Abstract
Smoking and subsequent development of COPD is an ever-increasing epidemic in Arabian Gulf and Middle East countries, with no signs of decline. The important fact to be highlighted is that this COPD epidemic of increasing incidence and prevalence is mostly unrecognized by patients, due to the common attribution of symptoms to “smoker’s cough”, and the underdiagnosis and undertreatment by physicians because the common signs and symptoms masquerade as asthma. Consequently, there are long-term adverse effects of missing the diagnosis. The purpose of this review article is to focus upon the status of COPD in Arabian Gulf and Middle East countries, stressing the increasing burden of smoking and COPD, to emphasize the specific factors leading to rise in prevalence of COPD, to bring to light the underdiagnosis and undermanagement of COPD, and to treat COPD in conformity with standard guidelines with local and regional modifications. This review ends with suggestions and recommendations to the health department to formulate policies and to generate awareness among the general public about the side effects of smoking and consequences of COPD.
Introduction
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines define COPD as
[…] a common preventable and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities caused by significant exposure to noxious particles or gases.Citation1
Although there is no permanent cure for COPD, reduction of risk factors can reduce the progression of the disease, with consequent health-related benefits. The most important risk factors for the development of COPD are smoking (cigarettes, water pipe), air pollution resulting from biomass fuel or coal and wood burning, previous tuberculosis, environmental/occupational exposures, and increasing age.Citation5 The worldwide increase in COPD prevalence is directly related to the increase in prevalence of smoking, especially in women and young adults.
Current status of COPD in Gulf Cooperative Council countries and Middle East–North Africa region
The prevalence of smoking and COPD has risen significantly in Gulf Cooperative Council (GCC) countries and the Middle East–North Africa (MENA) region, due to the lack of knowledge of the risks of smoking and COPD. It is worth noting that in the GCC–MENA region, COPD is largely underdiagnosed/wrongly diagnosed and hence inappropriately treated. Diagnosis of COPD is delayed until it is moderately or severely advanced, due to lack of standardized procedures to diagnose COPD, overreliance on clinicoradiological parameters by clinicians for diagnosis, low general public awareness, indifferent attitudes in physicians and the general public about smoking and COPD, high prevalence of smoking (active and passive) and environmental pollution, nonimplementation of health regulations for smoking cessation, and the dictum that “everything that wheezes is asthma” leading to misdiagnosis of COPD as asthma.
Smoking prevalence and smoking habits
A pandemic of smoking and COPD is sweeping across the Middle East and MENA region. The recently conducted BREATHE study revealed that the adjusted smoking rate in the general population of MENA countries was estimated to be 31.2% (ranging from 15.3% in Morocco to 53.9% in Lebanon). The smoking rate in men ranged from 29.7% in Morocco to 61% in Turkey, whereas in women it ranged from 1.4% in Morocco to 47.3% in Lebanon. Overall, the highest rates were detected in Jordan, Lebanon, Syria, and Turkey.Citation6
Many people in the GCC–MENA region consider smoking cigarettes and water pipes a socializing activity. In this region, tobacco is consumed in different forms, eg, water pipe (narghile/hookah) and medwakh (a pipe used to smoke dokha, a tobacco mixed with herbs and spices “dokha” in Arabic means “dizziness”), and the lack of health regulations to prohibit smoking and shisha in clubs, coffeehouses, and other public places is a major contributor to first and secondhand smoke.Citation7 Water-pipe smoking is highly prevalent in women and the young, due to less cultural stigma associated with water-pipe smoking. It is important to note that the nicotine exposure from daily water-pipe use is considered equivalent to 20 cigarettes/day,Citation8 and it has been found that water-pipe and medwakh smokers have dependence on nicotine and are unable to quit, despite repeated attempts. There is a clear misconception that the water pipe is harmless compared to cigarettes, because the smoke is filtered and the noxious smoking particles absorbed and dissolved in water.Citation9 We discuss regional smoking patterns and burden in later sections.
Other risk factors for COPD relevant to GCC–MENA
The development of COPD and its progression is multifactorial, including complex interplay of genetic/risk factors and environmental exposure. Considering the huge proportion of expatriates in the UAE population, this statement holds further true; however, most of the genetic factors that could be potential contributors to the development of COPD in the GCC–MENA region are unknown, due to a lack of phenotypic and genetic research studies on COPD.
Environmental factors
Outdoor air pollution, occupational exposure to dust and fumes, biomass-smoke inhalation, first- and secondhand smoke, and previous tuberculosis are important environmental factors associated with COPD in the GCC–MENA region. Although tobacco smoking is the most common and important cause for COPD development, the population-attributable fraction of smoking as an etiological cause of COPD ranges from 9.7% to 97.9%. A Swedish cohort observed that the population-attributable fraction of smoking as a cause of COPD was present in 76.2%,Citation10 suggesting that there are other factors contributing to COPD development.
Outdoor air pollution
Outdoor pollution coming mainly from emission of gases/fumes and pollutants from vehicular and industrial emission is an important public health problem in the fast-developed GCC–MENA region. In a community-based study, higher traffic density was found to be significantly associated with lower forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) values in women.Citation11 In a recent survey, Dubai Municipality found that the city ranked very high in vehicular pollution in the developed world, using an on-road vehicle emission-measurement device (measuring percentage scores for levels of harmful pollutants, including hydrocarbons, CO, nitrogen oxides, and CO2). Dubai’s statistical data showed that the number of motor vehicles had increased by an annual average of about 12% in Dubai every year, significantly contributing to air pollution. Frequent dust storms may also lead to acute exacerbation of COPD.
Rapid industrialization and urbanization
Very rapid construction of multistory buildings in the last 2 decades with the significant risk of exposure to harmful construction dust to laborers and nearby residents could have significantly contributed to the development of COPD, especially in the young.
Indoor air pollution
Indoor air pollutants contributing to COPD development are tobacco smoke, by-products of combustion of coal/wood/barbeque, such as nitrogen dioxide, carbon monoxide, volatile organic compounds, and biological allergens.Citation12 Among these, tobacco and biomass-smoke (wood, crop residue, barbeque)Citation13,Citation14 exposure contributes to the development of COPD. A recent meta-analysis showed biomass-smoke exposure to be a significant risk factor for developing COPD.Citation15 However, in view of the rapid urbanization and no or minimal use of biomass fuel, indoor air pollution would not be a significant contributing factor for the young, but could definitely be a contributory factor to COPD in the elderly when biomass fuel was the main source of cooking.
In the GCC–MENA region, traditional Arabian incense (bakhour) is a common source of indoor smoke, and has consistently been found to be associated with worsening of asthma and wheezing bronchitis, especially in children, in many studies. Unfortunately, there are no studies available to establish bakhour as a contributory or worsening cause of COPD; however, scientifically speaking, slow and incomplete combustion of bakhour produces continuous smoke-containing pollutants (toxic gases and chemical particles), including polycyclic aromatic hydrocarbons, CO, benzene, and isoprene, that accumulate easily in indoor environments if ventilation is inadequate or in crowded places,Citation16 and could be a potential indoor risk factor for COPD in the MENA region.
One study with the objective of characterization of particles and gases emitted from Arabian incense revealed that carbon monoxide and oxides of nitrogen concentration in time-weighted averages exceeded current government-regulation values and emissions from environmental tobacco smoke. Charcoal emissions were the main contributor to high CO and NOx concentrations. A significant cell-inflammatory response was observed to smoke components formed from incense burning. Our hazard evaluation suggests that incense burning contributes to indoor air pollution, and could be harmful to human health by worsening asthma and COPD.Citation17
Rationale for GCC–MENA joint statement
To produce this joint statement on COPD in the GCC–MENA region, we searched PubMed, Google Scholar, ResearchGate, Medline, SciSearch, Journal Citation Reports, Science Edition, Embase, Scopus, Directory of Open Access Journals, OAIster, and other research portals extensively for articles published on the burden of COPD in the MENA region, and found that there is scarce material available from the GCC–MENA region. BREATHE was the largest regional study done, with eleven countries participating, focusing on the epidemiology/pathogenesis/standardization of diagnosis/treatment and preventive strategies and covering all other important aspects of this chronic, debilitating, multisystem, inflammatory disease and considering the widely varied sociocultural, demographic, and ethnic background/factors in this part of the world, with its majority of immigrant populations from low/middle socioeconomic class and their different attitudes/beliefs, patterns of smoking, and compliance with treatment.Citation18
We decided to uncover various issues, with particular emphasis on local and regional factors covering all aspects of COPD in this GCC–MENA positional statement, and we recommend that all regional health authorities implement strict policies for the prevention/diagnosis and treatment of this debilitating disease in the GCC–MENA region as per standard evidence-based management strategies with regional modification to reduce the incidence of smoking and consequent COPD and to provide best standardized care to all COPD patients.
Incidence and prevalence of COPD in GCC–MENA region
More than 3 million people died of COPD in 2012, which is equal to 6% of all deaths globally that year. As per WHO estimates, 65 million people have moderately severe COPD. Estimates show that COPD in 2030 will be the third-leading cause of death worldwide.Citation19 More than 90% of COPD-related deaths occur in low- and middle-income countries. The primary cause of COPD is tobacco smoking (through tobacco use or secondhand smoke). COPD now affects men and women almost equally, due to increased use of tobacco and related products among women in high-income countries. COPD is not curable, but treatment can slow the progression of the disease.
A systemic meta-analysis of spirometry-based studies (n=123 studies) across the world showed growing prevalence of COPD globally, and produced the following results:
Global prevalence increased from 10.7% (227 million population) in 1990 to 11.7% (384 million population) in 2010 (68.9% increase).
Overall prevalence in men was 14.3% compared to 7.6% in women.
The Eastern Mediterranean Region (EMR) showed the highest increase in percentage, from 11.8% in 1990 to 13.4% in 2010 (118.7% increase in the prevalence of smoking).Citation20
Burden of COPD in MENA region
There is a scarcity of studies in Africa, Southeast Asia, and the EMR.Citation20 For the purpose of this review, PubMed was searched for articles published on the burden of COPD in the MENA region/EMR. Twelve studies were short-listed to assess the burden of COPD in the MENA region. BREATHE was the largest regional study done, with eleven countries participating.Citation21 It estimated the prevalence of COPD to be 3.6% in the MENA region, ranging from 1.9% in the UAE to 6.1% in Syria.Citation21 Al Zaabi et al estimated spirometry-based post-bronchodilator prevalence to be 3.7% in the UAE.Citation22 Another study in Dubai estimated COPD prevalence to be much higher – at 12.9%.Citation23 This difference in COPD prevalence can be explained, as the latter study resorted to prebronchodilator definition, which does not differentiate a substantial group of asthma patients from COPD, and hence overestimated the prevalence. Recent studies in Saudi Arabia have revealed COPD prevalence of 2.4%–14.2%.Citation21,Citation24,Citation25 The study with high estimated prevalence was conducted in high-risk patients (smokers presenting to primary health clinics) instead of the general population, hence overestimating the results.Citation24 This difference was also noted in Jordan, with COPD prevalence being 5.4% (BREATHE study in general population) vs 8.2% in a study done in male smokers by Al Omari et al.Citation21,Citation26
One recent Egyptian study conducted on a high-risk population categorized study subjects based on risk factors for COPD (smokers, occupational exposure, and biomass fuel-combustion exposure), and estimated threefold-higher prevalence of COPD (9.6% vs 3.5% in BREATHE).Citation21,Citation27 Another spirometry-based study in Egypt estimated the prevalence of COPD to be 6.6%.Citation21,Citation28 Similarly, COPD prevalence in Lebanon was 5.3% in a questionnaire-based BREATHE study, while it was 9.7% in another study using postbronchodilator definition of COPD.Citation21,Citation29 It is obvious that spirometry-based studies produce higher prevalence than questionnaire-based studies.
Burden of Lung Disease (BOLD) studies were conducted in the countries in the GCC–MENA region. Based on BOLD protocol-estimated prevalence of COPD in Tunisia, Saudi Arabia, and Morocco of 7.8%, 4.2%, and 12.6%, respectively, BREATHE COPD-prevalence estimates were 3.7%, 2.4%, and 2.2%, respectively.Citation25,Citation30,Citation31 Underreporting of mild symptoms, smoking prevalence (especially in females), criteria for diagnosis of COPD in BREATHE, and differences in methodology could partially account for this difference.
Estimated sex- and age-based prevalence of COPD also showed varied results. All studies reviewed demonstrated either statistically significant increased prevalence in males over femalesCitation21,Citation25,Citation29 or no difference.Citation22,Citation23,Citation28 This inconsistency can be explained by the wide variation in smoking prevalence in females within the region, differences in cultural background, and differences in exposure to other risk factors, eg, biomass fuel. Older age in some studies showed higher prevalence of COPD.Citation22,Citation28,Citation31 GOLD stage II (moderate) COPD was found to have the highest prevalence in a majority of the studies that staged the population.Citation22,Citation31
Discussion
What is common?
There is substantial variation in COPD trends, both globally and regionally. Smoking is the single commonest and most important risk factor for COPD. A comparison was made of the various studies done worldwide with those of the MENA region to assess the relationship between prevalence of smoking and prevalence of COPD. There were differences in the study groups, methodology, and definitions used, as already mentioned.
In 2015, over 1.1 billion people smoked tobacco. Although it is declining worldwide and in many countries, the prevalence of tobacco smoking appears to be increasing in the WHO EMR and the African region.Citation32 The BOLD study (from 14 countries worldwide) estimated smoking prevalence of 57.1% and COPD (stage 2 or higher) of 8.1%.Citation33 Similarly, PLATINO (from five Latin countries) estimated average smoking prevalence was 56.8%, with average COPD prevalence of 9.04%.Citation34
The Continuing to Confront COPD survey (updated version) from 13 countries estimated prevalence of smoking and COPD at 65% and 8.2%, respectively.Citation35 BREATHE (inspired by this survey) estimated smoking prevalence of 31.2% and COPD of 3.6% in the MENA region.Citation21 For the sake of comparison, smoking prevalence is almost double in the West compared to the MENA region, with COPD prevalence following the same trend. Needless to say, this is only a rough estimate to support the previously well-established fact that smoking is the commonest risk factor for COPD, with prevalence increasing proportionally.
What is different?
Comparison of studies
There is concern that the incidence and prevalence of COPD have been underestimated in the region, owing to overreliance on clinical diagnosis, with most of the time diagnoses not being based on spirometry or international guidelines.Citation36
Spirometry vs questionnaire-based
BOLD and PLATINO were both spirometry-based studies. Spirometry-based studies from the MENA region reviewed totaled eleven. The first study ever in the region was that of Al Zaabi et al in 2011, and results were comparable to the largest questionnaire-based BREATHE study in the MENA region.Citation21,Citation22 Three studies were based on the BOLD protocol: Al Ghobain et al in Saudi Arabia,Citation25 Daldoul et al in Tunisia,Citation30 and El Rhazi et al in Morocco.Citation31 The other studies have already been described herein.
Differences in study groups
Within the MENA region, significant variation in study groups can explain differences in COPD prevalence determined from the studies. For example, three studies were done in Saudi Arabia using different methodology, including smoking population, BREATHE study questionnaire, and BOLD protocol, which found COPD prevalence to be 14.2%, 2.4%, and 4.2%, respectively.
Comparison of risk factors
Cigarette smoking is the most important risk factor in the development and progression of COPD. However, a substantial number of COPD cases occur in the absence of smoking, especially among young adults and women and in developing countries. The population-attributable fraction of smoking as a cause of COPD was found to be less than 80% in a review by an ad hoc subcommittee of the American Thoracic Society, which revealed that a substantial burden of disease was attributable to nonsmoking and environmental risk factors.Citation37
Specific genetic syndromes are causally related to the development of COPD. Traffic and other outdoor pollution, secondhand smoke, biomass smoke, and occupational exposure are associated with COPD. Chronic uncontrolled asthma and sequelae of untreated chronic tuberculosis are associated with irreversible changes in lung parenchyma and airway remodeling with loss of lung function, but there is still uncertainty about whether there are important phenotypic differences compared with COPD, as is typically seen in clinical settings.Citation37
Tobacco smoking
As per WHO estimates in 2000, the rate of mortality from smoking in the adult population (age >30 years) was 19% in industrialized countries and 9% in developing countries.Citation38 Khattab et al estimated adjusted smoking prevalence to be 31.2% in the MENA region.Citation39 Highest rates were observed in Jordan, Lebanon, Syria, and Turkey. Significant variation was observed in the MENA region for estimated smoking prevalence between countries, ranging from 15.3% in Morocco to 53.9% in Lebanon.
Sex prevalence for smoking ranged from 29.7% in Morocco to 61% in Turkey in men and 1.4% in Morocco to 47.3% in Lebanon in women. Sex differences could be attributed to women having limited access to public places, religious and cultural beliefs, and social stigma. However, an increasing trend of smoking in women may be seen, due to increased marketing of cigarettes, social status, more professionally active women, and Westernization in the region.Citation39
Smoking in the MENA region (particularly water-pipe smoking) is part of the culture. Lack of rules and regulations to ban smoking in public places contributes to the high prevalence of smoking in the region.Citation40 Water-pipe smoking deserves special mention in this region, as it may increase the risk of COPD, lung carcinoma, and other chronic respiratory problems.Citation41–Citation43 Alzaabi et al screened 62,086 subjects in BREATHE, and found that water-pipe use was reported by 2,173 (3.5%, 95% CI 3.4%–3.6%), of whom 934 (43%) smoked both water pipes and cigarettes. The majority of water-pipe users were men (82%) and aged 40–49 years (53.7%). Over 90% of users smoked their water pipe for ≥1 hour per day.Citation42
The prevalence of water-pipe use is high in the EMR. A national survey in Kuwait showed that 57% of men and 69% of women had used a water pipe at least once. Water-pipe use is also common in Egypt, where 22% of 6,762 men from two rural villages reported current or past use.Citation27 Recent data from the EMR show that substantial numbers of adolescents and young adults are now smoking water pipes. In Syria, for example, about half of university students report having used a water pipe, and about a quarter of males currently use it. The picture is similar in Lebanon, where of 1,964 Beirut university students, 30.6% of men and 23.4% of women reported current, weekly water-pipe use in 2001. Across several EMR countries, about 10%–18% of those aged 13–15 years use tobacco products other than cigarettes, most likely water pipes.Citation41
A systematic review of water-pipe use on health outcomes published in 2016 showed chronic bronchitis and COPD were significantly associated (P<0.05) with water-pipe use.Citation21,Citation43–Citation46 Water-pipe use was significantly (P<0.05) associated with COPD in one study,Citation28 and was a significant (P<0.05) risk for developing COPD in another study.Citation43 A large study in UAE nationals found smoking prevalence to be commonest in younger groups, with an increasing trend in medwakh use among the young.Citation47 Passive smoking was also found to be an important risk factor for COPD in developed and developing countries.Citation37,Citation48
Factors other than smoking
The prevalence of COPD among nonsmokers is estimated to be 3%–15%, further validating the role of other risk factors.Citation33,Citation34,Citation37 A cross-sectional study in Lebanon revealed 3.4% of nonsmokers had COPD (GOLD stage I) and 11.75% chronic bronchitis. Lower prevalence in nonsmokers in the MENA region compared to other areas of the world could be attributed to a younger demographic in developing countries.Citation49 The Tunisian BOLD study estimated a prevalence of 4.7% (GOLD stage I) among nonsmokers (45% of COPD in the study were nonsmokers).Citation50
Outdoor pollution and urbanization
There is strong evidence of an association between outdoor pollution and decreased pulmonary function growth during childhood and adolescence, though there are few studies that have defined COPD by spirometry.Citation51–Citation53 SAPALDIA-cohort analysis examined the association between 11-year change in air quality and lung-function decline among 8,047 adult subjects (4,742 had complete follow-up), with significant decline with higher PM10, a measure of particulate matter in the air.Citation54
The AHSMOG study examined survey-based definitions of chronic bronchitis or emphysema only.Citation55,Citation56 The German SALIA study in women showed higher PM10 was related to an increased risk of COPD (GOLD stage I criteria).Citation57 There is also evidence of biological plausibility, in that exposure to air pollutants, such as particulate matter, O3, and NO2, can produce deleterious effects on the airway, irreversible loss of pulmonary function over time, and COPD.Citation58 A case-control study in Lebanon found a statistically significant association between outdoor pollution and chronic bronchitis. It concluded that living close to a busy road/power plant was linked to chronic bronchitis. Dubai was found to ranked highest in the developed world using a pollutant score on account of two variables: volume of cars and emission from each vehicle.Citation40 Rapid urbanization and motorization are responsible for this situation. COPD prevalence was found to be higher in urban dwellers (60% of all cases) in 2010 in a meta-analysis of WHO world regions.Citation20
Biomass fuel
In developing countries, a significant proportion of COPD cases occur among never- or nonsmokers, especially in women cooking with open-fire stoves. The fuel used in these stoves is collectively known as biomass (wood, animal dung, and crop residue). These stoves emit high levels of toxic air pollutants similar to those present in tobacco smoke, with exposure mostly occurring over the entire life span.Citation59 Approximately half the world’s population still uses solid fuels for cooking, and this biomass-fuel usage is even higher in rural areas (up to 80%). Particulate-matter concentrations greatly exceed most governmental standards for outdoor air.Citation37
Several case-control and cross-sectional studies, mostly from developing countries, have found a consistent direct correlation between cooking with biomass fuel and respiratory symptoms, chronic bronchitis, and chronic airflow obstruction, with evidence of an exposure–response relationship, eg, hours of cooking per day and number of years cooking with biomass.Citation60–Citation78 A study from Colombia found that biomass-stove use for ≥10 years was associated with a greater risk of COPD (GOLD stage I).Citation79 Exposure to woodsmoke (barbeques, cooking stoves) was shown to increase the risk of chronic bronchitis in smokers (current or past) in a study in the US.Citation18 In the MENA region, a study in the UAE showed that among COPD patients, biomass exposure was higher (33%) than other risk factors, including cigarette smoking (12% current, 12% past).Citation22 An Egyptian study showed a COPD (GOLD stage I) prevalence of 11.2% in women exposed to biomass fuel; 86.2% of the total females in the exposed group were nonsmokers.Citation27
Other exposure (occupational, Arabian incense)
Consistent direct associations have been observed between exposure to workplace pollutants and COPD in multiple high-quality epidemiological studies.Citation37 Prevalence of exposure to bakhour is high in the MENA region, particularly the Arabian Gulf. It has been found to be an exacerbating factor in asthma not proven to be associated with COPD.Citation22
Genetic factors
α1-Antitrypsin deficiency (AATD) accounts for 1%–2% of COPD cases, especially in the young.Citation40 There are very limited data available in the region to estimate the prevalence of AATD. One study in Saudi Arabia concluded higher-than-expected prevalence of the AATD allele in the Saudi population. Genetics has an important role to play in the prevalence of COPD, and more studies need to be conducted.Citation80
Asthma and tuberculosis
Many studies have the identified presence of irreversible airway obstruction in asthmatics, including nonsmokers, with evidence that longer asthma duration may lead to more severe chronic airway obstruction, due to accelerated loss of pulmonary function.Citation81–Citation84 Because airway obstruction and remodeling can lead directly to COPD, it is likely that asthma, with or without additional risk factors, can later develop into COPD. It remains uncertain, however, whether asthma patients with irreversible airway obstruction are phenotypically and pathologically similar to “typical” COPD patients or if they represent a separate subset of patients. There is limited but suggestive evidence of an association between tuberculosis and chronic airflow obstruction. The evidence is inadequate to show a causal relationship between tuberculosis and COPD worldwide and in the MENA region.
Sex
Across all WHO regions, COPD is more prevalent in men (14.3%) than women (7.6%), suggesting a higher-risk profile among men, with similar results seen in the MENA region: men (5.2%) vs women (1.8%).Citation20,Citation21 Recently, with increased tobacco consumption among women and higher risk of exposure to indoor pollution (biomass fuel used for cooking and heating) in low- and middle-income countries, sex differences in the disease may be reduced.Citation85 For example, in the revised Continuing to Confront COPD survey of North America and Europe done in 2013, women (7.1%) had a higher prevalence of COPD than men (6.2%) in the US only. The other countries showed similar patterns, with higher prevalence in men.Citation35
Burden of COPD in terms of cost and health care-resource consumption
COPD causes burdens in terms of mortality, morbidity, high health care costs, disability, and impaired quality of life.Citation12 The European Union (EU) reported that the direct cost from COPD was over €38.6 billion in 2005, representing about 3% of total health care expenditure.Citation86,Citation87 In 2010, the US government spent nearly $49.9 billion on COPD, including $29.5 billion spent on direct health care compared to $38 billion spent in 2005, with direct health care costs being $22 billion. Direct costs per patient in 2005 were $2,700–$5,900.Citation88,Citation89
The burden in low- and middle-income countries has been comparatively high, owing to relatively low COPD awareness, challenges with COPD diagnosis, and increased exposure to additional risk factors, like combustion of biomass fuel.Citation90 With increasing smoking prevalence in the MENA region, especially in the young, and increased life expectancy, the burden of resource consumption of COPD is likely to increase. The results of the BREATHE study were as follows (monetary quantification was not possible, due to wide variation in the region in terms of structure of health care provision).
Physician consultation was the most frequent cause of health care-resource use, followed by emergency visits and hospitalizations, with similar hierarchy seen in developed countries.Citation91–Citation96
Health care consumption showed proportional increase with GOLD severity.
Frequency of symptoms, exacerbations, and higher COPD-assessment test (CAT) scores resulted in higher burden in terms of resource consumption. Group D in CAT had the highest expenditure, with a sixfold increase in hospitalization and emergency visits. Exacerbation of COPD was the major reason for physician consultation, hospitalization, and emergency visits. This puts emphasis on having individualized approaches in high-risk patients to optimize management of COPD and prevent exacerbations.
The presence of comorbidities (mainly cardiovascular and diabetes) showed increased hospitalizations (P<0.0001) and hence increased consumption of resources, consistent with other studies.Citation97,Citation98
Variation was observed within the MENA region, which could be explained by differences in health care systems, eg, availability of health care facilities to the general population and reimbursement processes.Citation99
Diagnosis and assessment of COPD
The diagnosis of COPD should be considered in any patient who is complaining of dyspnea, cough, and sputum production, and who has a history of exposure to risk factors.Citation100,Citation101 To confirm the diagnosis, spirometry should be performed to detect the presence of airflow limitation.Citation102
Clinical presentation
Characteristic symptoms of COPD
Dyspnea
This is simply described as air hunger or increased effort to breathe. It is usually chronic, progressive, and increases with exertion.Citation103 Dyspnea is the most characteristic symptom of COPD, and it represents a major cause of disability and anxiety associated with COPD.
Cough
Chronic cough is the first COPD symptom to develop, initially intermittent, then it becomes every day. It may be productive or unproductive.Citation104
Sputum production
Commonly, small amounts of tenacious sputum are associated with coughing. The term “chronic bronchitis” refers to regular sputum production for ≥3 months in 2 consecutive years with an absence of other conditions that could explain it.Citation105
Wheezing and chest tightness
Wheezes and chest tightness are aspecific symptoms that can be variably present in COPD patients.
Other symptoms
Severe COPD may present with symptoms of cor pulmonale (ankle swelling), fatigue, weight loss, anorexia, anxiety, and depression.Citation106 These symptoms have an important prognostic value,Citation107 and can also be an indicator for other diseases (eg, tuberculosis, lung cancer), and thus should always be investigated.
Medical history
A detailed medical history is required in order to assess:
exposure to risk factors (smoking, occupational or environmental exposures);
asthma, allergy, sinusitis, or nasal polyps; respiratory infections in childhood; other respiratory diseases;
family history of smoking, COPD, or other chronic respiratory disease;
pattern of symptom development (COPD typically develops in adult life, and most patients develop progressive dyspnea over years, with chronic cough and sputum production);
history of exacerbations or previous hospitalizations for respiratory disorder;
presence of comorbidities, such as heart disease, osteoporosis, musculoskeletal disorders, and malignancies that may also contribute to limitation of activity;Citation108
impact of the disease on the patient’s life, including social and economic impact.
Physical examination
Physical signs as expiratory and inspiratory wheezes, coarse crepitations, and signs of hyperinflation may be present if lung function is significantly impaired. The absence of physical signs does not exclude the diagnosis.Citation109,Citation110
Spirometry
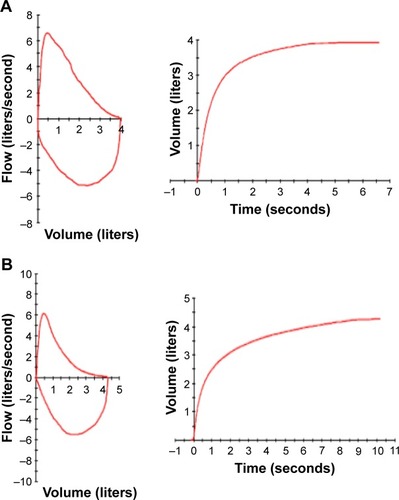
Spirometry is considered the most objective and reproducible test to detect airflow limitation. The test measures the volume of air forcibly exhaled from the point of maximal inspiration (FVC), the volume of air exhaled during the first second of this maneuver (FEV1), and the ratio of these two measurements (FEV1:FVC). Measurements are compared to reference values based on age, sex, height, and race.Citation111 Spirometry of a normal subject is shown in , and that of a COPD patient showing an obstructive pattern in . An obstructive pattern is defined by FEV1:FVC ratio below the fifth percentile of a predicted value, and the shape of the flow–volume curve is concave.
Assessment of COPD
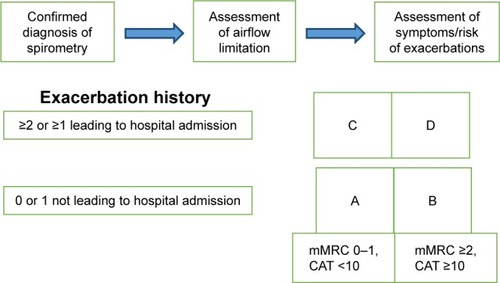
COPD is a complex disease, and hence the objectives of COPD assessment are to determine the severity of disease, risk of exacerbations, and hospital admissions, and its impact on the patient’s health status, quality of life, and risk of death. Knowledge of the previous combined parameters will guide optimum individualized therapy. The following aspects must be considered in COPD assessment: patient symptoms, spirometric abnormality, exacerbation risk, and presence/absence of comorbidities ().
Table 1 Classification of severity of airflow obstruction in COPD (based on postbronchodilator FEV1) in patients with FEV1/FVC <0.7
Assessment of symptoms
A simple measure of breathlessness using the modified Medical Research Council (mMRC) dyspnea scale was previously considered adequate for assessment of symptoms.Citation112,Citation113 However, since COPD has multiple symptomatic effects,Citation114 it is recommended to use more sophisticated tests with comprehensive symptom assessment. Questionnaires like the CAT (http://www.catestonline.org) and clinical COPD questionnaire (CCQ; http://www.ccq.nl) are practical and suitable for this purpose. However, because use of the mMRC is still widespread, an mMRC score ≥2 is still included as a cutoff point for separating “less breathlessness” from “more breathlessness”.Citation115,Citation116
CAT
This is an eight-item unidimensional measure of health-status impairment in COPD.Citation117 It was developed to be applied worldwide, and validated translations are available in many languages. The score ranges from 0 to 40, and CAT scores correlate very closely with the St George’s Respiratory Questionnaire.Citation118
CCQ
This is a ten-item self-administered subjective questionnaire developed to measure clinical control in patients with COPD.Citation119,Citation120 Although the concept of “control” in COPD remains controversial, the CCQ is short and easy to administer. It is reliable, available in many languages, and has been well validated.
Assessment of airflow limitation
Spirometry should be performed using techniques that meet recommended published standards after the administration of an adequate dose of a short-acting inhaled bronchodilator.Citation121 Spirometric measurements should be evaluated by comparison of the results with appropriate reference values based on standard demographic nomograms. The presence of postbronchodilator FEV1/FVC <0.7 confirms airflow obstruction in the patient.
Assessment of exacerbation risk
An exacerbation of COPD is defined as an acute worsening of underlying respiratory symptoms that result in requirement of additional therapy.Citation122 The best predictor of frequent exacerbations (two or more exacerbations per year or one or more leading to hospital admission) is the presence of frequent exacerbations in the past. Low frequency is defined as one (or zero) exacerbation per year or no hospital admission.Citation123 The risk of exacerbations increases significantly in severe and very severe disease. COPD exacerbations are associated with deterioration in health status, increased decline in lung function, and increased risk of death.Citation124
Assessment of comorbidities
Other diseases related to smoking and aging may coexist with COPD,Citation125 and COPD itself may lead to multiple systemic effects, including but not limited to cardiovascular disease, skeletal muscle dysfunction, metabolic syndrome, osteoporosis, depression, and lung cancer.Citation126,Citation127 Comorbidities should be looked for routinely and treated appropriately in any patient with COPD.
Combined COPD assessment
The combined assessment of COPD helps to categorize patients into one of four groups (), which helps guide therapy. To classify patients into a specific group, we perform the following steps.
Symptom assessment
If the patient has fewer symptoms (CAT <10) or less breathlessness (mMRC grade 0–1), then he/she belongs in the boxes on the left. If the patient has more symptoms (CAT ≥10) and/or more breathlessness (mMRC grade ≥2); then they belong in the boxes on the right.
Risk-of-exacerbation assessment
To detect if the patient has high or low risk, we choose the higher risk of the following three methods:
Assess the number of exacerbations the patient has had within the previous 12 months (zero or one indicate low risk [lower part of box], while two or more exacerbations indicate high risk [upper part of box]).
Determine whether the patient has had one or more hospitalization in the previous year for a COPD exacerbation, indicating high risk (upper part of box).
The latest GOLD 2017 document refines the ABCD assessment tool by separating spirometry grades from the “ABCD” groups.
Further clinical phenotyping
The Spanish guidelines for COPD (GesEPOC) recognize the importance of characterizing patients by clinical phenotype, helping to customize treatment according to the characteristics of each patient, and identifying clinical phenotypes that may help to identify group of patients with different mortality and treatment responses.Citation128–Citation130 GesEPOC identify four phenotypes, and these are of value in deciding which anti-inflammatory treatments should be given to patients: nonexacerbators, mixed COPD (asthma phenotype), exacerbators with chronic bronchitis phenotype, and exacerbators with emphysema phenotype.
Asthma–COPD overlap syndrome (ACOS)
Those patients who are characterized by persistent airflow limitation with several features, usually associated with asthma and several features usually associated with COPD. Asthma–COPD overlap syndrome (ACOS) is therefore identified in clinical practice by the features that it shares with both asthma and COPD.Citation131 Recently suggested criteria for the diagnosis of ACOS follow ().
Table 2 Criteria for diagnosis of asthma–COPD overlap syndrome
Role of eosinophils
The prevalence of eosinophilic inflammation in COPD patients is unknown. We do not know whether patients with sputum or blood eosinophilia represent a stable COPD phenotype over time. Prospective clinical trials are required to determine a cutoff threshold for blood eosinophils that predicts future exacerbation risk in COPD patients with an exacerbation history and to clarify the blood-eosinophil cutoff value that can be used in clinical practice. The most commonly reported value in the literature is 2%; however, there have been many other cutoff values reported.Citation132 The ACOS committee generally felt that a 2% threshold lacked sufficient specificity to diagnose eosinophilic airway inflammation. Until more evidence is available, the committee endorses a higher threshold of peripheral blood-eosinophil count of ≥300 cells/μL to increase the specificity of the biomarkers to diagnose eosinophilic airway inflammation as a minor criterion for ACOS.Citation133,Citation134 It has been found that many physicians in GCC–MENA follow the clinical phenotype approach of COPD treatment, and there is evidence that this approach has the potential to reduce the risk of exacerbation and assessment of different medium- and long-term mortality; however, large multicenter trials are needed to validate the evidence fully.
Additional investigations
Imaging
Radiological signs that may suggest COPD include signs of lung hyperinflation (flattened diaphragm and increase in retrosternal airspace volume), hyperlucency of the lungs, and rapid tapering of vascular markings (). However, radiological assessment in COPD patients, either chest X-ray or computed tomography (CT), may be helpful to exclude other diagnoses or to detect associated comorbidities (pulmonary fibrosis, pleural diseases, and cardiomegaly). Chest CT scans are recommended prior to lung-volume-reduction surgery in emphysema.Citation135
Table 3 Differential diagnosis of COPD
Lung volume and diffusion capacity
Body plethysmography may document increased residual volume and increased total lung capacity, which indicates air trapping and pulmonary hyperinflation. Measurement of lung CO-diffusion capacity provides information on the functional impact of emphysema in COPD.
Pulse oximetry and arterial blood-gas measurement
Pulse oximetry should be used to assess all stable patients with FEV1 <35% predicted, clinical signs suggestive of respiratory failure, or right-side heart failure. If peripheral saturation is <92%, arterial blood gases should be done and action taken accordingly.Citation136
Screening for α1-antitrypsin deficiency
Younger patients (<45 years) with lower-lobe emphysema should be screened. A serum concentration of AAT below 15%–20% of the normal value is highly suggestive of homozygous AATD.
Exercise testing
Objectively measured exercise impairment assessed by a reduction in self-paced walking distanceCitation137 or during incremental exercise testing in a laboratoryCitation138 is a powerful indicator of health-status impairment and predictor of prognosis.Citation139 Walking tests can be useful for assessing disability, and are used to assess the effectiveness of pulmonary rehabilitation.
Composite scores
Several variables may be combined in a composite score to try to identify disease severity. One of these scores is the BODE (body-mass index, obstruction, dyspnea, exercise) index, which has been found to be a better predictor of subsequent survival than any component alone.Citation140
Pharmacological management of COPD
The GOLD 2017 management strategy recommends a combined assessment of COPD based on symptoms and exacerbations to categorize patients into clinical phenotypes (groups A–D). These groups have distinct pharmacological treatment recommendations covering initial management and a stepwise approach to follow-up treatment. We have used the principles of GOLD 2017 to make pharmacological treatment recommendations using a format that will hopefully be easy to follow for the practicing clinician.
GOLD recognizes that some of its pharmacological treatment algorithms are only partly based on evidence. Most of our recommendations are similar to GOLD, but we highlight recommendations that differ and key areas where more evidence is needed. The evidence for the various pharmacological treatments for COPD is firstly summarized, followed by recommendations for initial and follow-up treatment.
Clinical evidence for pharmacological treatment
Long-acting muscarinic antagonists (LAMAs) improve symptoms, lung function, and exercise performance and reduce exacerbation rates.Citation141,Citation142 Long-acting β2-agonists (LABAs) also address these parameters, but have less effect on exacerbations than LAMAs.Citation143–Citation145 Long-acting bronchodilators provide a convenient treatment option, with prolonged duration of action compared to short-acting bronchodilators.
Dual-bronchodilator combination inhalers containing both a LAMA and LABA have a greater effect on lung function and symptoms than long-acting bronchodilator monotherapies.Citation146 LAMA–LABA combinations have a greater impact on exacerbations than long-acting bronchodilator monotherapies,Citation147 although only one study has been performed in COPD patients with a history of exacerbations.Citation148 Inhaled corticosteroid (ICS)–LABA combinations improve lung function and symptoms, and several clinical trials in COPD patients with exacerbation history have shown an effect on exacerbation prevention: recent studies have shown an approximately 25% exacerbation-rate reduction due to the ICS component.Citation143,Citation149,Citation150 Long-term use of ICS is associated with side effects, including pneumonia,Citation143,Citation149,Citation150 bone fractures,Citation151 cataracts,Citation152 diabetes,Citation153 and mycobacterial infection.Citation154 Pneumonia has been highlighted as a clinical concern, but the rate of these events is low and appears to be greater in certain subgroups, such as older patients and those with low body-mass index, low FEV1, and a history of pneumonia.Citation155,Citation156 Overall, the benefits of ICS–LABA combinations on exacerbation prevention outweigh the risks.
One clinical trial (the FLAME study) has compared ICS–LABA with LABA–LAMA in COPD patients with an exacerbation history. There was a greater impact of LABA–LAMA on exacerbations.Citation157 More studies are needed to confirm this finding in wider patient populations, and with different drugs within these classes.
Triple therapy refers to the combination of ICS plus LABA plus LAMA, either in a single combination or as separate inhalers. Triple therapy improves lung function and symptoms and reduces exacerbations compared to LAMA monotherapy or ICS–LABA treatment.Citation158,Citation159 There are currently insufficient data on the comparison of triple therapy and LAMA–LABA treatment.
Roflumilast (AstraZeneca, Cambridge, UK) is a PDE4 inhibitor that has anti-inflammatory effects, reducing exacerbations in COPD patients with FEV1 <50%, a history of exacerbations, and chronic bronchitis.Citation160 Long-term macrolide-antibiotic use reduces exacerbation rates, but it is unclear whether this is due to antibacterial or anti-inflammatory activity.Citation161 There are risks of increased bacterial resistance with long-term macrolide use, and the clinical phenotype of patients most likely to respond has not been conclusively described. The evidence for the use of mucolytics to prevent exacerbations is inconsistent.
Initial pharmacological treatment
COPD patients at initial diagnosis should be categorized as either “frequent exacerbators”, suffering two or more exacerbations (requiring oral steroids and/or antibiotics) or one hospitalization in the last year, or those with low exacerbation frequency (“infrequent exacerbators”), experiencing one (or zero) exacerbation in the previous year. Symptoms should be assessed using mMRC score or CAT score. Initial pharmacological treatment is described in , and key points are summarized in .
Table 4 Key principles of initial pharmacological management
Figure 3 Initial pharmacological treatment for new patient.
Abbreviations: mMRC, modified Medical Research Council (dyspnea scale); CAT, COPD-assessment test; LAMA, long-acting muscarinic antagonist; LABA, long-acting β2-agonist; ICS, inhaled corticosteroid; ACOS, asthma–COPD overlap syndrome.
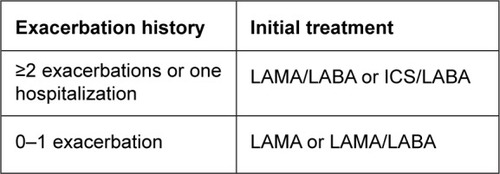
Infrequent exacerbators
COPD patients with low exacerbation frequency should initially receive long-acting bronchodilator treatment without the need for ICS. Patients should be started on a LAMA. Some patients with a very high degree of symptoms at initial presentation can be started with a LAMA–LABA combination inhaler, although the exact clinical characteristics favoring this approach have not been defined.
Frequent exacerbators
Frequent exacerbators should commence on either a LAMA–LABA or an ICS–LABA combination, both of which address symptoms and exacerbations. GOLD also proposes these options, but favors LAMA–LABA, due to the risk of pneumonia associated with ICS and the results of the FLAME trial. We do not follow this approach favoring LAMA–LABA, as the number of clinical trials showing that different ICS–LABA combinations reduce exacerbations far outweigh the number of exacerbation trials with LAMA–LABA combinations, and the results of FLAME require confirmation. Furthermore, many COPD patients have very low pneumonia risk.
GOLD advocates an individualized approach to COPD treatment, and identifies characteristics that favor ICS–LABA treatment response, such as the presence of asthma features or higher blood-eosinophil counts. We recommend an individual approach to decide the most appropriate treatment (ICS–LABA or LAMA–LABA), based on factors already described that predict ICS benefit or risk.
COPD patients with a low symptom burden (CAT score <10 or mMRC score 0–1) may be managed initially with a lower level of treatment than already described. For infrequent exacerbators, a short-acting BA and/or short-acting MA can be used initially, although often such patients need to step up to long-acting bronchodilator treatment. Frequent exacerbators with a low symptom burden may be managed initially with a LAMA.
Pharmacological treatment at follow-up
At follow-up, escalation of treatment should be based on whether the patient requires further treatment for symptoms only, or symptoms and exacerbations; it is uncommon for patients to suffer exacerbations without the need to treat symptoms. Follow-up pharmacological treatment is described in , and key points are summarized in .
Table 5 Key principles of follow-up pharmacological management
Figure 4 Follow-up treatment of COPD patients.
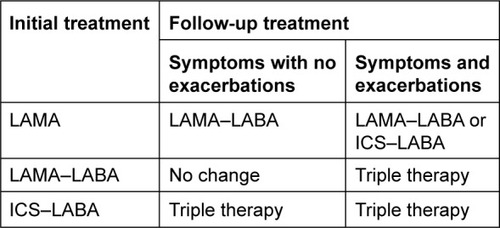
Treatment of symptoms only
The need to treat symptoms further should be based on an individual assessment of dyspnea, exercise capacity, and quality of life. Follow-up treatment of symptoms alone (without exacerbations) may require the addition of further long-acting bronchodilator treatment; there is no need to add an ICS. Patients treated with LAMA monotherapy can be escalated to a LAMA–LABA combination, while those treated with ICS–LABA can be escalated to triple therapy (ICS–LABA–LAMA). For patients with significant symptoms despite LAMA–LABA treatment, nonpharmacological approaches should be readdressed (eg, pulmonary rehabilitation, check for comorbidities), as well as checking inhaler technique and compliance.
Treatment of symptoms and exacerbations
Clinical trials have shown a benefit for various combination treatments (ie, triple therapy, ICS–LABA, and LABA–LAMA) in patients with a history of one or more exacerbations in the last year.Citation8–Citation10,Citation17,Citation19 The natural history of exacerbations is that a proportion of patients with one exacerbation in the previous year will experience none the following year.Citation162 We recommend that escalation of treatment is appropriate for patients with one exacerbation/year for each of the previous 2 years or two or more exacerbations (or one hospitalization) in the previous year.
Patients suffering with symptoms and exacerbations at follow-up can receive either an additional bronchodilator or ICS treatment. Patients treated with a LAMA can be escalated to a LAMA–LABA combination or switched to an ICS–LABA combination. Patients receiving ICS–LABA or LAMA–LABA can be escalated to triple therapy or treatment can be switched from one of these combinations to the other. Switching treatment is recommended in situations where the previous pharmacological treatment provided no or little clinical benefit. For patients established on triple therapy and still suffering with exacerbations, individual clinical characteristics can be used to decide to add further treatment: chronic bronchitis (add roflumilast or mucolytics as an alternative), evidence of bacterial infection or bronchiectasis (add azithromycin [Zithromax®; Pfizer, New York, NY, USA]).
Stepping down treatment
There are some situations in clinical practice where a step down in treatment should be considered. These are where an ICS–LABA combination has been inappropriately prescribed to a patient suffering with symptoms who has never experienced an exacerbation, for whom ICS withdrawal should be considered, and where the treatment of a patient has been escalated, but there has been either no or little clinical benefit, in which case a step down or switching of treatment should be considered.
Differences between drugs of the same class
The recommendations provided assume that the drugs within each class have similar effects. For the main drug classes recommended (LAMA, ICS–LABA, and LAMA–LABA), clinical trial data show generally similar effects of drugs (within a class) in terms of clinical efficacy, although direct comparisons in the same study are often lacking. On a practical level, the major differences between drugs within a class are the inhaler device and the need for once- or twice-daily delivery. It is important to check that the patient can use the inhaler device selected, and that the dosing regime is appropriate for the patient. Twice-daily dosing may be more suitable for patients with nighttime/early-morning symptoms, although an advantage for twice-daily vs once-daily dosing in this regard has not been definitively proven.
Blood eosinophils
Post hoc analysis of clinical trials in COPD patients with an exacerbation history has demonstrated that higher blood-eosinophil counts predict an increased effect of ICS on exacerbation-rate reduction.Citation163,Citation164 This observation has now been confirmed in a prospective randomized controlled trial (Vestbo et al, unpublished data, 2017). The blood-eosinophil cutoff values that should be used in clinical practice to predict a beneficial effect of ICS have yet to be defined.
Nonpharmacological management of COPD
As discussed earlier and in view of COPD being a systemic disease, comprehensive management of COPD includes nonpharmacological management incorporating various modalities to support already-declined lung functions, improve quality of life, and prevent morbidity and mortality consequent to COPD.
Self-management education
Education is an important component of the nonpharmacological aspect of COPD. Topics covered in education programs include smoking cessation, detailed knowledge about COPD, general and specific approaches to therapy and other aspects of COPD, strategies to minimize symptoms, primarily dyspnea, and initial management of exacerbations.Citation1 It is important to recognize that patient education alone does not change behavior or motivate patients, but if done appropriately can increase ability to cope with the stress of illness and improve health status. Self-help groups and group discussion may also help in encouraging COPD patients to follow a healthy lifestyle.
Pulmonary rehabilitation
GOLD 2017 guidelines stress that pulmonary rehabilitation should be a keystone in the management plan of COPD patients, underscoring its importance and effectiveness. Pulmonary rehabilitation is defined as a comprehensive intervention based on a thorough assessment of the COPD patient and delivering such interventions as exercise, behavior, and education to improve the quality of life of COPD patients.
The American Thoracic Society – European Respiratory Society joint statement states that all symptomatic patients, particularly those with systemic manifestations of COPD, benefit from rehabilitation, while the GOLD 2017 statement recommends it for patients with high symptoms burden and risk of exacerbation (groups B–D). These patients should be encouraged to participate in well-structured rehabilitation programs, taking into account individual patients’ characteristics and comorbidities.Citation165,Citation166 Patients planned for lung-volume-reduction surgery should also be included both preoperatively and postoperatively.Citation167 Evidence-based best practice for rehabilitation programs includes structured and supervised exercise training, smoking cessation, nutrition therapy, and self-management skills.
Adherence and cooperation are needed for rehabilitation programs, and patients who have active heart disease or are limited due to joint or orthopedic illness may be unable to join a program. Should active smokers be participating in such programs, whether they are motivated to adhere continues to be a field of investigation. Clearly, degree of FEV1 is not a contraindication, as the American College of Chest Physicians recommends rehabilitation for those with FEV1 <50%.Citation168 Pulmonary rehabilitation benefits include improving quality of life, body strength, and exercise tolerance. It also reduces exacerbation rate, and this in turn will affect the health care burden of COPD. However, the most interesting and debatable issue is rehabilitation’s impact on mortality, a question not resolved, yet with some indication that pulmonary rehabilitation may improve COPD mortality.Citation169
Exercise training
A combination of constant-load or interval training with strength training of upper- and lower-extremity muscle groups and goals of endurance training to 60%–80% of symptom-limited maximum work or heart rate is preferred,Citation170,Citation171 or to Borg dyspnea or fatigue score of 4–6.Citation172 Exercise training should be done after full optimization of LAMA–LABA therapy.
Smoking cessation
First and foremost in nonpharmacological management of COPD is that it includes smoking cessation. Data are currently clear that stopping smoking is important, as it decreases or halts decline in FEV1. In fact, the Lung Health Study revealed an improvement in lung function within a year or so of quitting smoking, and such data also push physicians to help patients to stop rather than reduce cigarette-smoke intake.Citation173 A Finnish study has shown a survival benefit among those who stop smoking, regardless of COPD stage.Citation174 Clearly, most attention has been focused on nicotine-replacement therapy, particularly combination-delivery systems (nicotine patch and nasal spray most preferred). There is also nicotine gum, lozenges, and inhalers. Research has shown nonpharmacologic approaches, such as behavioral counseling or hypnosis, especially when combined with nicotine patches, to be rewarding.Citation175
Nonnicotine therapy may include bupropion (Impax Laboratories, Inc., Hayward, CA, USA) and venlafaxine (Pfizer), which help in increasing quit rates. Electronic cigarettes’ role in smoking cessation is uncertain, and they are not US Food and Drug Administration-approved. A few studies have formally evaluated nicotine craving when using electronic cigarettes, with mixed results. Although patients support the use of electronic cigarettes in smoking cessation, more structured studies on safety and efficacy should be done to determine whether these products can play a role in smoking cessation.Citation176
Vaccination
Influenza vaccination is recommended for all patients with COPD. The pneumococcal vaccines PCV13 and PPSV23 are recommended for all patients >65 years of age. PPSV23 is also recommended for younger COPD patients with significant comorbidities, including heart and lung disease.Citation177
Nutritional support
Nutritional supplementation is recommended for malnourished COPD patients. There are positive effects on body weight, fat mass, and fat-free mass when nutritional supplementation is provided and when used during exercise training, and demonstrated significant improvements in 6-minute walk test, respiratory muscle strength, and health status.Citation178
Oxygen therapy
Long-term oxygen therapy is indicated for stable patients who have PaO2 ≤55 mmHg or SaO2 ≤88% with or without hypercapnia confirmed twice over a 3-weeks period, or those with PaO2 of 55–60 mmHg or SaO2 of 88%, evidence of pulmonary artery hypertension, and peripheral edema suggesting of congestive heart failure or polycythemia. Patients should be reevaluated after 2–3 months, with repeat arterial blood gas or SaO2 test done to assess need for continued oxygen therapy.
Ventilatory support
Long-term or nocturnal noninvasive ventilation may be considered in selected COPD patients, especially those with pronounced daytime hypercapnia and history of recurrent/recent hospitalization, although review data are not sufficient to support or refute this recommendation.Citation179
Interventional pulmonology/endoscopic lung-volume reduction
In selected COPD patients with heterogeneous emphysema and significant overinflation refractory to optimized medical treatment, endoscopic lung-volume reduction by placement of endobronchial valves and coils could be considered after thorough evaluation of the individual patient and at highly specialized centers.Citation180
Surgical intervention
In carefully selected COPD patients with large bulla with compressive effects or risk of spontaneous rupture surgical, bullectomy may be considered to relive the compressive effects of bullae. Some thoracic surgery centers in the GCC region are doing such surgeries after complete evaluation. Lung-volume-reduction surgery is another option in selected COPD patients. However, these modalities should be considered only after detailed assessment of patients, including extent and pattern of emphysema on high-resolution CT scan, presence of fissure integrity, absent collateral ventilation, and centers with high expertise.
Lung transplant
This is an option for very severe advanced COPD refractory to maximal pharmacological and nonpharmacological therapy, and following are the criteria for considering lung transplant: COPD patient with progressive disease, not a candidate for endoscopic or surgical lung-volume reduction, BODE index score of 5–6, PaCO2 >50 mmHg and/or PaO2 <60 mmHg, and FEV1 <25% predicted.Citation181 Other variables to be considered include more than three severe exacerbations in last year, exacerbation with hypercapnic respiratory failure, and pulmonary artery hypertension.Citation182
Joint committee recommendations and suggestions to curtail COPD in GCC–MENA region
Accurate information about the prevalence and types of tobacco use is essential to deliver effective public health policy. Implementation of effective public health strategies, including education and awareness of the general public, strict health regulations aimed at smoking reduction, and managing and preventing COPD, is very important. The main obstacle to this is the lack of data related to prevalence and burden of COPD with consequent prohibition in governmental health-planning strategies to curb COPD incidence and prevalence. There is a great deal of room for improvement in COPD care in the GCC–MENA region, and current trends suggest the following developments are possible and highly desirable.
More accurate data on illness, exacerbations, natural history, cost and deaths related to COPD, particularly in areas where no data are available, will provide a stronger foundation for fighting against COPD.
Studies of the effectiveness of current prevention, education, medication, rehabilitation, and terminal care will help to spread best practice and drive higher standards of COPD care.
New therapeutic modalities will inhibit the decline in lung function and improve the overall quality of life of COPD patients.
As smoking remains the key risk factor for COPD, measures that will reduce the burden of disease include more effective smoking-cessation interventions and techniques to encourage people to quit smoking, strict surveillance of harmful occupational exposure, and protection in early childhood against harmful exposure and events that affect the lung.
Health ministries and the general public need to be made aware of the high burden of COPD in the MENA region, and these countries should implement common strategies for effective prevention, diagnosis, and treatment of this disabling and life-threatening disease.
Research needs
Comprehensive research is needed in key areas related to COPD:
As smoking rates in UAE are increasing along with the rise in other risk factors for COPD, with resultant increased COPD incidence and prevalence, there is a need to know how this will affect the course, management, and prognosis of the disease, along with effects on health care systems.
Although spirometry is a prerequisite in COPD studies, more extensive characterization of the disease than that offered by spirometry is required. Novel imaging techniques and biomarkers offer the potential to characterize subgroups or phenotypes of COPD. Cohort studies should be conducted to assess the long-term natural history of COPD and its phenotypes.
No data are available about the prevalence, incidence, or natural history of various phenotypes of COPD, and their economic burden on the UAE (studies under progress, personal communication).
Our knowledge of the pathogenesis of COPD and how this can be modified is still limited. Novel molecular and genetic techniques offer promising possibilities for gaining important knowledge on disease mechanisms, which opens up possibilities for development of new drugs.
Suggestions to health authorities
Strict policies for control of sale of smoking products, prohibition of smoking near schools and public places, imposition of large penalties for violators, and increased tax prices on tobacco and related products should be implemented. Tobacco merchandisers should be obliged to distribute packages displaying graphics stating the negative effects of the smoking habit. However, it is not clear whether these measures would really cut down the prevalence of smoking and COPD morbidity and mortality.
Conclusion
A comprehensive assessment is required for the accurate diagnosis of COPD, assessment of symptoms, and risk of exacerbation. Phenotypic classification may further help. In the MENA region, studies are under way to assess the phenotypic variants of COPD and to correlate various aspects of COPD with phenotypic evaluation, which would give us better insight into the pathogenesis of COPD in the coming years. In the MENA region, most pulmonary physicians follow the standard GOLD recommendations; however with the recent availability of LAMA–LABA/ICS–LABA once daily, triple-combination LAMA–LABA–ICS, newer inhaler devices, and many pharmaceutical companies providing affordable inhalers with good efficacy, we hope that in future pharmacological management of COPD will be more in accordance with GOLD 2017.
In the context of nonpharmacologic management of COPD in the MENA region, there are many shortcomings: most patients are underdiagnosed/undertreated, are diagnosed by general practitioners (GPs) or internal medicine doctors, the unavailability of spirometry for confirmation of diagnosis, noncompliance among patients, low socioeconomic-class patients and patients from various sociodemographic backgrounds with different attitudes about disease and its management, absence of well-structured pulmonary rehabilitation programs, lack of dedicated/trained staff for delivering standard rehabilitation programs in most hospitals, and unaffordability of patients for noninvasive ventilation and oxygen machines.
In the last decade, there have been tremendous improvements in the care of COPD patients in the GCC region, but still the facilities for interventional pulmonology are in their infancy. In the near future, we anticipate some new upcoming state-of-the-art interventional pulmonology centers that will provide endoscopic lung-volume reduction for selected COPD patients. In the GCC region, few centers are doing surgical lung-volume reduction or bullectomy. These centers could broaden the scope of services to cater to increasing number of COPD patients who need surgical interventions. Governments should provide adequate professional training and facilities for the developments of such centers (interventional pulmonology/thoracic surgery and lung-transplant centers) in the GCC region.
Studies are ongoing to evaluate the harmful chemical constituents of shisha and medwakh, and to determine their causal association with COPD. Considering the increased awareness among patients and physicians over last decade, we hope that the nonpharmacological management of COPD will improve on a par with standard evidence-based practice done in other developed countries.
Acknowledgments
The joint committee of the GCC and the MENA region for COPD would like to convey its sincerest thanks for the support from AstraZeneca, Novartis, and Boehringer Ingelheim.
Disclosure
The authors report no conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease2017 Available from: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copdAccessed January 12, 2017
- MurrayCJLopezADMathersCDSteinCThe Global Burden of Disease 2000 project: aims, methods and data sources2001 Available from: http://www.who.int/healthinfo/paper36.pdfAccessed January 22, 2017
- World Health OrganizationThe Global Burden of Disease: 2004 UpdateGenevaWHO2008
- DevineJFChronic obstructive pulmonary disease: an overviewAm Health Drug Benefits200813442
- World Health OrganizationRisk factors for chronic respiratory diseasesBousquetJKhaltaevNGlobal Surveillance, Prevention and Control of Chronic Respiratory Diseases: A Comprehensive ApproachGenevaWHO20073755
- KhattabAJavaidAIraqiGSmoking habits in the Middle East and North Africa: results of the BREATHE studyRespir Med2012106Suppl 2S16S2423290700
- RiachyMRehayemCKhouryCAre narghile smokers different from cigarette smokers?Rev Mal Respir200825313318 French18449097
- NeergaardJSinghPJobJMontgomerySWaterpipe smoking and nicotine exposure: a review of the current evidenceNicotine Tob Res2007998799417943617
- MaziakWEissenbergTWardKDPatterns of waterpipe use and dependence: implications for intervention developmentPharmacol Biochem Behav20058017317915652393
- LindbergAErikssonBLarssonLGRönmarkESandströmTLundbäckBSeven-year cumulative incidence of COPD in an age-stratified general population sampleChest200612987988516608933
- KanHHeissGRoseKMWhitselELurmannFLondonSJTraffic exposure and lung function in adults: the Atherosclerosis Risk in Communities studyThorax20076287387917442705
- PolatliMKhederABWaliSChronic obstructive pulmonary disease and associated healthcare resource consumption in the Middle East and North Africa: the BREATHE studyRespir Med2012106Suppl 2S75S8523290706
- YinPJiangCQChengKKPassive smoking exposure and risk of COPD among adults in China: the Guangzhou Biobank Cohort studyLancet200737075175717765524
- EisnerMDBalmesJKatzPPTrupinLYelinEHBlancPDLifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary diseaseEnviron Health20054715890079
- HuGZhouYTianJRisk of COPD from exposure to biomass smoke: a metaanalysisChest2010138203120139228
- LinTCKrishnaswamyGChiDSIncense smoke: clinical, structural and molecular effects on airway diseaseClin Mol Allergy20086318439280
- CohenRSextonKGYeattsKBHazard assessment of United Arab Emirates (UAE) incense smokeSci Total Environ2013458–460176186
- El HasnaouiARashidNLahlouASalhiHDobleANejjariCChronic obstructive pulmonary disease in the adult population within the Middle East and North Africa region: rationale and design of the BREATHE studyRespir Med2012106Suppl 2S3S1523290702
- World Health OrganizationChronic obstructive pulmonary disease (COPD)2016 Available from: www.who.int/mediacentre/factsheets/fs315/enAccessed January 15,2017
- AdeloyeDChuaSLeeCWGlobal and regional estimates of COPD prevalence: systemic review and meta-analysisJ Glob Health2015502041526755942
- TageldinMANaftiSKhanJADistribution of COPD-related symptoms in the Middle East and North Africa: results of the BREATHE studyRespir Med2012106Suppl 2S25S3223290701
- Al ZaabiAAsadFAbdouJPrevalence of COPD in Abu Dhabi, United Arab EmiratesRespir Med201110556657021216136
- MahboubBAlzaabiASorianoJBCase-finding of chronic obstructive pulmonary disease with questionnaire, peak flow measurements and spirometry: a cross-sectional studyBMC Res Notes2014724124739210
- Al GhobainMAl-HajjajMSWaliSOPrevalence of chronic obstructive pulmonary disease among smokers attending primary healthcare clinics in Saudi ArabiaAnn Saudi Med20113112913321403413
- Al GhobainMAlhamadEHAlorainyHSAl KassimiFLababidiHAl-HajjajMSThe prevalence of chronic obstructive pulmonary disease in Riyadh, Saudi Arabia: a BOLD studyInt J Tuberc Lung Dis2015912521257
- Al OmariMKhassawnehBYKhaderYDauodASBergusGPrevalence of chronic obstructive pulmonary disease among adult male cigarettes smokers: a community-based study in JordanInt J Chron Obstruct Pulmon Dis2014975375825092972
- SaidAFEwisAAOmranAAMagdyMESaleebMFPrevalence and predictors of chronic obstructive pulmonary disease among high-risk EgyptiansEgypt J Bronchol201592733
- BadwayMSHamedAFYousefFMPrevalence of chronic obstructive pulmonary disease (COPD) in Qena GovernorateEgypt J Chest Dis Tuberc2016652934
- WakedMKhayatGSalamehPChronic obstructive pulmonary disease prevalence in Lebanon: a cross-sectional descriptive studyClin Epidemiol2011331532322253549
- DaldoulHDenguezliMJithooAPrevalence of COPD and tobacco smoking in Tunisia: results from the BOLD studyInt J Environ Res Public Health2013107257727124351745
- El RhaziKNejjariCBenJellounMCEl BiazeMAttassiMGarcia-LarsenVPrevalence of chronic obstructive pulmonary disease in Fez, Morocco: results from the BOLD studyInt J Tuberc Lung Dis20162013614126688540
- World Health OrganizationPrevalence of tobacco smoking Available from: http://www.who.int/gho/tobacco/use/enAccessed January 15, 2017
- JithooAEnrightPLBurneyPCase-finding options for COPD: results from the Burden of Obstructive Lung Disease StudyEur Respir J20134154855522743668
- MenezesAMPerez-PadillaRJardimJRChronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence studyLancet20053661875188116310554
- LandisSHMuellerovaHManninoDMContinuing to Confront COPD international patient survey: methods, COPD prevalence, and disease burden in 2012–2013Int J Chron Obstruct Pulmon Dis2014959761124944511
- KartLAkkoyunluMEBayramMCOPD: an underdiagnosed disease at hospital environmentWien Klin Wochenschr2014126737824249327
- EisnerMDAnthonisenNCoultasDAn official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med201018269371820802169
- EzzatiMLopezADEstimates of global mortality attributable to smoking in 2000Lancet200336284785213678970
- KhattabAJavaidAIraqiGSmoking habits in the Middle East and North Africa: results of the BREATHE studyRespir Med2012106Suppl 2S16S2423290700
- Al ZaabiAMahboubBHVatsMGIqbalMNVatsDMChronic obstructive pulmonary disease in Middle East and UAE: an unrecognized underestimated epidemicJ Lung Pulm Respir Res2016300089
- MaziakWWardKDSoweidRAEissenbergTTobacco smoking using a waterpipe: a re-emerging strain in a global epidemicTob Control20041332733315564614
- AklEAGaddamSGunukulaSKHoneineRJaoudePAIraniJThe effects of water pipe tobacco smoking on health outcomes: a systematic reviewInt J Epidemiol20103983485720207606
- SheJYangPWangYChinese water-pipe smoking and the risk of COPDChest201414692493124557573
- HaddadLKellyDLWeglickiLSBarnettTEFerrellAVGhadbanRA systematic review of effects of waterpipe smoking on cardiovascular and respiratory health outcomesTob Use Insights20169132827398028
- SalamehPWakedMKhouryFWaterpipe smoking and dependence are associated with chronic bronchitis: a case-control study in LebanonEast Mediterr Health J201218996100423301353
- MohammadYShaabanRAl-ZahabBAKhaltaevNBousquetJDubayboBImpact of active and passive smoking as risk factors for asthma and COPD in women presenting to primary care in Syria: first report by the WHO-GARD survey groupInt J Chron Obstruct Pulmon Dis2013847348224124359
- Al-HouqaniMAliRHajatCTobacco smoking using medwakh is an emerging health problem: evidence from a large cross-sectional survey in the United Arab EmiratesPLoS One20127e3918922720071
- SalamehPSalameJKhayatGExposure to outdoor air pollution and chronic bronchitis in adults: a case-control studyInt J Occup Environ Med2012316517723022867
- WakedMSalameJKhayatGSalamehPCorrelates of COPD and chronic bronchitis in nonsmokers: data from a cross-sectional studyInt J Chron Obstruct Pulmon Dis2012757758523055708
- DenguezliMDaldoulHHarrabiICOPD in nonsmokers: reports from the Tunisian population-based Burden of Obstructive Lung Disease studyPLoS One201611e015198127010214
- GaudermanWJAvolEGillilandFThe effect of air pollution on lung development from 10 to 18 years of ageN Engl J Med20043511057106715356303
- GaudermanWJVoraHMcConnellREffect of exposure to traffic on lung development from 10 to 18 years of age: a cohort studyLancet200736957157717307103
- Rojas-MartinezRPerez-PadillaROlaiz-FernandezGLung function growth in children with long-term exposure to air pollutants in Mexico CityAm J Respir Crit Care Med200717637738417446338
- DownsSHSchindlerCLiuLJReduced exposure to PM10 and attenuated age-related decline in lung functionN Engl J Med20073572338234718057336
- EulerGAbbeyDMagieAHodgkinJChronic obstructive pulmonary disease symptom effects of long-term cumulative exposure to ambient levels of total suspended particulates and sulfur dioxide in California Seventh-Day Adventist residentsArch Environ Health1987422132223662608
- AbbeyDBurchetteRKnutsenSMcDonnelWLebowitzMEnrightPLong-term particulate and other pollutants and lung function in nonsmokersAm J Respir Crit Care Med19981582892989655742
- SchikowskiTSugiriDRanftULong-term air pollution exposure and living close to busy roads are associated with COPD in womenRespir Res2005615216372913
- AndersenZJHvidbergMJensenSSCOPD and long-term exposure to traffic-related air pollution: a cohort studyAm J Respir Crit Care Med201118345546120870755
- SmithKRBiofuels, Air Pollution, and HealthNew YorkPlenum Press1987
- PandeyMBasnyatBNeupaneRChronic bronchitis and cor pulmonale in NepalLung India19886209210
- AlbalakRFrisanchoARKeelerGJDomestic biomass fuel combustion and chronic bronchitis in two rural Bolivian villagesThorax1999541004100810525559
- BeheraDAn analysis of effect of common domestic fuels on respiratory functionIndian J Chest Dis Allied Sci1997392352439654820
- BeheraDJindalSKRespiratory symptoms in Indian women using domestic cooking fuelsChest19911003853881864111
- BeheraDJindalSKMalhotraHSVentilatory function in nonsmoking rural Indian women using different cooking fuelsRespiration19946189928008994
- CetinkayaFGülmezIAydinTOztürkYOzesmiMDemirRPrevalence of chronic bronchitis and associated risk factors in a rural area of Kayseri, central Anatolia, TurkeyMonaldi Arch Chest Dis20005518919310948663
- DossingMKhanJal-RabiahFRisk factors for chronic obstructive lung disease in Saudi ArabiaRespir Med1994885195227972976
- EkiciAEkiciMKurtipekEObstructive airway diseases in women exposed to biomass smokeEnviron Res200599939816053933
- KirazKKartLDemirRChronic pulmonary disease in rural women exposed to biomass fumesClin Invest Med20032624324814596485
- PandeyMRDomestic smoke pollution and chronic bronchitis in a rural community of the hill region of NepalThorax1984393373396740536
- Pérez-PadillaRRegaladoJVedalSExposure to biomass smoke and chronic airway disease in Mexican women: a case-control studyAm J Respir Crit Care Med19961547017068810608
- RegaladoJPérez-PadillaRSansoresRThe effect of biomass burning on respiratory symptoms and lung function in rural Mexican womenAm J Respir Crit Care Med200617490190516799080
- ShresthaILShresthaSLIndoor air pollution from biomass fuels and respiratory health of the exposed population in Nepalese householdsInt J Occup Environ Health20051115016015875891
- MenezesAMVictoraCGRigattoMPrevalence and risk factors for chronic bronchitis in Pelotas, RS, Brazil: a population-based studyThorax199449121712217878555
- DuttDSrinivasaDKRottiSBSahaiAKonarDEffect of indoor air pollution on the respiratory system of women using different fuels for cooking in an urban slum of PondicherryNatl Med J India199691131178664820
- EllegardACooking fuel smoke and respiratory symptoms among women in low-income areas in MaputoEnviron Health Perspect19961049809858899378
- LiuSZhouYWangXBiomass fuels are the probable risk factor for chronic obstructive pulmonary disease in rural south ChinaThorax20076288989717483137
- DennisRJMaldonadoDNormanSBaenaEMartinezGWoodsmoke exposure and risk for obstructive airways disease among womenChest19961091151198549171
- Orozco-LeviMGarcia-AymerichJVillarJRamirez-SarmientoAAntoJMGeaJWood smoke exposure and risk of chronic obstructive pulmonary diseaseEur Respir J20062754254616507854
- CaballeroATorres-DuqueCAJaramilloCPrevalence of COPD in five Colombian cities situated at low, medium, and high altitude (PREPOCOL study)Chest200813334334917951621
- AljarallahBAliADowaidarMSettinAPrevalence of α-1-antitrypsin gene mutations in Saudi ArabiaSaudi J Gastroenterol20111725626021727732
- BrownPJGrevilleHWFinucaneKEAsthma and irreversible airflow obstructionThorax1984391311366701824
- UlrikCSBackerVNonreversible airflow obstruction in life-long nonsmokers with moderate to severe asthmaEur Respir J19991489289610573238
- CassinoCBergerKIGoldringRMDuration of asthma and physiologic outcomes in elderly nonsmokersAm J Respir Crit Care Med20001621423142811029356
- ConnollyCKChanNSPrescottRJThe relationship between age and duration of asthma and the presence of persistent obstruction in asthmaPostgrad Med J1988644224253211818
- BeagleholeRBonitaRAlleyneGUN High-Level Meeting on Non-Communicable Diseases: addressing four questionsLancet201137844945521665266
- European COPD CoalitionPrevalence in EU Available from: http://www.copdcoalition.eu/about-copd/prevalenceAccessed January 14, 2017
- ChapmanKRManninoDMSorianoJBEpidemiology and costs of chronic obstructive pulmonary diseaseEur Respir J20062718820716387952
- Centers for Disease Control and PreventionChronic obstructive pulmonary disease among adults: United States, 2011MMWR Morb Mortal Wkly Rep20126193894323169314
- FosterTSMillerJDMartonJPCaloyerasJPRussellMWMenzinJAssessment of the economic burden of COPD in the U.S.: a review and synthesis of the literatureCOPD2006321121817361502
- MehrotraAOluwoleAMGordonSBThe burden of COPD in Africa: a literature review and prospective survey of the availability of spirometry for COPD diagnosis in AfricaTrop Med Int Health20091484084819702594
- ShayaFTEl KhouryACSamantNDScharfSMUtilization of health care resources in a high-risk Medicaid population with chronic obstructive pulmonary diseasePhys Ther200631261268
- MenzinJBoulangerLMartonJThe economic burden of chronic obstructive pulmonary disease (COPD) in a U.S. Medicare populationRespir Med2008102124856
- ChungFBarnesNAllenMAssessing the burden of respiratory disease in the UKRespir Med20029696397512477209
- ManninoDMBradmanSThe epidemiology and economics of chronic pulmonary obstructive diseaseProc Am Thorac Soc2007450250617878461
- WoutersEFThe burden of COPD in the Netherlands: results from the Confronting COPD surveyRespir Med200397Suppl CS51S5912647943
- WoutersEFEconomic analysis of the Confronting COPD survey: an overview of resultsRespir Med200397Suppl CS3S14
- Simon-TuvalTScharfSMMaimonNBernhard-ScharfBJReuveniHTarasiukADeterminants of elevated healthcare utilization in patients with COPDRespir Res201112721232087
- DalalAAShahMLunacsekOHananiaNAClinical and economic burden of patients diagnosed with COPD with comorbid cardiovascular diseaseRespir Res201110515161522
- AlzaabiAMahboubBSalhiHKajinguWRashidNEl-HasnaouiAWaterpipe use in the Middle East and North Africa: data from the Breathe studyNicotine Tob Res Epub20161010
- KesslerRPartridgeMRMiravitllesMSymptom variability in patients with severe COPD: a pan-European cross-sectional studyEur Respir J20113726427221115606
- de los MonterosMJPeñaCHurtadoEJJareñoJMiravitllesMVariability of respiratory symptoms in severe COPDArch Bronconeumol2012483721944843
- ZwarNAMarksGBHermizOPredictors of accuracy of diagnosis of chronic obstructive pulmonary disease in general practiceMed J Aust201119516817121843115
- SimonPMSchwartzsteinRMWeissJWFenclVTeght-soonianMWeinbergerSEDistinguishable types of dyspnea in patients with shortness of breathAm Rev Respir Dis1990142100910142240820
- BurrowsBNidenAHBarclayWRChronic obstructive lung disease – II: relationship of clinical and physiologic findings to the severity of airways obstructionAm Rev Respir Dis19659166567814280940
- No authors listedDefinition and classification of chronic bronchitis for clinical and epidemiological purposesLancet196517757794165081
- ScholsAMSoetersPBDingemansAMMostertRFrantzenPJWoutersEFPrevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitationAm Rev Respir Dis1993147115111568484624
- ScholsAMSlangenJVolovicsLWoutersEFWeight loss is a reversible factor in the prognosis of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1998157179117979620907
- HolguinFFolchEReddSCManninoDMComorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001Chest20051282005201116236848
- KestenSChapmanKRPhysician perceptions and management of COPDChest19931042542588325079
- LoveridgeBWestPKrygerMHAnthonisenNRAlteration in breathing pattern with progression of chronic obstructive pulmonary diseaseAm Rev Respir Dis19861349309343777689
- PellegrinoRViegiGBrusascoVInterpretative strategies for lung function testsEur Respir J20052694896816264058
- BestallJCPaulEAGarrodRGarnhamRJonesPWWedzichaJAUsefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary diseaseThorax19995458158610377201
- NishimuraKIzumiTTsukinoMOgaTDyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPDChest20021211434144012006425
- JonesPWHealth status measurement in chronic obstructive pulmonary diseaseThorax20015688088711641515
- JonesPTabbererMChenWHCreating scenarios of the impact of COPD and their relationship to COPD assessment test (CATTM) scoresBMC Pul Med20111142
- JonesPWAdamekLNadeauGBanikNComparisons of health status scores with MRC grades in COPD: implications for the GOLD 2011 classificationEur Respir J20134264765423258783
- JonesPWCOPD assessment test: rationale, development, validation and performanceCOPD20131026927123547637
- van der MolenTWillemseBWSchokkerSten HackenNHPostmaDSJuniperEFDevelopment, validity and responsiveness of the clinical COPD questionnaireHealth Qual Life Outcomes200311312773199
- RedaAAKotzDKocksJWWesselingGvan SchayckCPReliability and validity of the clinical COPD questionnaire and chronic respiratory questionnaireRespir Med20101041675168220538445
- MillerMRHankinsonJBrusascoVStandardization of spirom-etryEur Respir J20052631933816055882
- BuistASMcburnieMAVollmerWMInternational variations in the prevalence of COPD (the BOLD study): a population based prevalence studyLancet200737074175017765523
- HurstJRWedzichaJAWhat is (and what is not) a COPD exacerbation: thoughts from the new GOLD guidelinesThorax20076219819917329557
- HurstJRVestboJAnzuetoASusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med20103631128113820843247
- Soler-CataluñaJJMartínez-GarcíaMASánchezPRSalcedoENavarroMOchandoRSevere acute exacerbations and mortality in patients with chronic obstructive pulmonary diseaseThorax20056092593116055622
- SorianoJBVisickGTMuellerovaHPayvandiNHansellALPatterns of comorbidities in newly diagnosed COPD and asthma in primary careChest20051282099210716236861
- LangePNyboeJAppleyardMJensenGSchnohrPVentilatory function and chronic mucus hypersecretion as predictors of death from lung cancerAm Rev Respir Dis19901416136172310094
- SkillrudDMOffordKPMillerRDHigher risk of lung cancer in chronic obstructive pulmonary disease: a prospective, matched, controlled studyAnn Intern Med19861055035073752756
- BurgelPRRocheNPaillasseurJLClinical COPD phenotypes identified by cluster analysis: validation with mortalityEur Respir J20124049549622855468
- MiravitllesMSoler-CataluñaJJCalleMSpanish COPD guidelines (GesEPOC): pharmacological treatment of stable COPDArch Bronconeumol20124824725722561012
- Garcia-AymerichJGómezFPBenetMIdentification and prospective validation of clinically relevant chronic obstructive pulmonary disease (COPD) phenotypesThorax20116643043721177668
- SinDDMiravitllesMManninoDMWhat is asthma-COPD overlap syndrome (ACOS)? Towards a consensus definition from a round table discussionEur Respir J20164866467327338195
- PavordIDLettisSLocantoreNBlood eosinophils and inhaled corticosteroid/long-acting β-2 agonist efficacy in COPDThorax20167111812526585525
- PascoeSLocantoreNDransfieldMTBarnesNCPavordIDBlood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomized controlled trialsLancet Respir Med2015343544225878028
- BafadhelMDaviesLCalverleyPMAaronSDBrightlingCEPavordIDBlood eosinophil guided prednisolone therapy for exacerbations of COPD: a further analysisEur Respir J20144478979124925917
- FishmanAMartinezFNaunheimKA randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysemaN Engl J Med20033482059207312759479
- KellyAMMcAlpineRKyleEHow accurate are pulse oximeters in patients with acute exacerbations of chronic obstructive airways disease?Respir Med20019533634011392573
- Pinto-PlataVMCoteCCabralHTaylorJCelliBRThe 6-min walk distance: change over time and value as a predictor of survival in severe COPDEur Respir J200423283314738227
- OgaTNishimuraKTsukinoMSatoSHajiroTAnalysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health statusAm J Respir Crit Care Med200316754454912446268
- JonesPWHealth status measurement in chronic obstructive pulmonary diseaseThorax20015688088711641515
- CelliBRCoteCGMarinJMThe body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary diseaseN Engl J Med20043501005101214999112
- TashkinDPCelliBSennSA 4-year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med20083591543155418836213
- BeehKMSinghDDi ScalaLDrollmannAOnce-daily NVA237 improves exercise tolerance from the first dose in patients with COPD: the GLOW3 trialInt J Chron Obstruct Pulmon Dis2012750351322973092
- CalverleyPMAndersonJACelliBSalmeterol and fluticasone propionate and survival in chronic obstructive pulmonary diseaseN Engl J Med200735677578917314337
- DecramerMLChapmanKRDahlROnce-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group studyLancet Respir Med2013152453324461613
- VogelmeierCHedererBGlaabTTiotropium versus salmeterol for the prevention of exacerbations of COPDN Engl J Med20113641093110321428765
- SinghDNew combination bronchodilators for chronic obstructive pulmonary disease: current evidence and future perspectivesBr J Clin Pharmacol20157969570825377687
- BatemanEDChapmanKRSinghDAclidinium bromide and formoterol fumarate as a fixed-dose combination in COPD: pooled analysis of symptoms and exacerbations from two six-month, multicentre, randomised studies (ACLIFORM and AUGMENT)Respir Res2015169226233481
- WedzichaJADecramerMFickerJHAnalysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group studyLancet Respir Med2013119920924429126
- WedzichaJASinghDVestboJExtrafine beclomethasone/formoterol in severe COPD patients with history of exacerbationsRespir Med20141081153116224953015
- SinghDCorradiMSpinolaMPetruzzelliSPapiAExtrafine beclometasone diproprionate/formoterol fumarate: a review of its effects in chronic obstructive pulmonary diseaseNPJ Prim Care Respir Med2016261603027309985
- LokeYKCavallazziRSinghSRisk of fractures with inhaled corticosteroids in COPD: systematic review and meta-analysis of randomised controlled trials and observational studiesThorax20116669970821602540
- WangJJRochtchinaETanAGCummingRGLeederSRMitchellPUse of inhaled and oral corticosteroids and the long-term risk of cataractOphthalmology200911665265719243828
- SuissaSKezouhAErnstPInhaled corticosteroids and the risks of diabetes onset and progressionAm J Med20101231001100620870201
- LeeCHKimKHyunMKJangEJLeeNRYimJJUse of inhaled corticosteroids and the risk of tuberculosisThorax2013681105111323749841
- CrimCDransfieldMTBourbeauJPneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPDAnn Am Thorac Soc201512273425490706
- FesticEScanlonPDIncident pneumonia and mortality in patients with chronic obstructive pulmonary disease: a double effect of inhaled corticosteroids?Am J Respir Crit Care Med201519114114825409118
- WedzichaJABanerjiDChapmanKRIndacaterol-glyco-pyrronium versus salmeterol-fluticasone for COPDN Engl J Med20163742222223427181606
- WelteTMiravitllesMHernandezPEfficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200918074175019644045
- SinghDPapiACorradiMSingle inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trialLancet201638896397327598678
- MartinezFJCalverleyPMGoehringUMBroseMFabbriLMRabeKFEffect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trialLancet201538585786625684586
- HanMKTayobNMurraySPredictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapyAm J Respir Crit Care Med20141891503150824779680
- HurstJRVestboJAnzuetoASusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med20103631128113820843247
- SiddiquiSHGuasconiAVestboJBlood eosinophils: a bio-marker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med201519252352526051430
- PascoeSLocantoreNDransfieldMBarnesNCPavordIDBlood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trialsLancet Respir Med2015343544225878028
- SpruitMASinghSJGarveyCAn official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitationAm J Resp Crit Care Med2013188e13e6424127811
- GarveyCBaylesMPHammLFPulmonary rehabilitation exercise prescription in chronic obstructive pulmonary disease: review of selected guidelinesJ Cardiopulm Rehabil Prev201667583
- RiesALMakeBJLeeSMThe effects of pulmonary rehabilitation in the National Emphysema Treatment TrialChest20051283799380916354848
- QaseemAWiltTJWeinbergerSEDiagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory SocietyAnn Intern Med201115517919121810710
- PuhanMAScharplatzMTroostersTSteurerJRespiratory rehabilitation after acute exacerbation of COPD may reduce risk for readmission and mortality: a systematic reviewRespir Res2005 Jun 865415943867
- OrtegaFToalJCejudoPComparison of effects of strength and endurance training in patients with chronic obstructive pulmonary diseaseAm J Resp Crit Care Med200216666967412204863
- GarberCEBlissmerBDeschenesMRQuantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuro-motor fitness in apparently healthy adults: guidance for prescribing exerciseMed Sci Sports Exerc2011431334135921694556
- HorowitzMBLittenbergBMahlerDADyspnea ratings for prescribing exercise intensity in patients with COPDChest1996109116911758625662
- ScanlonPDConnettJEWallerLASmoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease: the Lung Health StudyAm J Respir Crit Care Med200016138139010673175
- PelkonenMTukiainenHTervahautaMPulmonary function, smoking cessation and 30-year mortality in middle aged Finnish menThorax20005574675010950892
- CarmodyTPDuncanCSimonJAHypnosis for smoking cessation: a randomized trialNicotine Tob Res20081081181818569754
- OdumLEO’DellKASchepersJSElectronic cigarettes: do they have a role in smoking cessation?J Pharm Pract20122561161422797832
- TomczykSBennettNMStoeckerCUse of 13-valent pneumococcal conjugate vaccine and 23-valent vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practice (ACIP)MMWR Morb Mortal Wkly Rep20146382282525233284
- FerreiraIMBrooksDWhiteJGoldsteinRNutritional supplementation for stable COPDCochrane Database Syst Rev201212CD00099823235577
- StruikFMLacasseYGoldsteinRKerstjensHMWijkstraPJNocturnal non-invasive positive pressure for stable COPDCochrane Database Syst Rev2013CD00287823766138
- TiongLUDaviesRGibsonPGLung volume reduction surgery for diffuse emphysemaCochrane Database Syst Rev2006CD00100117054132
- WeillDBendenCCorrisPAA consensus document for the selection of lung transplant candidates – 2014: an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung TransplantationJ Heart Lung Transplant20153411525085497
- International Society for Heart and Lung TransplantationOverall lung transplantation statistics2016 Available from: https://www.ishlt.org/registries/slides.asp?slides=heartLungRegistryAccessed July 19, 2017