Abstract
Abstract presentation
An abstract, including parts of the results, has been presented at an oral session at the European Respiratory Society International Conference, London, UK, September 2016.
Background
Cardiovascular comorbidity contributes to increased mortality among subjects with COPD. However, the prognostic value of ECG abnormalities in COPD has rarely been studied in population-based surveys.
Aim
To assess the impact of ischemic ECG abnormalities (I-ECG) on mortality among individuals with COPD, compared to subjects with normal lung function (NLF), in a population-based study.
Methods
During 2002–2004, all subjects with FEV1/VC <0.70 (COPD, n=993) were identified from population-based cohorts, together with age- and sex-matched referents without COPD. Re-examination in 2005 included interview, spirometry, and 12-lead ECG in COPD (n=635) and referents [n=991, whereof 786 had NLF]. All ECGs were Minnesota-coded. Mortality data were collected until December 31, 2010.
Results
I-ECG was equally common in COPD and NLF. The 5-year cumulative mortality was higher among subjects with I-ECG in both groups (29.6% vs 10.6%, P<0.001 and 17.1% vs 6.6%, P<0.001). COPD, but not NLF, with I-ECG had increased risk for death assessed as the mortality risk ratio [95% confidence interval (CI)] when compared with NLF without I-ECG, 2.36 (1.45–3.85) and 1.65 (0.94–2.90) when adjusted for common confounders. When analyzed separately among the COPD cohort, the increased risk for death associated with I-ECG persisted after adjustment for FEV1 % predicted, 1.89 (1.20–2.99). A majority of those with I-ECG had no previously reported heart disease (74.2% in NLF and 67.3% in COPD) and the pattern was similar among them.
Conclusion
I-ECG was associated with an increased risk for death in COPD, independent of common confounders and disease severity. I-ECG was of prognostic value also among those without previously known heart disease.
Introduction
COPD and ischemic heart disease (IHD) are among the leading causes of death worldwide.Citation1 Cardiovascular disease is one of the most common comorbidities among subjects with COPD,Citation2,Citation3 and patients with COPD have a two to five times higher risk of not only coronary heart disease, heart failure, arrhythmias, and pulmonary/peripheral vascular diseaseCitation4,Citation5 but also an increased risk for stroke.Citation6
The overall mortality among subjects with COPD increases with disease severity, both when assessed as a decline in FEV1Citation7,Citation8 and by spirometric stagingCitation9 according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) document.Citation10 Studies on selected populations have shown that cardiovascular comorbidity is one of the major causes of death in mild-to-moderate COPD, whereas respiratory causes of death dominate among subjects with more severe COPD.Citation11 Population-based studies have also shown that a lower FEV1 itself, without taking COPD into account, is a predictor of cardiovascular death.Citation12
Most guidelines for diagnosis and treatment of COPD emphasize assessment of the presence of cardiovascular risk factors and comorbidities.Citation10 Early identification and treatment of cardiovascular risk factors are important to reduce morbidity and prevent mortality. A 12-lead ECG, registered during rest, is a relatively simple, cheap, and widely accessible diagnostic tool for detecting ischemic ECG abnormalities, and has been shown to improve risk prediction in asymptomatic subjects.Citation13 In the general population, there is an association between various ischemic ECG abnormalities (I-ECG) and all-cause mortalityCitation14–Citation16 as well as coronary heart disease events.Citation17,Citation18 Ischemic ECG findings are common in COPD,Citation19 with increasing prevalence by disease severity.Citation20,Citation21 Although ischemic ECG findings were associated with increased mortality in a study among patients with COPD discharged from hospital,Citation22 there is a lack of population-based studies evaluating the prognostic value of I-ECG among subjects with COPD.
The aim of this population-based study was to assess the impact of I-ECG on long-term mortality among subjects with COPD as compared to subjects with normal lung function (NLF).
Methods
Study population
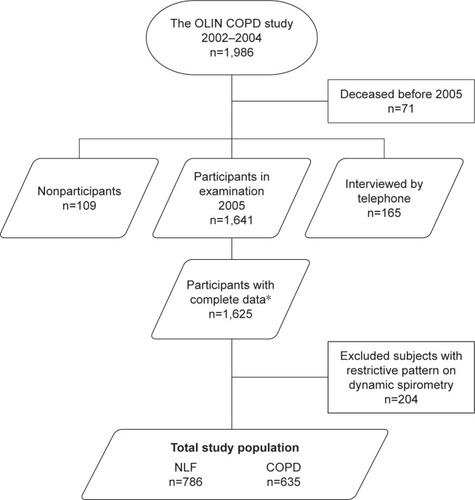
The inclusion of subjects in the OLIN COPD study has been described in detail previously.Citation20,Citation23 All subjects with obstructive lung function impairment (n=993) were identified from re-examination of four adult population-based cohorts in northern Sweden during the years 2002–2004, together with an age- and sex-matched reference population without obstructive lung function impairment. The study population (n=1986) has been invited to annual examinations with a basic program including spirometry and structured interview. The present study is based on data collected in 2005 when ECG recordings were performed in addition to the basic program. Mortality data were collected over 5 years, from 2005 to December 31, 2010 from the national population register. In total, 1,806 subjects participated of whom 1,625 had complete data on spirometry, structured interview, and ECG findings. Among those without airway obstruction, 204 subjects had restrictive pattern on dynamic spirometry and were excluded (). The Regional Ethics Review Board at Umeå University approved the study (dnr 04–045 M), which was carried out according to the Declaration of Helsinki, and all participants provided written informed consent.
Definitions
Height and weight were measured before spirometry. Body mass index (BMI) was calculated (weight/heightCitation2), and categorized into underweight (<20), normal weight (20–24.9), overweight (>25–29.9), and obese (≥30) subgroups. Smoking habits were divided into nonsmokers, ex-smokers (quit smoking since at least 12 months), and current smokers. Pack-years were calculated on baseline data (number of cigarettes smoked per day/20× number of years smoked). The variable “any ischemic heart disease” included self-reported angina pectoris, acute myocardial infarction, coronary intervention (coronary artery bypass surgery and/or percutaneous coronary intervention), and chronic heart failure. Cardiovascular drugs (including acetylsalicylic acid and lipid-lowering agents) were categorized into three groups; none, 1–2, and ≥3 drugs.
Electrocardiogram
Standard 12-lead ECGs were recorded before spirometry in supine position after sufficient rest. Two independent encoders classified ECG recordings according to the Minnesota Code (MC),Citation24 and both of them were blinded to COPD status and spirometry values. Disagreements in ECG coding were resolved by consensus. Ischemic abnormalities were defined as major Q/QS wave (MC 1-1, 1-2), major isolated STT abnormalities (MC 4-1, 4-2, 5-1, 5-2), minor Q/QS wave plus major ST-T (MC 1-3 plus 4-1, 4-2, 5-1, 5-2), and minor isolated Q/QS wave (MC 1-3), and also grouped together as “any ischemic ECG abnormalities” (I-ECG).
Lung function tests
The procedure of lung function testing has been previously described in detail.Citation20 Lung function tests were performed in accordance with the American Thoracic Society Guidelines.Citation25 COPD was defined as FEV1/VC <0.70, using the best values of FEV1 and VC pre- or post-reversibility testing, with VC defined as the highest of FVC and SVC. Severity of airflow limitation in COPD was classified according to the GOLD classification, with GOLD 1–4 based on FEV1 % predicted.Citation10 The reference population without obstructive lung function impairment, defined as FEV1/VC ≥0.70, was further divided into subjects with NLF (FEV1/VC ≥0.70, and VC ≥80% of predicted) and subjects with restrictive spirometric pattern (RSP) on dynamic spirometry (defined as FEV1/VC ≥0.70 and VC <80% of predicted). The OLIN reference values for spirometry were used.Citation26
Statistics
Statistical calculations were performed using SPSS version 23.0 (IBM, Armonk, NY, USA) and GraphPad Prism version 7.02 (GraphPad Software Inc, La Jolla, CA, USA). The chi-square test and Mantel–Haenszel test-for-trend were used for categorical variables and bivariate comparisons. Fisher’s exact test was used where appropriate. The independent sample t-test was used to compare means. The association between I-ECG abnormalities and mortality was analyzed using Kaplan–Meier curves and multivariate Poisson regression. A basic multivariable regression model was constructed containing the covariates age, sex, BMI-class, and smoking habits. Confidence intervals (CI) were set to 95% and P-values <0.05 were considered statistically significant.
Results
The study population () consisted of 786 subjects with NLF and 635 subjects fulfilling the spirometric criteria for COPD, whereof 40.7% (n=256) were GOLD 1, 51.1% (n=342) were GOLD 2, and 7.8% (n=49) were GOLD 3–4. The basic characteristics compared between NLF and COPD are presented in . Among all subjects fulfilling the spirometric criteria of COPD, 14.6% reported a physician diagnosis of COPD and, among those with GOLD ≥2, the corresponding proportion was 23.2%.
Table 1 Basic characteristics of the study population, comparing subjects with NLF and COPD
The prevalence of reported angina pectoris and coronary intervention was similar in COPD and NLF, whereas the prevalence of myocardial infarction and chronic heart failure was higher in COPD; the prevalence of the compound concept “any ischemic heart disease” was higher in COPD than in NLF (). Subjects with COPD also reported a higher use of cardiovascular drugs than those with NLF ().
Table 2 Reported comorbid conditions and use of heart medicines, comparing subjects with NLF and COPD
There were no differences in the prevalence of I-ECG when comparing subjects with NLF and those with COPD, neither in each of the ECG categories nor when grouped together as I-ECG (). The prevalence of I-ECG increased by GOLD grade: 11.2% in GOLD 1, 17.2% in GOLD 2, and 26.5% in GOLD 3–4 (test for trend, P=0.003). When compared with NLF, the prevalence of I-ECG was higher in GOLD 3–4 (P=0.010), but not in GOLD 1 or 2 subgroups.
Table 3 Ischemic ECG abnormalities classified according to the MC, comparing subjects with NLF and COPD
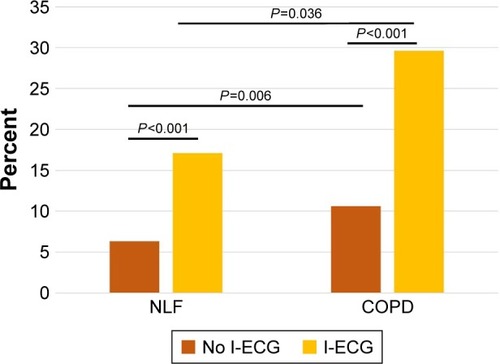
In the NLF group, the 5-year cumulative mortality was 17.1% among subjects with I-ECG and 6.3% among those without (P<0.001), corresponding to 29.6% and 10.6% in the COPD group (P<0.001; ). When comparing the 5-year cumulative mortality among subjects with and without I-ECG, respectively, by GOLD stage, it was 17.2% and 7.4% in GOLD 1 (P=0.071), 35.7% and 10.0% in GOLD 2 (P<0.001), and 30.8% and 36.1% in GOLD 3–4 (P=0.729). The 5-year survival among subjects categorized as NLF and COPD with and without I-ECG is illustrated by Kaplan–Meier curves (log-rank P<0.001; ).
Figure 2 Kaplan–Meier curves illustrating survival among subjects with NLF or COPD with and without ischemic ECG abnormalities (I-ECG) using log-rank; P<0.001.
Abbreviation: NLF, normal lung function.
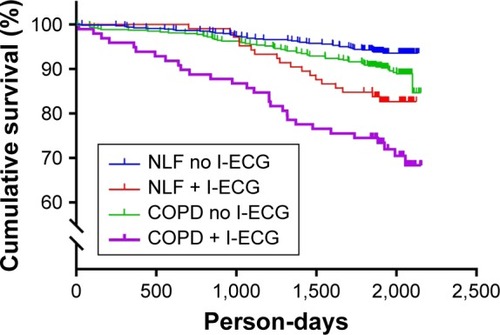
Figure 3 The 5-year cumulative mortality, comparing subjects with and without ischemic ECG abnormalities (I-ECG) among subjects with NLF and COPD, and comparing subjects with NLF and COPD, respectively, without I-ECG.
When I-ECG was analyzed as a risk factor for death, expressed as the mortality risk ratio [MRR (95% CI)] in the basic multivariate regression model adjusting for confounders, the MRR was 2.4 (1.5–3.9) for COPD with I-ECG and 1.65 (0.94–2.90) for NLF with I-ECG when compared to subjects with NLF without I-ECG (). The increased risk persisted even when smoking habits were replaced with pack-years, as well as when diabetes was included in the model.
Table 4 Ischemic ECG changes, I-ECG, as a risk factor for death, analyzed in a Poisson regression modelTable Footnotea
When analyzed separately among subjects with COPD in the basic regression model, I-ECG was associated with an increased MRR as compared with subjects without I-ECG independent of common confounders, 2.1 (95% CI: 1.3–3.2). The increased risk for death associated with I-ECG in COPD was independent of disease severity, when assessed as FEV1 % predicted in the model (). The MRR remained similar also when smoking habits were given as pack-years, and when diabetes was included in the model.
Table 5 Ischemic ECG changes, I-ECG, as a risk factor for death among subjects with COPD, analyzed in Poisson regression modelsTable Footnotea
The overlap between reported IHD and observed I-ECG was analyzed; 72.4% of subjects with NLF and I-ECG, and 67.3% of subjects with COPD and I-ECG had no previously reported heart disease. Among subjects without reported IHD, those with I-ECG had increased mortality compared to those without, both in COPD (25.8% vs 8.8%, P<0.001) and NLF (14.5% vs 4.3%, P<0.001). When the basic multivariate regression model was applied among those without reported IHD, I-ECG was associated with an increased MRR among subjects with COPD 3.01 (1.59–5.71) compared to NLF with no I-ECG. In subjects with NFL and I-ECG, the MRR was nonsignificantly increased 1.95 (0.94–4.06). When analyzed separately among subjects with COPD, I-ECG was associated with an increased MRR, to 1.95 (1.08–3.50), compared to those without I-ECG; however, when the FEV1 % predicted was added to the model, the significance was lost, as MRR was 1.69 (0.94–3.05).
Discussion
In this longitudinal population-based prospective study, I-ECG was indeed associated with increased crude mortality – both among subjects with NLF and COPD. However, only individuals with COPD and I-ECG had a signinficant increased risk for death as compared to NLF subjects without I-ECG, when adjusted for confounders. Among those with COPD, I-ECG almost doubled the risk for death when compared to those without I-ECG, independent of common confounders and disease severity, assessed as FEV1 % predicted. A high proportion – more than two-thirds of those with I-ECG – reported no previously known heart disease, and this was the case both among those with NLF and in those with COPD. Furthermore, I-ECG was associated with a significant increased risk for death, also among subjects with COPD without previously reported IHD.
Several large trials, including various selected COPD populations, have shown that IHD is a common cause of death among subjects with COPD.Citation11 One of the most well-known, feasible, and – for patients – comfortable ways to detect signs of IHD is by registration of ECG. Different I-ECG are associated with increased mortality in the general population,Citation15,Citation16,Citation27,Citation28 and the importance of ECG abnormalities among subjects with COPD was also highlighted in a recent review.Citation29 There are a few studies on the prognostic value of ischemic ECG findings among subjects with COPD; among participants in a COPD rehabilitation program, those with I-ECG had a worse clinical outcome than those without.Citation11 In another study published 20 years ago, an association between I-ECG and mortality among subjects with COPD was reported. However, this was a highly selected COPD population discharged from hospital after exacerbation, and no control group without COPD was included.Citation19,Citation22 Most studies within this area include highly selected COPD populations, not taking the large well-known under-diagnosis of COPDCitation30,Citation31 into account. To the best of our knowledge, this is the first population-based study evaluating the impact of I-ECG on mortality by comparing subjects with and without COPD.
Cardiovascular disease, including IHD, is one of the most common comorbid conditions among subjects with COPD,Citation21 but COPD can also be considered a comorbid condition in a patient with cardiovascular disease. According to a recently published large European study, almost a third of outpatients with IHD had lung function abnormalities compatible with COPD.Citation32 A corresponding pattern was found in a register-based Swedish study; the prevalence of COPD was more than five times higher among subjects with myocardial infarction and four times higher, among subjects with stroke when compared to those without corresponding disease.Citation6 There are several studies of subjects with IHD showing that those with concomitant COPD have worse outcome. Patients with myocardial infarction and concomitant COPD had an increased risk for death and hospital readmissions due to cardiovascular causes,Citation33 as well as an increased risk for death or cardiogenic shockCitation34 compared to those without COPD; these data are also supported by a quite recent Swedish publication.Citation35 Most current guidelines and consensus documents for diagnosis and treatment of COPDCitation10,Citation36 emphasize the importance of detecting comorbidities, such as IHD. Our results highlight the importance of detecting IHD among subjects with COPD; the combination of these conditions was related to worse outcome – not only as has been shown in selected study populations,Citation33–Citation35 but also as shown in this population-based study including mainly individuals with mild-to-moderate COPD.
What is the clinical implication of our results? COPD is vastly underdiagnosed, in generalCitation22 as well as among patients with IHD,Citation32 and, in the current study, merely one fifth of all subjects fulfilling the spirometric criteria for COPD reported a physician diagnosis of COPD. An increased awareness of the large COPD underdiagnosis is important to support an active approach to diagnostics in daily clinical practice. Furthermore, in both NLF and COPD with I-ECG, more than two-thirds did not report any previous IHD. Similarly, among those with COPD without known heart disease, I-ECG was related to increased risk for death. Whereas subjects with COPD and I-ECG share the risk factor smoking and have common symptoms such as dyspnea that may contribute to both misclassification and undiagnosed cardiovascular disease, the mechanisms underlying the observed increased risk for death are still unclear. Unknown underlying IHD of prognostic importance could, according to our results, be identified by a simple resting ECG and provide a basis for cardiopreventive measures. The value of ECG screening in subjects with elevated risk for CVD is unclear,Citation37 but recent studies have shown that ECG contributes to an improved risk prediction.Citation13,Citation38 Early identification of IHD and treatment of cardiovascular risk factors may have a beneficial impact on morbidity and prevent mortality. However, systemic inflammation has been suggested as a link between cardiovascular disease and COPD,Citation39 and the underlying pathophysiological mechanisms are not fully understood.
Subjects with a restrictive spirometric pattern on dynamic spirometry – RSP – comprise a heterogeneous group, as RSP is related to different underlying conditions such as idiopathic pulmonary fibrosis, thoracic deformities, obesity, and neuromuscular disorders. RSP is associated with an increased burden of cardiovascular risk factors and comorbidity.Citation40–Citation42 In the present study, subjects with RSP – in total 12.6% – were excluded from the reference population without airway obstruction, because the aim was to compare COPD and those with NLF. The cardiovascular risk profile related to RSP on dynamic spirometry is an important topic for future research; however, it was out of scope for this paper.
The strength of this study is the large population-based COPD cohort, with a distribution of disease severity comparable to other population-based studies,Citation5 and a spirometric classification of COPD based on post-bronchodilator spirometry. Furthermore, self-reports on cardiovascular diseases were available, and the ECGs were manually Minnesota coded by two independent coders. There are also limitations: ischemic abnormalities on ECG are often transient; ischemic T-waves may disappear after some months but can also remain for years; pathological Q-waves are often considered to be a permanent sign of transmural myocardial infarction, but in some cases they can disappear spontaneously during the years following a myocardial infractionCitation27; and, furthermore, re- and depolarization changes may reflect other, but less frequent, conditions such as cardiomyopathies, albeit classified as I-ECG by the MC. However, we expect that the distribution of transient ECG abnormalities was similar among those with COPD and NLF and, thus, not considered to affect the results. Although the study includes a rather large population-based COPD cohort, we acknowledge that both the number of subjects with I-ECG and the total number of fatal events are low, affecting the statistical power, especially in subgroup analyses. Nonetheless, the associations were strong enough to generate significant results while also generating hypotheses for future studies in larger population surveys. Further, limitations are that detailed data on medication were not available in 2005, which is why we could not control for specific medication use, and that data from medical records were not available to verify self-reported disease. Furthermore, the spirometric fixed ratio criterion to define COPD may overdiagnose COPD among the elderlyCitation43 and, during the last few years, the lower limit of normal has been more commonly used in epidemiological studies. Despite this, the fixed ratio criterion is used in most current clinical guidelines for diagnostics and treatment of COPD; thus, the results can be interpreted in the clinical setting.
Conclusion
This population-based study shows that although I-ECG are associated with a higher mortality both among subjects with NLF and COPD, only individuals with COPD and I-ECG had a significant increased risk for death when adjusted for confounders. Among those with COPD, the almost doubled risk for death associated with I-ECG was independent of disease severity, which was assessed as the FEV1 % predicted. Furthermore, I-ECG was associated with an increased risk for death among subjects without previously reported heart disease. These findings indicate that a simple resting ECG may be a valuable tool in the clinic setting to detect ischemic abnormalities of prognostic value among subjects with COPD, independent of previously known heart disease.
Author contributions
All authors have substantially contributed in the process of writing the manuscript and have approved the final version of the manuscript. In addition, UN is the corresponding author and has contributed to the interpretation of data and statistical analysis. AB and BJ contributed to the interpretation of data. HB has contributed to the interpretation of data and statistical analysis. BE contributed to data collection and interpretation of data. AL was responsible for the study design, and took part in data collection and interpretation of data. AL is also the guarantor and takes responsibility for the content of the manuscript and the accuracy of the data analysis.
Acknowledgments
The authors thank professors Eva Rönmark and Bo Lundbäck, the present and former head of the OLIN studies, for their support. Further, the authors thank research assistants Ann-Christine Jonsson and Sigrid Sundberg for collecting data. The authors also thank professors Abdonas Tamosiunas and Ricardas Radisauskas, Lithuanian University of Health Sciences for excellent assistance with the Minnesota coding of the ECGs.
The authors acknowledge the Swedish Heart-Lung Foundation, the Västerbotten County Council, and the Heart Foundation of Northern Sweden for research funding.
Disclosure
The authors report no conflicts of interest in this work.
References
- WHOThe top 10 causes of death [internet]Fact sheet N°310GenevaWHO2014 Available from: http://www.who.int/mediacentre/factsheets/fs310/en/Accessed October 05, 2016
- ManninoDMThornDSwensenAHolguinFPrevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPDEur Respir J200832496296918579551
- CamiciottoliGBigazziFMagniCPrevalence of comorbidities according to predominant phenotype and severity of chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis2016112229223627695310
- MüllerovaHAgustiAErqouSMapelDWCardiovascular comorbidity in COPD: systematic literature reviewChest201314441163117823722528
- FinkelsteinJChaEScharfSMChronic obstructive pulmonary disease as an independent risk factor for cardiovascular morbidityInt J Chron Obstruct Pulmon Dis2009433734919802349
- YinLLensmarCIngelssonEBackMDifferential association of chronic obstructive pulmonary disease with myocardial infarction and ischemic stroke in a nation-wide cohortInt J Cardiol2014173360160324704409
- MenezesAMPérez-PadillaRWehrmeisterFCPLATINO teamFEV1 is a better predictor of mortality than FVC: the PLATINO cohort studyPLoS One2014910e10973225285441
- LindbergALarssonLGMuellerovaHRönmarkELundbäckBUp-to-date on mortality in COPD – report from the OLIN COPD studyBMC Pulm Med201212122230685
- ManninoDMBuistASPettyTLEnrightPLReddSCLung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up studyThorax200358538839312728157
- From the Global Strategy for the Diagnosis MaPoCGlobal Initiative for Chronic Obstructive Lung Disease (GOLD)2017 Available from: http://www.goldcopd.orgAccessed June 30, 2017
- BerryCEWiseRAMortality in COPD: causes, risk factors, and preventionCOPD20107537538220854053
- SinDDWuLManSFThe relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literatureChest200512761952195915947307
- JørgensenPGJensenJSMarottJLJensenGBAppleyardMMogelvangRElectrocardiographic changes improve risk prediction in asymptomatic persons age 65 years or above without cardiovascular diseaseJ Am Coll Cardiol201464989890625169175
- OstörESchnohrPJensenGNyboeJHansenATElectrocardiographic findings and their association with mortality in the Copenhagen City Heart StudyEur Heart J1981243173287297572
- De BacquerDDe BackerGKornitzerMBlackburnHPrognostic value of ECG findings for total, cardiovascular disease, and coronary heart disease death in men and womenHeart199880657057710065025
- RollinAMauryPKeeFIsolated negative T waves in the general population is a powerful predicting factor of cardiac mortality and coronary heart diseaseInt J Cardiol201620331832426523363
- AuerRBauerDCMarques-VidalPHealth ABC StudyAssociation of major and minor ECG abnormalities with coronary heart disease eventsJAMA2012307141497150522496264
- ChouRAroraBDanaTFuRWalkerMHumphreyLScreening asymptomatic adults with resting or exercise electrocardiography: a review of the evidence for the U.S. Preventive Services Task ForceAnn Intern Med2011155637538521930855
- VanfleterenLEFranssenFMUszko-LencerNHFrequency and relevance of ischemic electrocardiographic findings in patients with chronic obstructive pulmonary diseaseAm J Cardiol2011108111669167422077976
- NilssonUJohanssonBErikssonBBlombergALundbäckBLindbergAIschemic heart disease among subjects with and without chronic obstructive pulmonary disease – ECG-findings in a population-based cohort studyBMC Pulm Med20151515626637314
- LangePMogelvangRMarottJLVestboJJensenJSCardiovascular morbidity in COPD: a study of the general populationCOPD20107151020214458
- Antonelli IncalziRFusoLDe RosaMCo-morbidity contributes to predict mortality of patients with chronic obstructive pulmonary diseaseEur Respir J19971012279428009493663
- LindbergALundbäckBThe Obstructive Lung Disease in Northern Sweden Chronic Obstructive Pulmonary Disease Study: design, the first year participation and mortalityClin Respir J20082Suppl 1647120298352
- PrineasRJCrowRSBlackburnHWThe Minnesota code manual of electrocardiographic findings: standards and procedures for measurement and classificationBoston, MAJohn Wright-PSG1982
- Standardization of Spirometry, 1994 Update. American Thoracic SocietyAm J Respir Crit Care Med19951523110711367663792
- BackmanHLindbergAOdénAReference values for spirometry – report from the Obstructive Lung Disease in Northern Sweden studiesEur Clin Respir J2015226375
- CollSBetriuAde FloresTSignificance of Q-wave regression after transmural acute myocardial infarctionAm J Cardiol198861107397423354435
- KurlSMakikallioTHLaukkanenJAT-wave inversion and mortality riskAnn Med2015471697325613172
- GoudisCAKonstantinidisAKNtalasIVKorantzopoulosPElectrocardiographic abnormalities and cardiac arrhythmias in chronic obstructive pulmonary diseaseInt J Cardiol201519926427326218181
- LamprechtBSorianoJBStudnickaMDeterminants of underdiagnosis of COPD in national and international surveysChest2015148497198525950276
- JohnstonAKManninoDMHaganGWDavisKJKiriVARelationship between lung function impairment and incidence or recurrence of cardiovascular events in a middle-aged cohortThorax200863759960518245145
- FranssenFMSorianoJBRocheNLung function abnormalities in smokers with ischemic heart diseaseAm J Respir Crit Care Med2016194556857627442601
- CampoGGuastarobaPMarzocchiAImpact of COPD on long-term outcome after ST-segment elevation myocardial infarction receiving primary percutaneous coronary interventionChest2013144375075723392738
- WakabayashiKGonzalezMADelhayeCImpact of chronic obstructive pulmonary disease on acute-phase outcome of myocardial infarctionAm J Cardiol2010106330530920643237
- AndellPKoulSMartinssonAImpact of chronic obstructive pulmonary disease on morbidity and mortality after myocardial infarctionOpen Heart201411e00000225332773
- MiravitllesMVogelmeierCRocheNA review of national guidelines for management of COPD in EuropeEur Respir J201647262563726797035
- MoyerVAU.S. Preventive Services Task Force. Screening for coronary heart disease with electrocardiography: U.S. Preventive Services Task Force recommendation statementAnn Intern Med2012157751251822847227
- ShahAJVaccarinoVJanssensACAn electrocardiogram-based risk equation for incident cardiovascular disease from the National Health and Nutrition Examination SurveyJAMA Cardiol20161777978627487404
- SinDDAnthonisenNRSorianoJBAgustiAGMortality in COPD: role of comorbiditiesEur Respir J20062861245125717138679
- ErikssonBLindbergAMüllerovaHRönmarkELundbäckBAssociation of heart diseases with COPD and restrictive lung function – results from a population surveyRespir Med201310719810623127573
- FordESWheatonAGManninoDMPresley-CantrellLLiCCroftJBElevated cardiovascular risk among adults with obstructive and restrictive airway functioning in the United States: a cross-sectional study of the National Health and Nutrition Examination Survey from 2007–2010Respir Res20121311523237325
- BackmanHErikssonBHedmanLRestrictive spirometric pattern in the general adult population: methods of defining the condition and consequences on prevalenceRespir Med201612011612327817808
- HardieJABuistASVollmerWMEllingsenIBakkePSMørkveORisk of over-diagnosis of COPD in asymptomatic elderly never-smokersEur Respir J20022051117112212449163