 4 phenotypes, each with a different prognosis and therapeutic approach: the non-exacerbator phenotype (with emphysema or chronic bronchitis); the asthma-COPD overlap syndrome (ACOS); the exacerbator phenotype with emphysema; and the exacerbator phenotype with chronic bronchitis.Citation3
Tobacco use is widely accepted as the major risk factor for the development of COPD.Citation4 Exposure to tobacco smoke may also act as a differential factor in the various phenotypic expressions of the disease, and evidence suggests that a phenotype derived from smoking may be different from one caused by other environmental factors.Citation5–Citation7 Active smoking has been associated with COPD severity, quality of life, and comorbidities, although some results are controversial. For example, some authors report a positive relationship between active smoking and a higher rate of admission due to acute exacerbation, but others have not confirmed this finding.Citation8–Citation11 The impact of smoking on patients’ quality of life has also been a topic of discussion,Citation12–Citation17 with previous reports indicating a lower mentalCitation13,Citation14 and physicalCitation16 functional status in current smokers and an improvement of health-related quality of life after smoking cessation,Citation15,Citation17 although other studies have shown the opposite results.Citation18,Citation19
The primary objective of this study, conducted under the auspices of a large trial aimed primarily at describing the distribution and characteristics of the different COPD phenotypes in Spain, was to examine the cross-sectional prevalence of active smoking among the various COPD phenotypes; the secondary objective was to determine the relationships between the smoking habit, COPD phenotypes, and major demographic and clinical variables, such as quality of life, disease severity, and presence of comorbidities.
Patients and methods
Study design and sample
This was an observational, cross-sectional, multicenter study performed in Pulmonology departments and other specialist units (Internal Medicine, Family and Community Medicine, and Occupational Medicine) throughout Spain. The aim of this study was to determine the prevalence and characteristics of the 4 COPD phenotypes established by GesEPOCCitation3 in Spain. Each investigator was requested to include the first 6–8 unselected (consecutive) patients that fulfilled the selection criteria during the 90-day recruitment period. The study was approved by the Clinical Research Ethics Committee of the Hospital San Pedro de Alcántara in Cáceres, and patients gave written informed consent before participating. In this study, we examined the rates of active smoking and its relationship with COPD phenotype and factors influencing disease prognosis (severity, comorbidities, and quality of life). Patients were included if they were over the age of 35 years and had a clinical and functional diagnosis of COPD according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) or GesEPOC criteria at least 6 months before participation in the study. Patients with symptoms of COPD exacerbation or who had had an exacerbation within the previous 6 weeks which could interfere with their perception of quality of life and functional status, those with any type of chronic respiratory disease other than COPD, or who had difficulty reading or understanding the study questionnaires were excluded from participation.
Sociodemographic and clinical variables
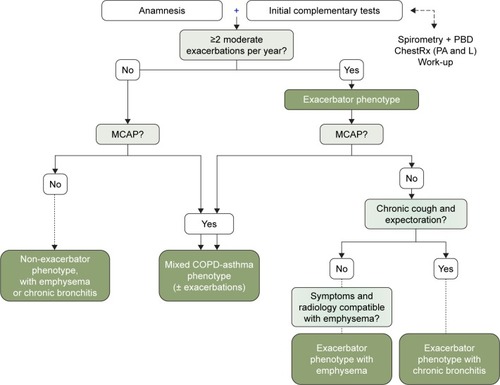
Each investigator was trained in the study procedures and questionnaires and used a case report form (CRF) to collect the demographic details of each patient and the clinical variables required for the study. The GesEPOC algorithm was used to determine COPD phenotype.Citation3 This algorithm, classifies the phenotype according to the following rules: A) patients with <2 exacerbations in the previous year were classified as non-exacerbators; B) patients with a previous diagnosis of asthma were considered as ACOS; C) exacerbators with emphysema that have a clinical/radiological/functional diagnosis of emphysema; and D) exacerbators who experienced cough with expectoration for >3 months of the year over 2 consecutive years were classified as exacerbators with chronic bronchitis. The schematic algorithm was provided to all the investigators ().
Figure 1 Diagnostic algorithm of the clinical phenotypes.
Abbreviations: COPD, chronic obstructive pulmonary disease; MCAP, mixed COPD-asthma phenotype; ACOS, asthma-COPD overlap syndrome; PBD, bronchodilator test.
Patients were asked about their smoking habit and were classified as nonsmokers, ex-smokers, and active smokers. COPD severity was measured using the BODEx index.Citation20 Quality of life was assessed with the COPD Assessment Test (CAT).Citation21 Finally, comorbidities present at the time of data collection were evaluated.
Statistical analysis
COPD phenotype distribution was calculated using a descriptive analysis, with percentages and binomial 95% confidence intervals (95% CIs). Qualitative variables were compared using Fisher’s exact test, and quantitative groups and variables were compared with the Student’s t-test (for 2 groups) or the one-way analysis of variance (ANOVA) (for 3 or more groups). A logistic regression method was used to model the probability of being a smoker in relation to comorbidities (arrhythmia, coronary artery disease, heart failure, hypertension, dyslipidemia, diabetes mellitus, osteoporosis, and dementia), CAT score, serious exacerbations, and BODEx index. After stepwise analysis, the adjusted odds ratios (ORs) were calculated using multivariate logistic regression. Only significant associations are shown in the tables. All calculations were performed using the SAS statistical package version 9.4 for Windows, establishing a level of significance of 0.05.
Results
A total of 268 respiratory medicine specialists, 177 primary care physicians, 34 internists, and 1 occupational medicine specialist participated in this study. They recruited 1,610 patients (see and S1 for patients’ characteristics), predominantly men (82.13%) with a mean age of 66.71 years (95% CI: 66.24–67.19). Of these, 55.41% (95% CI: 52.98–57.84) stated they were active smokers, with a smoking index of 65.02 pack-years (95% CI: 59.62–71.41), and a mean forced expiratory volume in 1 second (FEV1) (%) of 54.70% (95% CI: 53.90–55.51). Seventy-one percent of patients had dyspnea grade ≤2, a mean BODEx index of 2.75 (95% CI: 2.65–2.85), and a mean CAT score of 22.04 (95% CI: 21.43–22.66). Finally, 83.79% had comorbidities, the most common being arterial hypertension (62.90%) and diabetes mellitus (31.7%).
Table 1 Characteristics of the whole study population and stratification by smoking habit
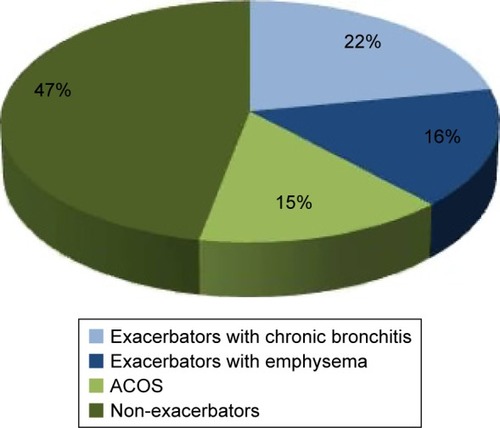
With regard to phenotype distribution, 46.70% (95% CI: 44.26–49.14) were classified as non-exacerbators, 14.53% (95% CI: 12.81–16.25) as ACOS, 16.37% (95% CI: 14.56–18.18) as exacerbators with emphysema, and 22.40% (95% CI: 20.36–24.43) as exacerbators with chronic bronchitis (). Significant differences were found in the distribution of sexes by phenotype; the most pronounced being in the ACOS phenotype, in which there were almost twice as many women as men (21.7% vs 13.0%, respectively, P=0.002). Mean age among the phenotypes was significantly lower in the ACOS than that of exacerbators with chronic bronchitis ().
Table 2 Patient characteristics and COPD severity according to phenotype and smoking habit
Active smoking
The frequency of active smoking by phenotype and its impact on quality of life, disease severity, and comorbidities were compared (). The group of nonsmokers accounted for only 2.9% of the total patient population; therefore, we decided to perform the remaining analyses on the basis of 2 groups only (active/nonsmokers + ex-smokers [nonactive smokers]). Even so, it is interesting to note that overall 9.1% of the female population had never smoked, whereas only 0.6% of all men had never smoked; this difference was statistically significant (P<0.001). Active smokers were predominant among the exacerbator phenotypes with emphysema and with chronic bronchitis (58.91% and 57.67%, respectively, P=0.03). The impact of active smoking on patients’ quality of life was found to be significantly positive, according to the CAT scores (20.51 [95% CI: 19.62–21.41] vs 23.33 [95% CI: 22.50–24.15; P<0.001]). This relationship remained significant for each of the items making up the CAT scale. When this correlation was analyzed for the different phenotypes, we found that while active smokers consistently had a better CAT score, it was statistically significant only in the more common phenotypes, that is, non-exacerbators and exacerbators with chronic bronchitis. In contrast, when the relationship between active smokers and dyspnea severity was analyzed, active smokers were seen to have a significantly greater dyspnea grade (1.83 [95% CI: 1.77–1.899] vs 1.69 [95% CI: 1.62–1.76]; P=0.002). When this relation was assessed according to the different phenotypes, it was only maintained in the group of exacerbators with chronic bronchitis (2.21 [95% CI: 2.09–2.33] vs 1.92 [95% CI: 1.80–2.00]; P=0.001). No differences were found in the BODEx score with respect to active smoking (2.82 [95% CI: 2.68–2.96] vs 2.67 [95% CI: 2.52–2.82]; P=0.73). Finally, no differences were observed in the presence of comorbidities according to smoking habit, although active smokers tended to have a higher comorbidity burden; this difference was significant among patients classified as exacerbators with chronic bronchitis ().
Multivariate analysis showed that higher CAT scores were negatively associated, whereas BODEx score was positively associated with active smoking (). Among comorbidities, osteoporosis was the only one associated with the probability of an individual being a smoker (OR =1.61, Wald 95% CI: 1.45–2.26).
Table 3 Factors associated with active smoking in the multivariate analysis
Discussion
The results of this study are derived from a larger study designed to describe phenotype distribution among patients with COPD throughout Spain. In comparison with other studies of Spanish series,Citation22,Citation23 we found that while the proportion of patients with ACOS in our cohort was still around 15%, a greater proportion of patients were described as exacerbators (around 40% compared to 20% reported in other studies), and fewer were stable phenotypes (45% vs 65%). It is difficult to determine the reason for this discrepancy as the definition used to characterize the exacerbator phenotype was the same in all studies (presence of at least 2 exacerbations in the last year or 1 hospitalization for acute exacerbation). The difficulty in precisely defining “exacerbation” may have contributed to this discrepancy. Evidence shows that exacerbations are often underreported because many patients with COPD may have difficulty in recognizing their symptoms as exacerbation, and accordingly, tend not to report them.Citation24,Citation25 Langsetmo et al, for example, evaluated the incidence of reported and unreported exacerbations in a cohort of 421 patients with COPD and showed that less than one third of the exacerbations were reported, being that the number of symptoms at onset was the most important predictor of reporting.Citation24 However, Hurst et al, in one of the largest studies conducted to determine susceptibility to exacerbations in stable patients with COPD over a period of 3 years, established that the incidence of exacerbations was 22% in patients with moderate COPD, rising to 47% in severe cases.Citation25 According to the study authors, this incidence would have been much greater if untreated exacerbations had been computed along with treated events. In any case, the clinical implication of these results could be important. An action plan to help patients recognize changes in their symptoms, to implement self-care, and to self-initiate prescription in the event of an exacerbation has been suggested as a promising strategy.Citation26
Existing evidence shows not only that smoking is the primary etiological cause of COPD, but also that it can also lead to faster decline in lung function if the habit continues; even so, the proportion of smokers among patients with COPD remains higher than among the general population.Citation27 Recent studies performed in the USA found smoking rates between 35% and 45% among adult patients with COPD.Citation28,Citation29 In our series, the prevalence of active smokers is 55%, contrasting with reports recently published in Spain that set it at about 25%.Citation22,Citation23 Perhaps these differences reflect differences in data collection methods. Different ways of classifying a smoker as active may have a significant influence on the results of studies conducted in these patients, and hinder comparison among the different papers.Citation30 However, a good level of agreement has been shown between smoking statuses based on self-reported and serum cotinine measurements.Citation31 In any case, the proportion of active smokers among patients with COPD is still unacceptably high, particularly when we consider that one of the key objectives established by GOLD guidelines in the management of these patients is smoking cessation.Citation1
Results on smoking and phenotype distribution among patients with COPD show that a significantly higher proportion of patients with exacerbator phenotype are active smokers. Previous data indicate that patients with greater susceptibility to viral infections are more prone to exacerbations, and this susceptibility appears to be associated with their smoking habit.Citation32 The viral load during acute exacerbations of COPD has been detected as significantly higher than in stable state.Citation33 Moreover, a recent study has demonstrated an upregulated expression of ICAM-1 (major receptor for 60% of human rhinoviruses and Haemophilus influenza, two major pathogens in COPD) in the respiratory tract of smokers and especially in the airway epithelium in subjects with chronic airflow obstruction. This finding could reinforce the relation between active smoking, viral infection, and frequency of exacerbations.Citation34 However, conflicting data about this association still persists. For example, Hurst et al,Citation25 in a study that evaluated the impact of certain factors (ie, demographic and clinical characteristics, lung function, and laboratory and biomarkers values) on susceptibility to exacerbations found that while there was some relationship between active smoking and susceptibility to exacerbations, this association disappeared after multivariate analysis, showing that the link was not independent and may have been influenced by interactions with other variables, such as previous exacerbations, disease severity, health status, history of gastroesophageal reflux, and white cell count.Citation25 This discrepancy has also been observed in series recently published in Spain.Citation22,Citation23 Although Miravitlles et alCitation22 did report a higher rate of smoking among exacerbators, Cosio et alCitation23 failed to identify such an association. In our series, patients over the age of 50 years, who were significantly heavier smokers, were predominant in these phenotypes, and this may have affected the results.
When the impact of smoking on patients’ quality of life was analyzed, we were surprised to find that former smokers obtained a poorer CAT score. Moreover, the fact that the groups that smoked most, that is, older patients and exacerbators, reported a worse quality of life makes this finding even more difficult to understand. Although most studies have found an inverse relationship between smoking and quality of life, this has not always been the case. Some studies have found that while many smokers attempt to give up their habit, many others continue to smoke as they associate the habit with psychological well-being, relaxation, and relief of stress.Citation18,Citation19 These factors may lead to a mistaken perception of quality of life on the part of the patients themselves. Wijnhoven et al also observed that quality of life was positively affected by active smoking. These authors associated it with the fact that patients with a better perception of their disease (less symptomatic) may wait longer before quitting smoking than patients with more severe symptoms.Citation35 These differences might perhaps not have been revealed if the dynamics of this variable over time had been evaluated. Shields et al in a longitudinal study that analyzed the dynamics of smoking cessation between former smokers and nonsmokers concluded that a relatively long period of time was needed before quitters achieved level of comfortable life similar to those of individuals who have never smoked. Thus, the authors assert that it is difficult to extract conclusions on these measurements from cross-sectional studies.Citation15 Ståhl et al also failed to find differences in quality of life associated with smoking habit among patients with COPD and suggest that the degree of disease severity is a metric with greater impact in this entity.Citation36 The discordance is deepened further by the fact that dyspnea severity is significantly associated with active smoking. Several studies have cast doubts on the equivalence between dyspnea grade and CAT score in the evaluation of disease involvement.Citation37,Citation38 Indeed, recent evidence shows that the isolated use of CAT as a tool to establish the impact and severity of COPD may be inappropriate, given the difficulty of establishing definite cutoff points for a sufficiently sensitive and specific classification of patients according to their severity.Citation39 Our results appear to support this premise. Finally, we should mention the limited representation among our series of nonsmokers, which prevented us from analyzing the influence of this population on the final results.
COPD is often associated with other diseases and a great deal has been written about the systemic effects of the disease itself and how these clearly increase pressure on health care resources and the risk of death. COPD was associated with other diseases in up to 80% of the patients in our series, the most common comorbidity being arterial hypertension; these data coincide with the findings of most previously published studies.Citation40,Citation41 When comorbidities were analyzed according to the use of tobacco, it was found that patients with osteoporosis were more likely to be active smokers. Osteoporosis has been recognized as one of the major noncardiovascular comorbidities associated with COPD, occurring at rates of 4%–59%, depending on the diagnostic method employed.Citation42 Many explanations have been given for this association, including age, sex, smoking habit, limited daily activity, chronic hypoxemia, or corticosteroid treatment. Smoking has consistently been associated with low bone density in healthy volunteers;Citation43 therefore, it is unsurprising that this relationship is stronger in patients with COPD, in whom other factors also combine to raise the risk of developing osteoporosis. It is also interesting to note the relationship that has been established between the emphysema phenotype and an increased risk of osteoporosis suggests a common mechanism between these two conditions.Citation43,Citation44 We ourselves did not find any specific relationship between phenotypes and osteoporosis, although active smokers were more predominant in phenotypes in which this disease may occur (nonexacerbators and exacerbators with emphysema).
Conclusion
Our study provides a general overview of how smoking, phenotype, and prognostic factors, such as quality of life or comorbidities, are interwoven in a large sample of Spanish patients with COPD. Some disparity between our data and those derived from similar recently published series may be due to the limitations to which all studies of this kind are subject (cross-sectional, participation of different specialists). Nevertheless, the results underline the importance of active smoking in the characterization of the disease.
Acknowledgments
This study was funded by Grupo Ferrer (Barcelona, Spain). The authors would like to thank David Calbet for providing statistical analysis and Jose L Lorenzo for manuscript writing assistance.
Supplementary materials
Table S1 Description of patient population included in the study
Disclosure
The authors report no conflicts of interest in this work.
References
- RabeKFHurdSAnzuetoAGlobal Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2007176653255517507545
- HanMKAgustíACalverleyPMChronic obstructive pulmonary disease phenotypes. The future of COPDAm J Respir Crit Care Med2010182559860420522794
- MiravitllesMSoler-CataluñaJJCalleMSpanish COPD Guidelines (GesEPOC): Pharmacological treatment of stable COPDArch Bronconeumol201248724725722561012
- World Health OrganizationWHO report on the global tobacco epidemic2015 Available from: http://www.who.int/tobacco/global_report/2015/en/Accessed January 9, 2017
- MiravitllesMCalleMSoler-CataluñaJJClinical phenotypes of COPD: identification, definition and implications for guidelinesArch Bronconeumol2012483869822196477
- GolpeRSanjuán LópezPCano JiménezECastro AñónOPérez de LlanoLADistribution of clinical phenotypes in patients with chronic obstructive pulmonary disease caused by biomass and tobacco smokeArch Bronconeumol201450831832424576449
- Izquierdo-AlonsoJLRodriguez-GonzálezmoroJMde Lucas-RamosPPrevalence and characteristics of three clinical phenotypes of chronic obstructive pulmonary disease (COPD)Respir Med2013107572473123419828
- HunterLCLeeRJButcherIPatient characteristics associated with risk of first hospital admission and readmission for acute exacerbation of chronic obstructive pulmonary disease (COPD) following primary care COPD diagnosis: a cohort study using linked electronic patient recordsBMJ Open201661e009121
- Garcia-AymerichJMonsoEMarradesRMEFRAM InvestigatorsRisk factors for hospitalisation for a chronic obstructive pulmonary disease exacerbation. EFRAM studyAm J Respir Crit Care Med200116461002100711587986
- MiravitllesMGuerreroTMayordomoCSánchez-AgudoLNicolauFSegúJLFactors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple regression analysis. The EOLO Study GroupRespiration200067549550111070451
- BahadoriKFitzGeraldJMLevyRDFeraTSwistonJRisk factors and outcomes associated with chronic obstructive pulmonary disease exacerbations requiring hospitalisationCan Respir J2009164e43e4919707601
- TillmannMSilcockJA comparison of smokers’ and ex-smokers’ health-related quality of lifeJ Public Health Med19971932682739347449
- WoolfSHRothemichSFJohnsonREMarslandDWIs cigarette smoking associated with impaired physical and mental functional status? An office-based survey of primary care patientsAm J Prev Med199917213413710490056
- McClaveAKDubeSRStrineTWMokdadAHAssociations between health-related quality of life and smoking status among a large sample of U.S. adultsPrev Med200948217317919103219
- ShieldsMGarnerREWilkinsKDynamics of smoking cessation and health-related quality of life among CanadiansHealth Rep2013242311
- HolahanCKHolahanCJNorthRJHayesRBPowersDAOckeneJKSmoking status, physical health-related quality of life, and mortality in middle-aged and older womenNicotine Tob Res201315366266922965789
- TaylorGMcNeillAGirlingAFarleyALindson-HawleyNAveyardPChange in mental health after smoking cessation: systematic review and meta-analysisBMJ2014348g115124524926
- McEwenAWestRMcRobbieHMotives for smoking and their correlates in clients attending Stop Smoking treatment servicesNicotine Tob Res200810584385018569758
- FidlerJAWestRSelf-perceived smoking motives and their correlates in a general population sampleNicotine Tob Res200911101182118819640835
- Soler-CataluñaJJMartínez-GarcíaMASánchezLSTorderaMPSánchezPRSevere exacerbations and BODE index: two independent risk factors for death in male COPD patientsRespir Med2009103569269919131231
- JonesPWHardingGBerryPWiklundIChenWHKline LeidyNDevelopment and first validation of the COPD Assessment TestEur Respir J200934364865419720809
- MiravitllesMBarrechegurenMRomán-RodríguezMFrequency and characteristics of different clinical phenotypes of chronic obstructive pulmonary diseaseInt J Tuberc Lung Dis201519899299826162367
- CosioBGSorianoJBLópez-CamposJLDistribution and Outcomes of a Phenotype-Based Approach to Guide COPD Management: Results from the CHAIN CohortPLoS One2016119e016077027684372
- LangsetmoLPlattRWErnstPBourbeauJUnderreporting exacerbation of chronic obstructive pulmonary disease in a longitudinal cohortAm J Respir Crit Care Med2008177439640118048806
- HurstJRVestboJAnzuetoAEvaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) InvestigatorsSusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- NiciLDonnerCWoutersEATS/ERS Pulmonary Rehabilitation Writing CommitteeAmerican Thoracic Society/European Respiratory Society statement on pulmonary rehabilitationAm J Respir Crit Care Med2006173121390141316760357
- Centers for Disease Control and PreventionMMWR20126146938943 Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6146a2.htmAccessed January 9, 201723169314
- SchauerGLWheatonAGMalarcherAMCroftJBSmoking prevalence and cessation characteristics among U.S. adults with and without COPD: findings from the 2011 Behavioral Risk Factor Surveillance SystemCOPD201411669770424841392
- CheruvuVKOdhiamboLAMowlsDSZulloMDGudinaATHealth-related quality of life in current smokers with COPD: factors associated with current smoking and new insights into sex differencesInt J Chron Obstruct Pulmon Dis2016112211221927695308
- RyanHTrosclairAGfroererJAdult current smoking: differences in definitions and prevalence estimates – NHIS and NSDUH, 2008J Environ Public Health2012201291836822649464
- JainRBComparative analysis of two tobacco surveillance questionnaires used in NHANES: accuracy of self-reported smoking statusToxicol Environ Chem2016981137148
- CohenSTyrrellDARussellMAJarvisMJSmithAPSmoking, alcohol consumption, and susceptibility to the common coldAm J Public Health1993839127712838363004
- GeorgeSNGarchaDSMackayAJHuman rhinovirus infection during naturally occurring COPD exacerbationsEur Respir J2014441879624627537
- ShuklaSDMahmoodMQWestonSThe main rhinovirus respiratory tract adhesion site (ICAM-1) is upregulated in smokers and patients with chronic airflow limitation (CAL)Respir Res201718628056984
- WijnhovenHAKriegsmanDMHesselinkAEPenninxBWde HaanMDeterminants of different dimensions of disease severity in asthma and COPD: pulmonary function and health-related quality of lifeChest200111941034104211296166
- StåhlELindbergAJanssonSAHealth-related quality of life is related to COPD disease severityHealth Qual Life Outcomes200535616153294
- JonesPWAdamekLNadeauGBanikNComparisons of health status scores with MRC grades in COPD: implications for the GOLD 2011 classificationEur Respir J201342364765423258783
- KimSOhJKimYIDifferences in classification of COPD group using COPD Assessment Test (CAT) or modified Medical Research Council (mMRC) dyspnea scores: a cross-sectional analysesBMC Pulm Med2013133523731868
- MittalRChhabraSKGOLD classification of COPD: discordance in criteria for symptoms and exacerbation risk assessmentCOPD20171411627723367
- CamiciottoliGBigazziFMagniCPrevalence of comorbidities according to predominant phenotype and severity of chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis2016112229223627695310
- GershonASMecredyGCGuanJVictorJCGoldsteinRToTQuantifying comorbidity in individuals with COPD: a population studyEur Respir J2015451515925142481
- LehouckABoonenSDecramerMJanssensWCOPD, bone metabolism, and osteoporosisChest2011139364865721362651
- PapaioannouAKennedyCCCranneyARisk factors for low BMD in healthy men age 50 years or older: a systematic reviewOsteoporos Int200920450751818758880
- BonJFuhrmanCRWeissfeldJLRadiographic emphysema predicts low bone mineral density in a tobacco-exposed cohortAm J Respir Crit Care Med2011183788589020935108