Abstract
Background
Smoking increases the risk of community-acquired pneumonia (CAP) and is associated with the development of COPD. Until now, it is unclear whether CAP in COPD is due to smoking-related effects, or due to COPD pathophysiology itself.
Objective
To evaluate the association between COPD and CAP by smoking status.
Methods
In total, 62,621 COPD and 191,654 control subjects, matched by year of birth, gender and primary care practice, were extracted from the Clinical Practice Research Datalink (2005–2014). Incidence rates (IRs) were estimated by dividing the total number of CAP cases by the cumulative person-time at risk. Time-varying Cox proportional hazard models were used to estimate the hazard ratios (HRs) for CAP in COPD patients versus controls. HRs of CAP by smoking status were calculated by stratified analyses in COPD patients versus controls and within both subgroups with never smoking as reference.
Results
IRs of CAP in COPD patients (32.00/1,000 person-years) and controls (6.75/1,000 person-years) increased with age and female gender. The risk of CAP in COPD patients was higher than in controls (HR 4.51, 95% CI: 4.27–4.77). Current smoking COPD patients had comparable CAP risk (HR 0.92, 95% CI: 0.82–1.02) as never smoking COPD patients (reference), whereas current smoking controls had a higher risk (HR 1.23, 95% CI: 1.13–1.34) compared to never smoking controls.
Conclusion
COPD patients have a fourfold increased risk to develop CAP, independent of smoking status. Identification of factors related with the increased risk of CAP in COPD is warranted, in order to improve the management of patients at risk.
Introduction
Community-acquired pneumonia (CAP) is characterized by an acute infection of the pulmonary parenchyma with onset in the out-of-hospital setting.Citation1 CAP incidence increases with age, smoking and the presence of comorbidities,Citation1,Citation2 varying between 1.5 and 11.0 per thousand adult population.Citation3,Citation4 In COPD, high incidence rates (IRs) of CAP, up to 22.4 per 1000 person-years, have been reported.Citation5 Worse outcomes ie, higher mortality ratesCitation6,Citation7 and longer length of hospital stayCitation6 were observed, as well as more pronounced hypoxemia,Citation7 hypercapnia,Citation6,Citation7 tachypneaCitation6 and increased symptoms such as dyspnea and purulent sputum.Citation7
Smoking individuals have a twofold increased CAP risk.Citation1,Citation4 Besides, smoking has been associated with increased susceptibility to infections in healthy subjectsCitation8 and COPD patients, triggering exacerbations.Citation9 Until now, it is unclear whether CAP development in COPD is due to smoking-related increased susceptibility to infections, or due to COPD pathophysiology itself. Müllerova et alCitation5 observed no association between current smoking and CAP incidence in COPD.
As smoking has been identified as a risk factor for both COPD and CAP, it is important to compare COPD patients to smoking and nonsmoking controls, to assess smoking-related effects, and distinguish possibly from additional risks associated with COPD and its pathophysiology. Particularly, it is important to take changes in smoking status over time into account, since smokers are known to undertake several attempts to quit smoking.Citation10,Citation11 Therefore, the aim of the present study was to evaluate the association between COPD and CAP by smoking status.
Methods
Source population
A population-based cohort study, with data derived from the world’s largest primary care database, Clinical Practice Research Datalink (CPRD), was conducted. CPRD contains computerized medical records of 674 primary care practices in the UK, collected since January 1987, representative for the total population.Citation12 Coded data are collected on demographics, prescription details, clinical events, preventive care provided, tests, immunizations, specialist referrals, hospital admissions, discharge summaries and details regarding death.Citation12 The period of data collection for the present study included the period in which the quality and outcomes framework (QOF) was effective (January 2005–January 2014).Citation13 CPRD data have been widely used to study CAP,Citation14,Citation15 COPDCitation16,Citation17 and other respiratory diseases.Citation18,Citation19 CPRD data have been shown to be accurate and valid.Citation20
Study population
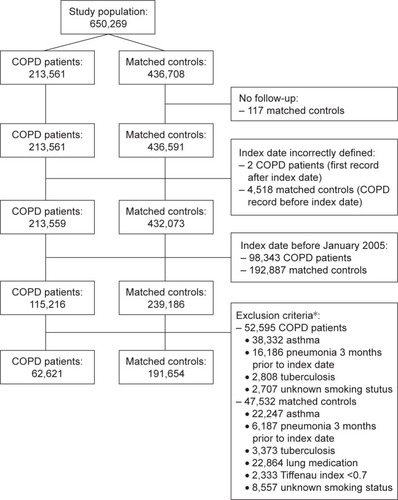
Two cohorts were extracted. Cohort I: patients aged ≥40 years with a first ever recorded COPD read code, assigned by the general practitioner (see Supplementary materials, Table S1). COPD diagnosis defined start of follow-up (index date). Cohort II: randomly selected controls, without COPD diagnosis, matched by year of birth, gender and practice, using incidence density sampling. Controls were assigned the index date of their matched COPD patient. Controls with lung medication or lung function with Tiffenau index <0.7 before start of follow-up were excluded.Citation21 From both cohorts, individuals with history of asthma, history of pneumonia 3 months prior to index date, active tuberculosis or use of tuberculosis medication and unknown smoking status were excluded.
Outcome
The primary outcome was physician-recorded pneumonia diagnosis, identified by read codes (Table S2). All patients were followed from index date to end of data collection, date of transfer out of the practice, patient’s death or outcome of interest, whichever came first. Follow-up time was divided into fixed intervals of 90 days.
Exposure of interest
Smoking status was determined prior to each interval (90-day) and stratified into three subcategories: never, current and former smoking. Smoking was defined by read codes (Table S3), which have provided valid estimates for the prevalence of current and never smoking.Citation22 When the most recent smoking status was “never”, and the patient had quit smoking, his status was classified as former smoking.
Potential confounders
Potential confounders were time-dependently assessed, except for gender and body mass index (BMI). Time-dependent potential confounders were collected at the start of each time interval (90-day): age, history of pneumonia, cerebrovascular disease, dementia, malignancy (excluding nonmelanoma skin cancer), chronic renal disease, diabetes mellitus (use of insulin and/or blood glucose lowering medicines), cardiovascular diseases (heart failure, ischemic heart disease, coronary artery disease, myocardial infarction) and chronic liver disease. Moreover, proxies of the underlying severity of COPD, including number of exacerbations in the year before, and use of the following drugs 6 months before, were collected: short-acting beta-2 agonists, long-acting beta-2 agonists, inhaled corticosteroids, xanthine derivatives, short-acting inhaled anticholinergics, long-acting anticholinergics, cromoglycates, oxygen or systemic glucocorticoids.Citation23 Analyses were adjusted for exposure to antipsychotics, acid suppressants or immunosuppressants in the past 6 months, as well as influenza or pneumococcal vaccination the year before. The most recent forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC) and Tiffenau index (ratio FEV1/FVC) were reviewed in the time frame ever before.
Statistical analysis
Data analyses were performed using SAS 9.3. IRs were estimated by dividing the total number of CAP cases by cumulative person-time at risk. Cox proportional hazard models estimated hazard ratios (HRs) of CAP in COPD patients versus controls. Analyses were stratified to gender, age and smoking status during follow-up.
Determinants of CAP within COPD were evaluated during follow-up: age, gender, smoking status and most recently recorded FEV1. HRs were estimated within each smoking status stratified for most recent level of airflow obstruction. HRs of CAP stratified by time-varying smoking status were assessed within COPD patients and controls separately. Never smoking was used as reference.
All analyses used time-varying Cox regression analysis. HRs were adjusted for gender and time-varying age and potential confounders (specified in previous section). Confounders were entered into the final model when independently changing the beta coefficient for current smoking by at least 5%, or when consensus was reached within the research team, supported by clinical evidence from literature. A test of interaction was performed to compare effects between the defined stratifications.Citation24 The study protocol was approved by Independent Scientific Advisory Committee, 14_055.
Results
In total, 254,277 subjects were included in the present analysis (). Of these, 62,621 had COPD. describes baseline characteristics: almost half were female, mean age was 67 years, follow-up time on average was 3.6–4.0 years. At baseline, smoking status differed: COPD patients were frequently former or current smokers, while controls were more often never smokers. FEV1 data were available for <50% of COPD patients, most classified as mild-to-moderate airflow obstruction.
Table 1 Baseline characteristics of patients with COPD and matched controls
CAP incidence
Around 3.04% (n=7,730) of the total population was diagnosed with CAP during follow-up: 3,819 (6.10%) COPD patients and 3,911 (2.04%) controls. Table S4 shows IRs of CAP in COPD patients (32.00 per 1000 person-years) and controls (6.75 per 1000 person-years). IRs increased with age and female gender. In COPD patients, IR was highest in never smokers (39.51 per 1000 person-years), while former smokers had the lowest IR (28.31 per 1000 person-years). In controls, IR was highest in current smokers (7.82 per 1000 person-years).
CAP risk in COPD patients versus controls
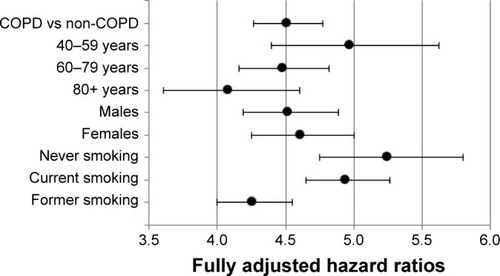
COPD patients had a fourfold increased CAP risk compared to controls (fully adjusted [adj.] HR 4.51 [4.27–4.77]; ; Table S4). Stratum-specific CAP risk in COPD was significantly higher (test of interaction HR 1.22 [1.03–1.45]) in younger patients (aged 40–59; fully adj. HR 4.97 [4.40–5.62]), then in elderly patients (aged ≥80; fully adj. HR 4.08 [3.61–4.60]). After stratification to smoking status, the HR of CAP was five times higher in never smoking COPD patients, compared to never smoking controls (fully adj. HR 5.25 [4.75–5.80]). The same for current smoking COPD patients versus current smoking controls (fully adj. HR 4.94 [4.65–5.26]). Former smoking COPD patients had a fourfold increased CAP risk compared to former smoking controls (fully adj. HR 4.26 [4.00–4.55]).
Figure 2 Stratum-specific risk of CAP in patients with COPD compared to matched controls, stratified by age, gender and smoking status.
Abbreviations: BMI, body mass index; CAP, community-acquired pneumonia; ICS, inhaled corticosteroids.
CAP risk and smoking status
Within COPD, CAP risk in current smokers (fully adj. HR 0.92 [0.82–1.02]) was comparable to the risk in never smokers (reference; ; Table S5). Former smoking COPD patients had a lower risk (fully adj. HR 0.81 [0.73–0.90]). Current smokers had a significantly higher CAP risk as compared to former smokers (P<0.001). CAP risk increased by older age (fully adj. HR aged 60–79, 2.26 [2.00–2.57]; aged ≥80, 6.06 [4.86–7.55]) and female gender (fully adj. HR 1.30 [1.22–1.39]). The level of airflow obstruction showed a trend toward increased CAP risk for very severe obstruction (fully adj. HR 1.30 [0.98–1.73]) in comparison to mild obstruction (reference). A subanalysis was performed by smoking status, stratified for level of airflow obstruction (Table S6). No clear trend of increased risk by severity of airflow obstruction was observed within never, current or former smokers.
Figure 3 Risk of CAP in patients with COPD, stratified by age, gender, smoking status and the level of airflow obstruction.
Abbreviations: BMI, body mass index; CAP, community-acquired pneumonia; ICS, inhaled corticosteroids.
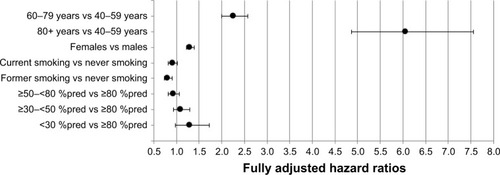
Within controls, CAP risk was highest in current smokers (fully adj. HR 1.23 [1.13–1.34]), while the risk in former smokers (fully adj. HR 1.07 [0.99–1.16]) was comparable with the risk in never smokers (reference; Table S7). Older age (fully adj. HR aged 60–79 years, 2.54 [2.23–2.90]; aged ≥80 years, 8.85 [7.10–11.00]) and female gender (fully adj. HR 1.26 [1.18–1.35]) showed increased CAP risk.
Discussion
COPD patients had a fourfold increased CAP risk in comparison with matched controls. Current smoking had no additional impact on CAP risk in COPD. In controls, CAP risk was elevated in current smokers. The risk of CAP increased in both COPD patients and controls with older age and female gender.
CAP risk and IRs
COPD patients had higher CAP IRs and increased CAP risk compared to controls, in line with former research.Citation4,Citation25 The observed IR in COPD patients was higher than reported by Müllerova et alCitation5 (22.4/1000 person-years), but in accordance with the incidence reported by DiSantostefano et al (30.9/1000 person-years).Citation26 In controls, Capelastegui et alCitation27 observed an IR of 3.1/1000 adults per year, while our observed IR was twice as high (6.8/1000 person-years). A possible explanation of the higher IRs observed, might be the rising incidence in European countries.Citation28 A number of factors are associated with this phenomenon: populations grow older, and lifestyle factors and comorbid conditions related to CAP become more prevalent.Citation28 Older age might also be a potential factor in our study, as the average age was 67 years. This was also shown by Millett et alCitation29 with an incidence of 8.0/1000 person-years in controls ≥65 years old.
Gender differences appeared, as females had a slightly larger CAP risk than males. This is in contrast with former research.Citation2,Citation30,Citation31 Reasons for increased risk in females could not be further delineated in this study, but clearly warrant further research. Changes in female lifestyle and risk behavior have been reported in previous decades and might represent important factors.Citation32,Citation33 Furthermore, former research showed that respiratory symptoms were more often reported by females than males,Citation34 with higher hospitalization incidences in studies concerning milder CAP cases.Citation35,Citation36
Smoking status and CAP risk
Current smoking was associated with increased CAP risk in controls, in line with former research.Citation37–Citation39 Smoking has been related to structural changes in the respiratory tract and a decrease in immune response,Citation40 which might result in microbial invasion of the bronchial tree, triggering CAP.
In COPD patients, CAP risk was comparable between never and current smokers. It was expected that, in accordance with controls, current smokers were at increased CAP risk. However, Müllerova et alCitation5 also observed no difference in CAP risk between nonsmoking and current smoking COPD patients. Moreover, Myint et alCitation41 observed a larger proportion of current smokers in COPD without CAP than in CAP–COPD. Maybe, inaccurate recording of smoking status by general practitioners influenced the present results, although smoking is a QOF indicator, rewarding general practitioners to record patients’ smoking status every year when diagnosed with COPD. However, smoking is the major risk factor of COPD, but the majority of persistent smokers do not develop COPD. This suggests that the vulnerability to cigarette smoke varies between individuals. The mechanisms behind this are at the moment not completely understood.Citation42 Never smokers may also develop COPD, but by a different pathway than exposure to smoking,Citation43 for example, by occupational/environmental exposures, alpha-1-antitrypsin deficiency or due to factors early in life which affect the respiratory health in the long-term.Citation44 Pathophysiological differences between subgroups of COPD might contribute to the observed differences in CAP risk. Besides, it is likely that a combination of factors is responsible for disease development, or not. For example, smoking has been associated with CAP development, but is also associated with a lower socioeconomic status, poor diet, alcohol consumption and reduced physical activity,Citation45 which, in turn, are also risk factors for CAP.Citation4 Furthermore, there are theoretically three general mechanisms related to the increased CAP risk of smoking: 1) tobacco-induced physiological and structural changes, 2) tobacco-induced increase in bacterial virulence, 3) tobacco-induced dysregulation of immune function.Citation8 These three mechanisms are also key features in COPD, and probably the smoking-effect related to both the development of CAP and COPD, does not sum-up. Overall, many mechanisms might be associated with the observed results, but further research is warranted to explore exact pathways involved.
We also observed no difference concerning severity of airflow obstruction stratified by smoking status and CAP risk. IRs increased with worse airflow obstruction, but only showing a trend toward increased risk in very severe airflow obstruction. Conflicting results were reported before, some observing increased CAP risk in severe and very severe airflow obstruction,Citation46 while others observed no difference in CAP risk by airflow obstruction.Citation41 There may be reverse causation underlying this lack of relationship; those with worse airflow obstruction stopped smoking, while others continued their smoking habits. However, this is less likely, as smoking status over time was taken into account, correcting for possible confounding. Furthermore, never and current smoking COPD patients had a comparable risk, which stresses the fact that the observed results are not due to smoking cessation.
Strengths and limitations
The current study includes a large population-based cohort study, providing anonymous longitudinal medical records of primary care patients.Citation12,Citation47 This study design makes the current results generalizable to a larger population. In addition, by taking smoking status over time into account, the results are representative of real life setting.
In contrast, several methodological issues could have influenced the results. First, primary care databases rely on the quality of information included in records.Citation12 This depends on the accuracy of individuals responsible for entering data. However, inclusion of a large number of patients will minimize potential bias. Second, we did not use spirometry to confirm COPD diagnosis, as this was available for only one-third of patients. Misclassification of exposure is likely to be nondifferential and may have led to a bias toward null.Citation48 This implies that the fourfold increased risk of CAP in COPD versus non-COPD may have been under-estimated. In addition, CAP diagnosis was not confirmed by chest X-ray, but based on clinical features, probably causing CAP overestimation.Citation49 This may have led to a nondifferential misclassification of the outcome and a bias toward the null value. However, it has a small impact on our main findings: the true HR of CAP among smokers versus nonsmokers in COPD would have been lower than the nonsignificant 8% reduced risk, and will still not support our main hypothesis that smoking increases the risk of CAP in COPD. In addition, ICS use was not separately analyzed, but only included as a potential confounder, as this would go beyond the primary aim of the objective. However, ICS risk in CAP is of high interest, often showing an increased risk, although probably depending on the specific ICS and dose.Citation50
Clinical implications
The results of the present analyses highlight the fact that CAP remains a major health issue, impacting both socially and economically.Citation51 Smoking cessation is an important aspect in the management of CAP and especially in control subjects, this strategy might be beneficial and lead to a decreased risk to develop CAP.Citation38 In contrast, for patients with COPD, this association seems less clear, but as earlier described, further research is necessary to assess mechanisms associated with the increased risk to develop CAP. For clinical practice it remains important to not underestimate the impact of CAP in patients with COPD. The management of CAP is at the moment focused on treating the disease, and prevention by vaccination,Citation51 while prevention of lifestyle factors, such as smoking, alcohol consumption and bodyweight, is at least as important.Citation4,Citation38,Citation39,Citation52,Citation53
Conclusion
COPD patients have a fourfold increased risk to develop CAP, independent of smoking status. Identification of factors related with the increased risk of CAP in COPD is warranted, in order to improve the management of patients at risk.
Acknowledgments
The abstract of this manuscript was presented as an oral presentation during the conference of the European Respiratory Society in September 2016 in London. The abstract was published in the “ERS International Congress 2016 Abstracts” (http://erj.ersjournals.com/content/48/suppl_60/OA1504).
Disclosure
The authors report no conflicts of interest in this work.
References
- HerreroFSOlivasJBMicrobiology and Risk Factors for Community-acquired PneumoniaSemin Respir Crit Care Med201233322023122718208
- Vila-CorcolesAOchoa-GondarORodriguez-BlancoTRaga-LuriaXGomez-BertomeuFGroup ESEpidemiology of community-acquired pneumonia in older adults: a population-based studyRespir Med2009103230931618804355
- LimWSBaudouinSVGeorgeRCBTS guidelines for the management of community acquired pneumonia in adults: update 2009Thorax200964Suppl 3iii1iii5519783532
- TorresAPeetermansWEViegiGBlasiFRisk factors for community-acquired pneumonia in adults in Europe: a literature reviewThorax201368111057106524130229
- MüllerovaHChigboCHaganGWThe natural history of community-acquired pneumonia in COPD patients: a population database analysisRespir Med201210681124113322621820
- BraekenDCWFranssenFMESchuetteHIncreased severity and mortality of CAP in COPD: results from the German Competence Network, CAPNETZChronic Obstr Pulm Dis201522131140
- MolinosLClementeMGMirandaBASTURPAR GroupCommunity-acquired pneumonia in patients with and without chronic obstructive pulmonary diseaseJ Infect200958641742419329187
- BagaitkarJDemuthDRScottDATobacco use increases susceptibility to bacterial infectionTob Induc Dis200841219094204
- BauerCMMorissetteMCStampfliMRThe influence of cigarette smoking on viral infections: translating bench science to impact COPD pathogenesis and acute exacerbations of COPD clinicallyChest2013143119620623276842
- van EerdEAMvan RossemCRSpigtMGWesselingGvan SchayckOCPKotzDDo we need tailored smoking cessation interventions for smokers with COPD? A comparative study of smokers with and without COPD regarding factors associated with tobacco smokingRespiration201590321121926022403
- GetsiosDMartonJPRevankarNSmoking cessation treatment and outcomes patterns simulation: a new framework for evaluating the potential health and economic impact of smoking cessation interventionsPharmacoeconomics201331976778023821436
- HerrettEGallagherAMBhaskaranKData resource profile: Clinical Practice Research Datalink (CPRD)Int J Epidemiol201544382783626050254
- RolandMLinking physicians’ pay to the quality of care – a major experiment in the United kingdomN Engl J Med2004351141448145415459308
- Wvan der ZandenRde VriesFLalmohamedAUse of dipeptidylpeptidase-4 inhibitors and the risk of pneumonia: a population-based cohort studyPLoS One20151010e013936726468883
- MillettERDe StavolaBLQuintJKSmeethLThomasSLRisk factors for hospital admission in the 28 days following a community-acquired pneumonia diagnosis in older adults, and their contribution to increasing hospitalisation rates over time: a cohort studyBMJ Open2015512e008737
- MullerovaHShuklaAHawkinsAQuintJRisk factors for acute exacerbations of COPD in a primary care population: a retrospective observational cohort studyBMJ Open2014412e006171
- Garcia RodriguezLAWallanderMATolosaLBJohanssonSChronic obstructive pulmonary disease in UK primary care: incidence and risk factorsCOPD20096536937919863366
- de VriesFSetakisEZhangBvan StaaTPLong-acting {beta}2-agonists in adult asthma and the pattern of risk of death and severe asthma outcomes: a study using the GPRDEur Respir J201036349450220351036
- ZhangBde VriesFSetakisEvan StaaTPThe pattern of risk of myocardial infarction in patients taking asthma medication: a study with the general practice research databaseJ Hypertens20092771485149219491706
- HerrettEThomasSLSchoonenWMSmeethLHallAJValidation and validity of diagnoses in the general practice research database: a systematic reviewBr J Clin Pharmacol201069141420078607
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management, and prevention of Chronic Obstructive Pulmonary Disease2015 Available from: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.htmlAccessed February 17, 2015
- BoothHPPrevostATGullifordMCValidity of smoking prevalence estimates from primary care electronic health records compared with national population survey data for England, 2007 to 2011Pharmacoepidemiol Drug Saf201322121357136124243711
- de VriesFvan StaaTPBrackeMSCooperCLeufkensHGLammersJWSeverity of obstructive airway disease and risk of osteoporotic fractureEur Respir J200525587988415863646
- AltmanDGBlandJMInteraction revisited: the difference between two estimatesBMJ2003326738221912543843
- RyanMSuayaJAChapmanJDStasonWBShepardDSThomasCPIncidence and cost of pneumonia in older adults with COPD in the United StatesPLoS One2013810e7588724130749
- DiSantostefanoRLSampsonTLeHVHindsDDavisKJBakerlyNDRisk of pneumonia with inhaled corticosteroid versus long-acting bronchodilator regimens in chronic obstructive pulmonary disease: a new-user cohort studyPLoS One201495e9714924878543
- CapelasteguiAEspanaPPBilbaoAStudy of community-acquired pneumonia: incidence, patterns of care, and outcomes in primary and hospital careJ Infect201061536437120692290
- ThomsenRWRiisANorgaardMRising incidence and persistently high mortality of hospitalized pneumonia: a 10-year population-based study in DenmarkJ Intern Med2006259441041716594909
- MillettERQuintJKSmeethLDanielRMThomasSLIncidence of community-acquired lower respiratory tract infections and pneumonia among older adults in the United Kingdom: a population-based studyPLoS One201389e7513124040394
- GutierrezFMasiaMMireteCThe influence of age and gender on the population-based incidence of community-acquired pneumonia caused by different microbial pathogensJ Infect200653316617416375972
- ManninoDMDavisKJKiriVAChronic obstructive pulmonary disease and hospitalizations for pneumonia in a US cohortRespir Med2009103222422918945605
- RahoEvan OostromSHVisserMGeneration shifts in smoking over 20 years in two Dutch population-based cohorts aged 20–100 yearsBMC Public Health20151514225884440
- BilalUBeltranPFernandezENavas-AcienABolumarFFrancoMGender equality and smoking: a theory-driven approach to smoking gender differences in SpainTob Control201625329530025701858
- LamprechtBVanfleterenLEStudnickaMSex-related differences in respiratory symptoms: results from the BOLD StudyEur Respir J201342385886024000253
- MarrieTJHuangJQLow-risk patients admitted with community-acquired pneumoniaAm J Med2005118121357136316378779
- AliyuZYAliyuMHMcCormickKDeterminants for hospitalization in “low-risk” community acquired pneumoniaBMC Infect Dis200331112809564
- AlmirallJBolibarISerra-PratMNew evidence of risk factors for community-acquired pneumonia: a population-based studyEur Respir J20083161274128418216057
- AlmirallJGonzalezCABalanzoXBolibarIProportion of community-acquired pneumonia cases attributable to tobacco smokingChest1999116237537910453865
- BaikICurhanGCRimmEBBendichAWillettWCFawziWWA prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and womenArch Intern Med2000160203082308811074737
- ArcaviLBenowitzNLCigarette smoking and infectionArch Intern Med2004164202206221615534156
- MyintPKLoweDStoneRABuckinghamRJRobertsCMUK National COPD Resources and Outcomes Project 2008: patients with chronic obstructive pulmonary disease exacerbations who present with radiological pneumonia have worse outcome compared to those with non-pneumonic chronic obstructive pulmonary disease exacerbationsRespiration201182432032721597277
- PanZYuHLiaoJLProbing cellular and molecular mechanisms of cigarette smoke-induced immune response in the progression of chronic obstructive pulmonary disease using multiscale network modelingPLoS One2016119e016319227669518
- SalviSSBarnesPJChronic obstructive pulmonary disease in nonsmokersLancet2009374969173374319716966
- CarraroSScheltemaNBontLBaraldiEEarly-life origins of chronic respiratory diseases: understanding and promoting healthy ageingEur Respir J20144461682169625323240
- AlmirallJBlanquerJBelloSCommunity-acquired pneumonia among smokersArch Bronconeumol201450625025424387877
- CrimCCalverleyPMAndersonJAPneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study resultsEur Res J2009343641647
- Clinical Practice Research Datalink Available from: http://www.cprd.comAccessed January 20, 2016
- RothmanKJGreenlandSLashTLModern EpidemiologyLippincott Williams and Wilkins; PhiladelphiaLippincott Williams & Wilkins2008137144
- MandellLAWunderinkRGAnzuetoAInfectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adultsClin Infect Dis200744Suppl 2S27S7217278083
- FinneyLBerryMSinganayagamAElkinSLJohnstonSLMalliaPInhaled corticosteroids and pneumonia in chronic obstructive pulmonary diseaseLancet Respir Med201421191993225240963
- WelteTTorresANathwaniDClinical and economic burden of community-acquired pneumonia among adults in EuropeThorax2012671717920729232
- GrauIArdanuyCCalatayudLSchulzeMHLinaresJPallaresRSmoking and alcohol abuse are the most preventable risk factors for invasive pneumonia and other pneumococcal infectionsInt J Infect Dis201425596424853638
- CecereLMWilliamsECSunHSmoking cessation and the risk of hospitalization for pneumoniaRespir Med201210671055106222541719