Abstract
Background and purpose
While the efficacy and safety of combined tiotropium and olodaterol in patients with COPD was established in a large clinical trial program, it is important to assess whether clinical data can be applied to geographic patient groups, particularly for East Asian patients who may have a different phenotypic profile to the global trial population. This study aimed to compare the lung function and safety profiles of tiotropium/olodaterol and monocomponents in East Asian and global populations from the TONADO® trials.
Materials and methods
In the replicate, double-blind, parallel-group, active-controlled, randomized, 52-week, Phase III TONADO studies, patients received tiotropium/olodaterol, tiotropium, or olodaterol. We assessed the forced expiratory volume in 1 second (FEV1) area under the curve from 0 to 3 hours (AUC0–3) response and trough FEV1 response at 24 weeks for the approved doses, tiotropium/olodaterol 5/5 μg, tiotropium 5 μg, and olodaterol 5 μg. Treatment-emergent adverse events were recorded throughout treatment and ≤21 days after study medication.
Results
In the East Asian population, 1,152 patients were randomized (5,163 overall). After 24 weeks, FEV1 AUC0–3 and trough FEV1 responses were greater (P<0.0001) with tiotropium/olodaterol 5/5 μg in both populations versus tiotropium or olodaterol. The East Asian population showed slightly greater trough FEV1 treatment differences between tiotropium/olodaterol 5/5 μg and tiotropium compared to the overall population. Generally, no increase in adverse events was seen with tiotropium/olodaterol 5/5 μg compared to tiotropium and olodaterol in either population.
Conclusion
The efficacy and safety profile of tiotropium/olodaterol 5/5 μg has been demonstrated for both East Asian and global populations.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Plain language summary
Why was the study done? Drugs are known to be processed differently in East Asian people compared with Caucasians, which may affect how new therapies perform in this geographic group. We wanted to find out whether a combined treatment for COPD (tiotropium/olodaterol) in East Asian patients works as well and as safely as in the global population.
What did the researchers do and find? We analyzed results from two large identical clinical trials (TONADO®) in which patients were given either tiotropium/olodaterol 5/5 μg or the monocomponent tiotropium 5 μg or olodaterol 5 μg alone, and found that the drugs worked as well and as safely in East Asian patients as in the global population.
What do the results mean? This analysis provides interesting and robust data regarding the East Asian patients included in two large clinical trials of tiotropium/olodaterol for the treatment of COPD. Our findings give reassurance to doctors prescribing tiotropium/olodaterol to patients with COPD that being of East Asian origin should not make a difference on how well tiotropium/olodaterol works and how safe it is.
Introduction
COPD is characterized by progressive, persistent airflow limitation, often accompanied by exacerbations and comorbidities. COPD and its accompanying comorbidities remain a leading cause of death worldwide.Citation1 The effects of pathophysiologic hallmarks of COPD, such as chronic airflow limitation and hyperinflation, create a need for safe, reliable maintenance therapies.
Long-acting bronchodilators are recommended for maintenance therapy in patients with moderate to very severe COPD.Citation1 Tiotropium is an established once-daily, long-acting muscarinic antagonist that has been shown to improve the lung function and patient-reported outcomes such as dyspnea and quality of life, and reduce exacerbations in patients with COPD.Citation2–Citation8 Olodaterol is a long-acting β2-agonist (LABA) that provides 24-hour bronchodilation and symptomatic benefits in patients with COPD.Citation9–Citation12 The advantages of combining tiotropium and olodaterol as a long-acting bronchodilator have been demonstrated in an extensive clinical trial program (TOviTO®),Citation13–Citation17 and the combination is now available globally at a dose of 5/5 μg for once-daily use via the Respimat® inhaler (Boehringer Ingelheim, Ingelheim am Rhein, Germany).
Long-term efficacy and safety results of treatment with tiotropium/olodaterol in a global population with moderate to very severe COPD over 52 weeks versus the monocomponents have been published from two replicate Phase III trials (TONADO 1 and 2).Citation13 Tiotropium/olodaterol has also been shown to improve the lung function to a greater extent in patients with Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2 compared to GOLD 3–4 disease, stressing the importance of providing early maintenance therapy in patients with COPD.Citation18
There is a concern that global clinical trial data may not be applicable to East Asian patients due to lower average body weight and potentially different metabolism and elimination profiles, compared to a predominantly Caucasian population.Citation19 For example, in the PHILO trial of the blood thinner ticagrelor, Asian patients had notably worsened outcomes compared to other ethnic populations.Citation20 In cancer patients, the incidence of severe neutropenia following treatment with the chemotherapy drug docetaxel was demonstrated to be 19 times greater than in non-Asian patients in Phase II and III clinical trials,Citation21 leading to the proposal of dose reduction in Asian patients treated with docetaxel.Citation22 A 10% dose reduction in Asian patients with head and neck squamous cell cancer receiving induction chemotherapy with dose-modified docetaxel, cisplatin, and 5-fluorouracil achieved similar survival figures compared to the original recommended dose regimen.Citation23 For inhaled drugs, in particular, the response and long-term safety profiles may be different in regional patient populations according to different population characteristics such as phenotype physiology, genetics, disease severity, and distribution of other risk factors in the target COPD population.Citation24,Citation25
In the clinical pharmacology programs for tiotropium/olodaterol, tiotropium, and olodaterol, there was no observed influence of patient race,Citation13 although the lung function benefits and safety were not specifically analyzed in East Asian patients. This paper will describe the lung function and safety profiles of tiotropium/olodaterol and the monocomponents in the East Asian population from the large TONADO studies compared to the overall population.
Materials and methods
Study design
The detailed methodology of TONADO has been previously published.Citation13 Briefly, this trial was a pair of replicate, double-blind, parallel-group, active-controlled, multicenter, randomized, Phase III studies (registered with ClinicalTrials.gov: NCT1431274 [Study 1237.5] and NCT1431287 [Study 1237.6]). Eligible patients were ≥40 years of age with a diagnosis of COPD, a smoking history of >10 pack-years, post-bronchodilator forced expiratory volume in 1 second (FEV1) <80% of predicted normal, and post-bronchodilator FEV1/forced vital capacity (FVC) <70%.
Patients received tiotropium/olodaterol 2.5/5 μg or 5/5 μg, tiotropium 2.5 or 5 μg, or olodaterol 5 μg over 52 weeks. This analysis focused on the approved doses of tiotropium/olodaterol 5/5 μg, tiotropium 5 μg, and olodaterol 5 μg, which are the worldwide marketed product doses of tiotropium/olodaterol (Spiolto® Respimat), tiotropium (Spiriva® Respimat), and olodaterol (Striverdi® Respimat) in COPD. All treatments were administered once daily via the Respimat inhaler. Salbutamol (albuterol) (100 μg per actuation) was provided as rescue medication for all study participants and use was recorded in a diary. All studies were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation’s Harmonised Tripartite Guideline for Good Clinical Practice and local regulations. This work observed appropriate ethical guidelines relating to clinical trials involving human participants and with approval from institutional review boards (Coordinating Investigator’s [Prof Roland Buhl] Independent Ethics Committee: Ethikkommission bei der Landesärztekammer Rheinland-Pfalz, Deutschhausplatz 3, 55116 Mainz, Germany). Patients provided written informed consent for this study.
Study outcomes and assessments
For the overall study, primary end points included FEV1 area under the curve from 0 to 3 hours (AUC0–3) response (change from baseline), trough FEV1 response, and St George’s Respiratory Questionnaire (SGRQ) total score, all measured at 24 weeks. Secondary end points included a Transition Dyspnea Index focal score, also measured at 24 weeks, and further end points included FEV1 response over time measured at 5 minutes post-dose on Day 1 and at Weeks 12, 24, and 52. These and others have been reported elsewhere.Citation13
Subgroup analysis
The East Asian population was selected by Asian race from countries of mainland China, Taiwan, Japan, and South Korea; India was not included in countries defining the East Asian population. The overall population was defined as all patients worldwide, including the East Asian population.
In this prespecified exploratory analysis, we only report lung function end points (FEV1 AUC0–3, trough FEV1, and FVC), SGRQ total score, and safety. Transition Dyspnea Index focal score data, which was also exploratory in nature, are not included. P-values associated with treatment comparisons for the East Asian population were non-alpha controlled. P-values associated with treatment comparisons for the primary and key secondary end points for the overall population were confirmatory.
Treatment-emergent adverse events (AEs) were recorded, regardless of causality, throughout the trial and during a follow-up period of 21 days after study medication discontinuation. Vital signs were recorded at screening, baseline, and after 12, 24, and 52 weeks of treatment, and measured before the pre-dose and 60-minute post-dose pulmonary function tests. Twelve-lead electrocardiogram recordings were performed at screening, study withdrawal, pre-dose, and 40 minutes post-dose at baseline and at Weeks 12, 24, and 52.
Serious AEs were reviewed by an independent external expert adjudication committee, which was blinded to treatment, to determine if any of the deaths, hospitalizations, or intubations were related to respiratory, cardiovascular, cerebrovascular, or other diseases.
All randomized patients who received at least one dose of treatment were included in the safety analysis set (treated set). The frequencies of investigator-reported AEs (coded using the Medical Dictionary for Regulatory Activities) were analyzed descriptively. Analyses were performed on pooled data from the TONADO studies and were based on treatment-emergent AEs, including those AEs that occurred after the first dose of trial medication and within 21 days after the last dose of trial medication.
Results
Patients
A total of 6,887 patients were enrolled in the study across 40 different countries.Citation13 In the overall population, 5,163 (75.0%) patients were randomized to receive treatment, with 84.6% of patients completing the trials and 15.4% prematurely discontinuing study medication. In this article, we report on a total of 3,100 patients in the two studies, who were treated with tiotropium/olodaterol 5/5 μg (1,029; 33.2%), tiotropium 5 μg (1,033; 33.3%), or olodaterol 5 μg (1,038; 33.5%). Of these, 2,175 (70.2%) patients classified their race as white, 807 (26.0%) as Asian, 40 (1.3%) as black/African American, and 20 (0.6%) as American Indian/Alaska Native. Information on race could not be collected for 58 (1.9%) patients. Regionally, the overall population was divided between Western Europe (26.9%), East Asia (22.9%), North America (19.5%), Eastern Europe (16.3%), Latin America (8.9%), India (3.0%), and Australia/New Zealand/South Africa (2.5%).
In the East Asian population, 1,525 patients were enrolled, with 1,152 (75.5%) randomized to receive treatment. Of these, 1,151 patients received treatment. The trial was completed by 89.2% of patients, while 10.8% prematurely discontinued. For this analysis, a total of 709 (22.9%) patients were treated with the marketed product doses of tiotropium/olodaterol 5/5 μg, tiotropium 5 μg, or olodaterol 5 μg. The distribution of treatments was similar to the overall population, with 228 (32.2%) patients receiving tiotropium/olodaterol 5/5 μg, 243 (34.3%) receiving tiotropium 5 μg, and 238 (33.6%) receiving olodaterol 5 μg.
Some differences in demographics and baseline information were observed between the East Asian and overall populations (). The East Asian population contained a higher proportion of men, a lower mean weight and body mass index, and a lower percentage of current smokers than the overall population. The East Asian population reported less usage of pulmonary medication, particularly, inhaled corticosteroids, LABAs, and short-acting β-agonists, but did show an increased use of xanthines. There were also fewer comorbidities at baseline in the East Asian population and a shorter history of COPD diagnosis. Post-bronchodilator lung function at screening was slightly lower in the East Asian population, although this population also had slightly more patients with COPD severity of GOLD 4.
Table 1 Demographic and baseline patient characteristics
Lung function
After 24 weeks, the adjusted mean FEV1 AUC0–3 and trough FEV1 responses were significantly greater (P<0.0001) with tiotropium/olodaterol 5/5 μg versus monocomponents in both the East Asian and overall populations (). The East Asian population showed slightly greater trough FEV1 treatment differences between tiotropium/olodaterol 5/5 μg and tiotropium 5 μg, when compared to the overall population ().
Figure 1 Treatment differences for (A) FEV1 AUC0–3 response, (B) trough FEV1 response, and (C) FVC AUC0–3 response after 24 weeks in the East Asian and overall populations.
Abbreviations: AUC0–3, area under the curve from 0 to 3 hours; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; O, olodaterol; T, tiotropium.
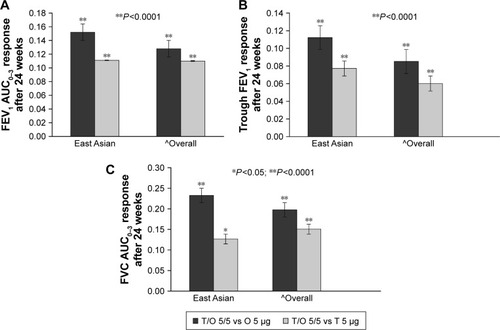
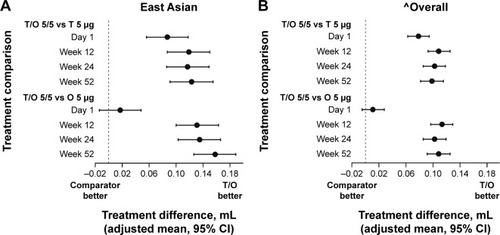
The secondary end point of the FVC AUC0–3 response significantly improved (P<0.05) in both the East Asian and overall populations with tiotropium/olodaterol 5/5 μg versus tiotropium 5 μg and olodaterol 5 μg (). FEV1 response over time favored tiotropium/olodaterol 5/5 μg compared to the monocomponents in both groups, and again treatment differences were larger for the East Asian population compared to the overall population ().
Figure 2 FEV1 treatment comparisons at 5 minutes post-dose at Day 1, Week 12, Week 24, and Week 52: (A) East Asian population and (B) overall population.
Abbreviations: FEV1, forced expiratory volume in 1 second; O, olodaterol; T, tiotropium.
Quality of life
Symptomatic benefit of tiotropium/olodaterol 5/5 μg was demonstrated by statistically significant improvements in mean SGRQ total score versus both monotherapies in the overall population after 24 weeks; in the East Asian population, this benefit was observed versus olodaterol 5 μg ().
Table 2 SGRQ score treatment comparison after 24 weeks of treatment
Similarly, responder rates, which are defined as a reduction of SGRQ total score of ≥4 units from baseline, were significantly greater for tiotropium/olodaterol 5/5 μg versus both monotherapies in the overall population; in the East Asian population, this was observed versus olodaterol 5 μg treatment ().
Table 3 Responder analysis of SGRQ total score treatment comparisons after 24 weeks of treatment
Safety
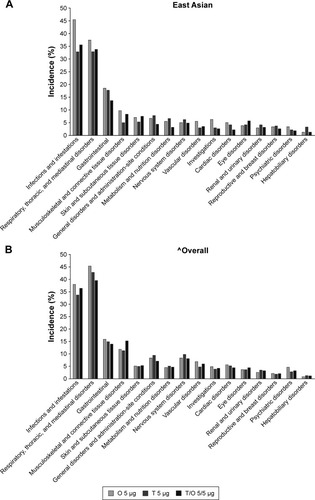
The incidence of AEs was similar in the East Asian and overall populations and was generally balanced across all treatment groups (). A similar number of AEs were considered treatment related in the East Asian and overall populations (7.1% versus 6.6%, respectively). The majority of AEs in both populations were mild to moderate in severity, and similar proportions of patients discontinued treatment due to AEs across treatment groups and between populations. In the East Asian population, there was a lower incidence of serious AEs and fatal AEs compared to the overall population, although the numbers were low. The most frequent System Organ Class events reported in both populations were infections and infestations, and respiratory, thoracic, and mediastinal disorders (). The East Asian population had a higher incidence of infections and infestations (including upper respiratory tract infections, bronchitis, and pneumonia) and a lower incidence of respiratory, thoracic, and mediastinal disorders (including COPD exacerbation); musculoskeletal and connective tissue disorders (including back pain); and nervous system disorders (including headache) when compared to the overall population.
Table 4 Summary of AEs
Figure 3 AEs occurring with an incidence of >3% by System Organ Class: (A) East Asian population and (B) overall population.
Abbreviations: AEs, adverse events; O, olodaterol; T, tiotropium.
Incidences of clinically relevant cardiovascular AEs were low in both populations (). The East Asian population reported slightly lower incidences of tachyarrhythmia and ischemic heart disease than the overall population.
Table 5 Clinically relevant cardiovascular AEs
Respiratory AEs in the East Asian and overall populations were broadly comparable between treatment groups (). The East Asian population had slightly lower incidence of lower respiratory disorders, including COPD exacerbation, compared to the overall population. Incidences of upper and lower respiratory tract infections, including bronchitis and pneumonia, were higher in the East Asian population, but comparable among the treatment groups.
Table 6 Clinically relevant respiratory AEs
There was generally no increase in incidence of AEs, cardiac AEs, or respiratory AEs with tiotropium/olodaterol 5/5 μg compared to tiotropium 5 μg and olodaterol 5 μg in either population.
Discussion
As mentioned earlier, differences in efficacy and safety in clinical studies of different therapies have been reported between Asian and non-Asian populationsCitation20–Citation23 and demonstrate the need to carefully assess all therapies in Asian populations.
This study assessed lung function and safety in a subset of East Asian patients in the large, replicate, 52-week TONADO studies, in comparison with the overall population. Minor differences were found in several population characteristics and baseline demographics, such as sex, weight, and pulmonary medication use at baseline. The East Asian population also had a lower incidence of comorbidities and a lower proportion of current smokers than the overall population. This finding may be due to a higher prevalence of smoking reported in Europe for both men and women, while a very high prevalence of smoking was reported only among men in East Asia.Citation26 The sex difference in smoking prevalence in the East Asian population may explain the low number of females included in the East Asian subgroup compared with the overall population. However, this did not seem to impact other demographics such as age or severity of disease, which may be expected as female COPD patients are often older than males or have more severe disease if not differentiated by age. One final difference between populations was the treatments used at baseline. Short-acting muscarinic antagonist, short-acting β-agonist, inhaled corticosteroid, and LABA were used less frequently in the East Asian population compared with the overall population, while the use of xanthines was higher. As the severity of disease was relatively consistent across the populations as per the GOLD stages, these treatment differences likely reflect differences in clinical practice in East Asia compared with other regions included in the overall population.
Lung function, as measured by FEV1 and FVC responses, improved substantially with tiotropium/olodaterol 5/5 μg in the East Asian population compared to monocomponents, which was consistent with the overall population.Citation13 No significant differences in safety outcomes were seen between the two populations. Minor differences in clinically relevant cardiovascular outcomes were noted, with the East Asian population reporting lower incidences of tachyarrhythmia and ischemic heart disease; this was possibly influenced by fewer comorbidities and current smokers at baseline in the East Asian population. Minor differences were also seen in clinically relevant respiratory events, with higher incidences for upper respiratory tract infections, bronchitis, and pneumonia seen in East Asian patients.
There was a significant reduction in SGRQ total score and responder rates for tiotropium/olodaterol 5/5 μg versus monotherapy for the overall population. In the East Asian population, SGRQ improvements were observed versus olodaterol 5 μg, but not tiotropium 5 μg. This was an exploratory analysis, and the lower number of East Asian patients included in this study limits the interpretation of this result. It is, however, also worth noting that improvements in SGRQ score from baseline have previously been described in placebo-treated patients from the Asia-Pacific area, which were accredited to a “trial effect”, showing sustained improvements irrespective of whether patients were receiving active study treatment.Citation27 Participation in a clinical trial in some Asian regions, in particular China, may have led to improved overall health care of patients. This observation should be considered for our findings, where the percentage of responders for treatment with tiotropium monotherapy was slightly raised compared with the overall population (51.9% versus 48.7%).
Generally, no increase in AEs was seen with tiotropium/olodaterol 5/5 μg compared to the monocomponents in either of the populations. These findings reflect the overall safety analysis of the TONADO study.Citation13 Interestingly, the incidences of most AE categories were lower for tiotropium/olodaterol 5/5 μg compared to monocomponents in both populations (). These data demonstrate that tiotropium/olodaterol 5/5 μg offers an acceptable safety profile in East Asian populations as well as in the overall population. These data mirror the safety data reported with olodaterol alone, where no safety concerns were identified up to 1 year at doses up to twice the recommended dose in Japanese and other Asian patients compared to Caucasian patients.Citation28 Tiotropium 5 μg alone has also been shown to significantly improve the lung function and demonstrated an acceptable safety profile in Chinese patients with COPD.Citation29
The pattern of AEs seen in the East Asian and overall populations is also likely influenced by comorbidities, which have a high prevalence among patients with COPD.Citation30 The most common comorbidities include cardiovascular diseases, metabolic disorders, osteoporosis, skeletal muscle dysfunction, anxiety/depression, cognitive impairment, gastrointestinal diseases, and respiratory conditions, which can have unique effects on COPD management and treatment.Citation31 The prevalence of AEs in this study is probably influenced by the presence of abundant comorbidities typically seen in patients with COPD.
This study had some limitations. Due to the high risk for events merely from preexisting comorbidities, it is usually difficult to assess a causal relationship between inhaled medications and AEs in patients with COPD. The East Asian population also contained a smaller number of patients, but the data are reassuring and suggest no obvious concerns for using tiotropium/olodaterol 5/5 μg in the East Asian population.
Overall, the efficacy/safety profile and positive benefit–risk of tiotropium/olodaterol 5/5 μg, as established for the overall population, have been demonstrated for the East Asian subpopulation. Physicians should feel confident prescribing tiotropium/olodaterol 5/5 μg to patients in the East Asian population.
Author contributions
The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors. They take full responsibility for the scope, direction, content, and editorial decisions relating to the manuscript; were involved at all stages of development; and have approved the submitted manuscript. All authors contributed to the concept/design of the study, data acquisition, or analysis and interpretation of the data. CB, OJ, and UB were involved in the analysis and planning for this manuscript; collated the data; and were involved with the interpretation of the study. MI, SHL, and KHL were study investigators and participated in the coordination and interpretation of the study. YZ performed the statistical analyses and was involved in the interpretation of the study. RB was the coordinating investigator of the study and participated in the design, implementation, and interpretation of the study.
Acknowledgments
The authors received no compensation related to the development of the manuscript. Medical writing assistance was provided by Laura Badtke, PhD, of Complete HealthVizion. This work was supported by Boehringer Ingelheim Pharma GmbH & Co. KG. Medical writing assistance was contracted and compensated by Boehringer Ingelheim Pharma GmbH & Co. KG.
Disclosure
RB reports grants from Boehringer Ingelheim, Novartis, and Roche, and personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Novartis, Roche, GlaxoSmithKline, and Takeda. MI reports personal fees from Nippon Boehringer Ingelheim, AstraZeneca, Novartis Pharma KK, and Kyorin. OJ and UB are employees of Boehringer Ingelheim Pharma GmbH & Co. YZ is an employee of Boehringer Ingelheim Pharmaceuticals, Inc. The authors report no other conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2016 Report Available from: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/Accessed April 27, 2016
- CasaburiRMahlerDAJonesPWA long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary diseaseEur Respir J200219221722411866001
- O’DonnellDEFlugeTGerkenFEffects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPDEur Respir J200423683284015218994
- MaltaisFHamiltonAMarciniukDImprovements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPDChest200512831168117816162703
- TashkinDPCelliBSennSA 4-year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med2008359151543155418836213
- BatemanEDTashkinDSiafakasNA one-year trial of tiotropium Respimat plus usual therapy in COPD patientsRespir Med2010104101460147220620037
- YohannesAMWillgossTGVestboJTiotropium for treatment of stable COPD: a meta-analysis of clinically relevant outcomesRespir Care201156447748721255503
- VogelmeierCHedererBGlaabTTiotropium versus salmeterol for the prevention of exacerbations of COPDN Engl J Med2011364121093110321428765
- FergusonGTFeldmanGJHofbauerPEfficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2–4 COPD: results from two replicate 48-week studiesInt J Chron Obstruct Pulmon Dis2014962964524966672
- KochAPizzichiniEHamiltonALung function efficacy and symptomatic benefit of olodaterol once daily delivered via Respimat® versus placebo and formoterol twice daily in patients with GOLD 2–4 COPD: results from two replicate 48-week studiesInt J Chron Obstruct Pulmon Dis2014969771425045258
- FeldmanGJBernsteinJAHamiltonANivensMCKorduckiLLaForceCThe 24-h FEV1 time profile of olodaterol once daily via Respimat® and formoterol twice daily via Aerolizer® in patients with GOLD 2–4 COPD: results from two 6-week crossover studiesSpringerplus20143141925187881
- LangePAumannJHamiltonATetzlaffKTingNThe 24-hour lung function time profile of olodaterol once daily versus placebo and tiotropium in patients with moderate to very severe chronic obstructive pulmonary diseaseJ Pulm Respir Med201444196
- BuhlRMaltaisFAbrahamsRTiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2–4)Eur Respir J201545496997925573406
- CazzolaMRoglianiPOraJMateraMGOlodaterol + tiotropium bromide for the treatment of chronic obstructive pulmonary diseaseExpert Rev Clin Pharmacol20158552953926294073
- SinghDFergusonGTBolitschekJTiotropium + olodaterol shows clinically meaningful improvements in quality of lifeRespir Med2015109101312131926320402
- BeehKMWestermanJKirstenAMThe 24-h lung-function profile of once-daily tiotropium and olodaterol fixed-dose combination in chronic obstructive pulmonary diseasePulm Pharmacol Ther201532535925956072
- BeehKMDeromEEchave-SustaetaJThe lung function profile of once-daily tiotropium and olodaterol via Respimat® is superior to that of twice-daily salmeterol and fluticasone propionate via Accuhaler® (ENERGITO® study)Int J Chron Obstruct Pulmon Dis20161119320526893551
- FergusonGTFlezarMKornSEfficacy of tiotropium + olodaterol in patients with chronic obstructive pulmonary disease by initial disease severity and treatment intensity: a post hoc analysisAdv Ther201532652353626112656
- SahooUBasic principlesClinical Research in Asia: Opportunities and ChallengesCambridgeWoodhead Publishing Ltd20124546
- SerebruanyVLTomekAPyaYBekbossynovaMKimMHInferiority of ticagrelor in the PHILO trial: play of chance in East Asians or nightmare confirmation of PLATO-USA?Int J Cardiol201621537237627128564
- YanoRKonnoAWatanabeKPharmacoethnicity of docetaxel-induced severe neutropenia: integrated analysis of published phase II and III trialsInt J Clin Oncol20131819610422095245
- HuangCELuCHChenPTEfficacy and safety of dose-modified docetaxel plus cisplatin-based induction chemotherapy in Asian patients with locally advanced head and neck cancerJ Clin Pharm Ther201237334234721950487
- WangHMLinCYHsiehCHInduction chemotherapy with dose-modified docetaxel, cisplatin, and 5-fluorouracil in Asian patients with borderline resectable or unresectable head and neck cancerJ Formos Med Assoc2017116318518927133181
- European Medicines AgencyICH Topic E 11 Clinical Investigation of Medicinal Products in the Paediatric Population Available: http://www.ema.europa.eu/docs/en_GB/document_library/Scien-tific_guideline/2009/09/WC500002926.pdfAccessed September 12, 2016
- YasudaSUZhangLHuangSMThe role of ethnicity in variability in response to drugs: focus on clinical pharmacology studiesClin Pharmacol Ther200884341742318615002
- NgMFreemanMKFlemingTDSmoking prevalence and cigarette consumption in 187 countries, 1980–2012JAMA2014311218319224399557
- JonesPWAndersonJACalverleyPMHealth status in the TORCH study of COPD: treatment efficacy and other determinants of changeRespir Res20111217121627828
- Boehringer Ingelheim International GmbHSpiolto Respimat 2.5 microgram/2.5 microgram, inhalation solution. Summary of product characteristics, labelling and package leaflet 2015 Available from: http://mri.medagencies.org/download/NL_H_3157_001_FinalPI.pdfAccessed March 1, 2016
- TangYMasseyDZhongNSEvaluation of the efficacy and safety of tiotropium bromide (5 microg) inhaled via Respimat in Chinese patients with chronic obstructive pulmonary diseaseChin Med J (Engl)2013126193603360724112149
- DecramerMJanssensWChronic obstructive pulmonary disease and comorbiditiesLancet Respir Med201311738324321806
- NegewoNAGibsonPGMcDonaldVMCOPD and its comorbidities: impact, measurement and mechanismsRespirology20152081160117126374280
