?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Tidal expiratory flow limitation (EFLT) is frequently found in patients with COPD and can be detected by forced oscillations when within-breath reactance of a single-breath is ≥0.28 kPa·s·L−1. The present study explored the association of within-breath reactance measured over multiple breaths and EFLT with 6-minute walk distance (6MWD), exacerbations, and mortality.
Methods
In 425 patients, spirometry and forced oscillation technique measurements were obtained on eight occasions over 3 years. 6MWD was assessed at baseline and at the 3-year visit. Respiratory symptoms, exacerbations, and hospitalizations were recorded. A total of 5-year mortality statistics were retrieved retrospectively. We grouped patients according to the mean within-breath reactance , measured over several breaths at baseline, calculated as mean inspiratory–mean expiratory reactance over the sampling period. In addition to the established threshold of EFLT, an upper limit of normal (ULN) was defined using the 97.5th percentile of
, of the healthy controls in the study; 6MWDs were compared according to
, as normal, ≥ ULN < EFLT, or ≥ EFLT. Annual exacerbation rates were analyzed using a negative binomial model in the three groups, supplemented by time to first exacerbation analysis, and dichotomizing patients at the ULN.
Results
In patients with COPD and baseline below the ULN (0.09 kPa·s·L−1), 6MWD was stable. 6MWD declined significantly in patients with
. Worse lung function and more exacerbations were found in patients with COPD with
, and patients with
had shorter time to first exacerbation and hospitalization. A significantly higher mortality was found in patients with
and FEV1 >50%.
Conclusion
Patients with baseline had a deterioration in exercise performance, more exacerbations, and greater hospitalizations, and, among those with moderate airway obstruction, a higher mortality.
is a novel independent marker of outcome in COPD.
Introduction
COPD is a major cause of morbidity and mortality, leading to an estimated 3.1 million deaths globally in 2012.Citation1 Although symptoms and exacerbation rate have been added to our assessment system of COPD,Citation2 spirometry remains an important tool in assessing severity and prognosis in this disease. Changes in the forced expiratory volume in 1 second (FEV1) are still the best indicator of disease progression and the risk of dying from COPD.Citation2,Citation3 Yet, on an individual level, FEV1 is an unreliable marker of morbidity, especially in early disease,Citation4–Citation6 and there is a need to identify alternative objective tests that can aid in stratifying the risk of further patient deterioration.
Patients who exhibit tidal expiratory flow limitation (EFLT) might be such a subgroup. EFLT occurs when increases in driving pressure fail to increase expiratory flow during resting tidal breathing and is most often seen in patients with severe COPD,Citation7,Citation8 although it can occur with only moderate airway obstruction. Previous studies of EFLT in COPD have focused on its association with operating lung volume, exercise tolerance,Citation9,Citation10 and its relation to breathlessness.Citation11–Citation13 It is not known whether EFLT can provide longer-term prognostic information in COPD.
EFLT can be detected by the forced oscillation technique (FOT).Citation14,Citation15 When peripheral airways collapse on expiration, oscillatory pressure signals are prevented from reaching the alveoli. As this happens, the oscillatory compliance is reduced. Consequently, expiratory reactance (Xrsexp) becomes more negative than the inspiratory reactance (Xrsinsp), leading to a within-breath reactance difference (ΔXrs). In the absence of EFLT, reactance measured in inspiration and in expiration is almost identical. The within-breath reactance difference (ΔXrs) cut-off of 0.28 kPa·s·L−1 has been defined and validated to identify flow-limited breaths.Citation14,Citation15 EFLT is not only linked to dyspnea in COPD,Citation11,Citation16 but also to COPD exacerbations, where ΔXrs have been found to increase at the onset of COPD exacerbations and decrease when the exacerbation resolves.Citation17
In this study, we used forced oscillation technique to define EFLT in 425 patients with COPD. We hypothesized that the presence of EFLT, assessed by increased mean ΔXrs , would relate to changes in 6MWD, the risk of COPD exacerbations, and, possibly, mortality. We explored these hypotheses using baseline
as a predictor for future events. Patients with COPD with abnormally high
, both above the upper limit of normal (ULN) and above the established threshold of EFLT, were investigated to determine whether they differed from those without evidence of tidal expiratory flow limitation.
Methods
Study design and patients
The current data derive from the Bergen cohort of the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study,Citation18 with additional patients enrolled from our clinical catchment area. Written informed consent was obtained from all study subjects. The study was approved by the regional ethics committee, REK vest (REK 165.08), and performed in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines (ClinicalTrials.gov; No: NTC00292552; www.ClinicalTrials.gov).Citation39
Inclusion criteria for patients with COPD were: age 40–75 years, FEV1 <80% predicted, FEV1/FVC <0.7, and a smoking history of ≥10 pack-years. Study patients were evaluated every 6 months for 3 years with an additional visit at 3 months after baseline, totaling eight visits. The American Thoracic Society-Division of Lung Disease (ATS-DLD-78) questionnaire and the modified Medical Research Council dyspnea scale score (mMRC) were used to record respiratory symptoms.Citation19,Citation20
Post-bronchodilator spirometry and oscillatory lung mechanics during tidal breathing were performed after inhalation of 0.4 mg salbutamol (GlaxoSmithKline, Ventolin, London, UK) according to American Thoracic Society/European Respiratory Society (ATS/ERS) international standards at each visit using a Jaeger MasterScope CT Impulse Oscillation System (Jaeger, Hoechberg, Germany).Citation21,Citation22 Local reference values were used to determine the FEV1% predicted.Citation23 The FOT measurements were performed with the patient seated, cheeks supported, and wearing a nose clip. We performed three continuous measurements of 30 seconds, totaling 90 seconds of tidal volume breathing. Acceptability of measurements was determined using the ERS 2003 task force recommendations.Citation22
reactance was averaged over the 90-second sample, containing several breaths, and was calculated as follows:
Xrsinsp at 5 Hz – mean Xrsexp at 5 Hz. Two cut-offs were investigated: the ULN defined as the 97.5th percentile of healthy controls in the Bergen cohort of the ECLIPSE study (
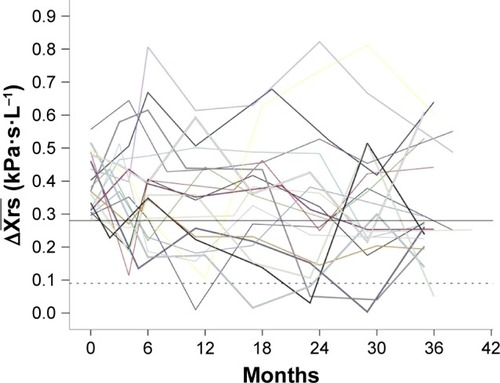
; Figure S1),Citation13 and at the established EFLT defining threshold for within-breath reactance, 0.28 kPa·s·L−1, derived from single-breath analysis.Citation14,Citation15 To illustrate the variability of our multiple-breath measurement,
was plotted against time in a subset of the patients defined EFLT at baseline with complete visits (N=20).
Study outcomes
Distance walked during the 6-minute walk test (6MWT) was used to assess exercise performance. The 6MWT was supervised by a trained technician and performed in a 30-m, straight hospital corridor according to agreed standards at baseline and at the end of the study, at the 3-year visit.Citation24
Exacerbations were defined as a worsening of respiratory symptoms over 2 days or more that required systemic corticosteroids or antibiotics, alone or in combination (“moderate exacerbations”), or exacerbations resulting in hospitalization (“severe exacerbations”). Assessment of exacerbations was performed retrospectively by the study physician at the half-yearly visits, over the 3-year study period.
Mortality statistics were retrieved on August 25, 2011, approximately 5 years after the conclusion of the baseline visit by checking vital status in our local patient file system, which is linked to the Norwegian Causes of Death Registry. The Causes of Death Registry includes deaths of all residents, regardless of whether they die in Norway or abroad, and is assumed to have information on >98% of all deaths.Citation25
Statistics
IBM SPSS version 22, Stata 13.1, and R 3.2.3 GUI 1.66 were used for different aspects of statistical analyses. Data are presented as mean ± standard deviation, median (quartiles), mean (95% confidence interval), and absolute count or percentage. Means were compared using independent samples t-tests, paired samples t-tests, or Mann–Whitney U test when appropriate.
Patients were categorized into three groups according to : normal, ≥ ULN < EFLT, and ≥ EFLT at baseline. We report the change in the 6-minute walk distance in these three groups from baseline to the 3-year visit in notched boxplots. Differences in walking distance between baseline and the end of the study were compared using paired samples t-test, grouping the patients according to the
and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) grade.Citation2
The rate of exacerbations in the three groups was analyzed as suggested by Keene et al using a negative binomial regression model, accounting for the yearly variability among test subjects in the exacerbation rate over the 3-year study period, adjusting for FEV1, age, sex, and a binary classifier identifying frequent exacerbators in the year prior to inclusion.Citation26 Parameter effects of the baseline explanatory variables are reported as annual rate ratios (RRs), 95% confidence interval (CI), and P-values. Moderate and severe exacerbations requiring hospitalization were also investigated on the basis of the occurrence of at baseline by time to first event analysis. Groups were compared using a log-rank test. Results are displayed as 1-minus-survival plots.
Survival analysis was performed with Kaplan–Meier survival analysis, comparing distributions with the log-rank test, grouping patients by the occurrence of at baseline, and by dichotomizing FEV1 at 50% of that predicted.
Results
Baseline characteristics
In this population of patients with COPD, 60% were men and had moderate to very severe airway obstruction. Women were, on average, 2 years younger and had lower tobacco exposure (Table S1); 50% of patients with COPD had normal , 31% were classified as being abnormal with
, and 18% had
above the threshold of EFLT (). From the normal to the abnormal group, lung function worsened with a decline in FEV1 and FVC, and an increase in body mass index (BMI) and mMRC. This difference was most marked between the normal and EFLT group. For IC, we only found a significant difference between the normal and the EFLT group.
Table 1 Baseline characteristics in COPD patients with different levels of (N=425)
was not a stable measurement. Many patients defined as EFLT at baseline fell below the EFLT threshold during the course of the study (). Six percent of the consequent
measurements were found to be normal; 23% of the patients dropped out of the study before the final visit, the main reason being death during the study period ().
Table 2 Deaths and dropouts during the 3-year study period (N=96)
Relationship of EFLT to study outcomes
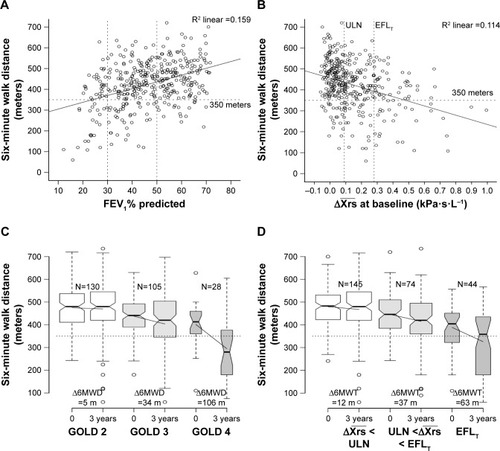
was as poorly correlated with baseline 6-minute walking distance as FEV1 (). On a group level, 6MWD declined with increasing
, being 455 m in the normal, 410 m in the abnormal, and 363 m in the EFLT group (). In patients who completed both the baseline and the final visit, changes in 6MWD were compared. No significant change between the baseline and the final 3-year visit was seen in the 6MWD of patients with normal
. Patients with
declined by a mean of 37 m (95% CI 15–58), P=0.001, and patients with EFLT declined by 63 m (95% CI 31–95), P<0.001 (). Grouping by GOLD grade produced similar boxplots. GOLD 2 patients remained stable whereas a significant decline was seen in GOLD 3–4 grades ().
Figure 2 (A) Six-minute walk distance (6MWD) in patients with COPD (N=388) at different levels of FEV1% predicted (A) and (B) at baseline. Dotted lines set at 350 m (horizontal) and at
, upper limit of normal (ULN), and
, the threshold for tidal expiratory flow limitation (EFLT). The solid lines represent the regression lines. (C) 6MWT at baseline and the 3-year visit presented by notchplots with quartiles, 95% central range and outliers among COPD patients of GOLD II–IV grades and categorized according to
(D) as normal (white), below EFLT, but above ULN (light gray), and > EFLT threshold (gray). Non-overlapping notched areas are likely to represent significant differences between groups. Solid lines are drawn between the mean 6MWD at baseline and the 3-year visit.
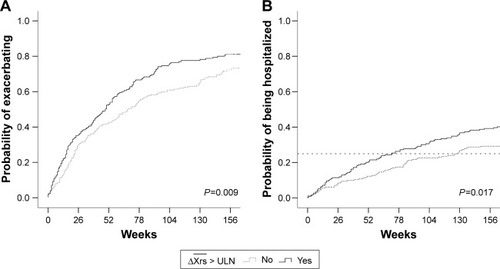
A total of 1,289 moderate to severe exacerbations were recorded throughout the study. By negative binomial regression, estimated annual exacerbations rates were 0.7 in patients with normal , 1.3 in patients with COPD with
, and 1.6 in patients with EFLT. Annual exacerbation RR compared to the normal group was 1.28 for ULN patients and 1.30 for EFLT patients (). A time to first exacerbation analysis was performed in patients with COPD with and without evidence of
at baseline. Significant differences were found in both time to first exacerbation (P=0.009) and in time to first hospitalization (P=0.017; ). Median time to first moderate or severe exacerbation was 76 weeks in patients with COPD with normal
, compared with only 55 weeks in COPD patients with
at baseline (). The time until 25% of the patients were hospitalized was 126 weeks in patients with normal
and 72 weeks in patients with
.
Table 3 Annual rate ratios estimated by negative binomial regression (N=395)
Table 4 Exacerbations, hospitalizations, and deaths in COPD patients with and without at baseline (N=425)
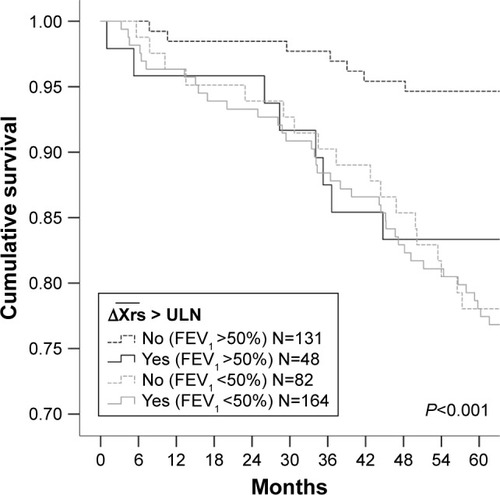
Mortality
At the 5-year census, 72 (17%) of the 425 patients with COPD had died. Deaths were significantly more frequent in patients with ≥ ULN than in patients with normal , but not significantly different between the ≥ ULN and the EFLT, groups (). Mortality was higher in patients with FEV1 <50% (). The difference in mortality between patients with
in the normal range and ≥ ULN was driven by increased mortality in patients with FEV1 >50% (). Mortality was 17% in
patients with FEV1 >50%, compared to 5% in patients with normal ΔXrs (P<0.001; ). Mortality in patients with ΔXrs ≥ ULN was similar to what was found in patients with COPD with much more advanced airway obstruction.
Discussion
Using data from the ECLIPSE study, we investigated associations between increased at the ULN and at the threshold of EFLT with decline in 6MWD, risk of later exacerbations, and all-cause mortality. No change in 6MWD was found in patients with COPD with
in the normal range. All patients with COPD with
deteriorated significantly in 6MWD. Patients with COPD with
had increased risk for both moderate and severe exacerbations, and, in the patients with only moderate airway obstruction, FEV1 >50%, a significantly higher mortality. This study Xrs demonstrates that
provides valuable extra information in addition to FEV1 when characterizing the effects of small-airway obstruction on key COPD outcomes.
We defined the ULN at the 97.5th percentile of healthy controls included in our study. Data describing the control group have previously been published.Citation13,Citation18 The FOT threshold identifying a single flow-limited breath has been defined when within-breath reactance was >0.28 kPa·s·L−1.Citation14,Citation15 We interpreted our continuous spectrum measurements performed over several breaths as showing evidence of flow limitation when measurements between the ULN and the threshold of EFLT were found. These borderline measurements are thought to represent a mixture of normal and flow-limited breaths.
Exercise limitation is extremely common in COPD, and even small decreases in the 6MWD over time identify patients with increased risks of dying.Citation27 When EFLT is present, patients can only increase their minute ventilation during exercise by increasing breathing frequency or by dynamic hyperinflation, allowing end-expiratory lung volume to rise.Citation28 Dynamic hyperinflation can be induced by self-paced walking exercise. This is usually assessed by changes in operating lung volume.Citation12,Citation29 We did not measure lung volume during the 6MWT, but reasoned that patients with COPD showing evidence of flow limitation at baseline, either as having or the threshold of EFLT, were more likely to have a worse exercise tolerance at the same visit. This was true on a group level. The EFLT group had the lowest 6MWD, but neither FEV1 % predicted nor any cut-off level of
could identify patients with COPD with a short walking distance (<350 m), as proposed by Spruit et al.Citation30 No significant decline was seen in patients with
in the normal range; in patients with COPD with
it declined 37 m, and in patients with EFLT, it declined by 63 m. The minimal clinically important difference on an individual level has been suggested to be 30 m in an ERS/ATS systematic review;Citation31 consequently, the differences seen over time between our subgroups defined by
and EFLT are likely to be clinically important.
Exacerbation and hospitalization rates at our site were similar to those reported in the completed ECLIPSE study and in the TORCH study.Citation32,Citation33 By negative binomial regression, the and EFLT were both significant predictors for higher rates of exacerbations with very similar RR, demonstrating that the increased risk of exacerbations by EFLT is identified already at ULN. In the time to first event analysis of patients with
, we found a shorter time to first exacerbation and a shorter time to first hospitalization than that of the COPD patients with
in the normal range. A previous study on hospitalized patients showed that many, but not all, have FOT-defined EFLT during a COPD exacerbation.Citation17 FOT measurements performed during hospitalized COPD exacerbations show that
measurements improve during resolution of an exacerbation, although many patients remain EFLT at discharge.Citation17 At present, there is a shortage of readily measurable biomarkers that can predict the risk of future exacerbations. Our data suggest that, after adjusting for lung function and exacerbation history, EFLT measurement can identify patients who are more likely to develop exacerbations. Further prospective studies to confirm these observations and explore their mechanism are merited.
Patients with COPD with an FEV1 >50% predicted and evidence of EFLT, defined as baseline ΔXrs ≥ ULN, had significantly higher risk of dying within the 5-year follow-up. Similar associations were not found in the patients with more advanced airway obstruction and FEV1 <50%. However, COPD is a complex, heterogeneous disease, and respiratory impairment is associated with higher incidence of comorbidities, such as hypertension, cardiovascular disease, and diabetes.Citation34 If there is an association between and mortality, the higher risk of adverse outcomes associated with comorbid disease could mask such an association with advanced disease. Although the association between death and
was highly significant, this sub-analysis should be interpreted with caution due to the relatively small number of deaths in patients with FEV1 >50% predicted ().
There are certain limitations of this study. The FOT threshold identifying a single flow-limited breath has been defined at .Citation14,Citation15 It is not known whether the use of this threshold may lead to different results when applied to values measured over a continuous spectrum of several breaths. Previously published data show that the end-expiratory lung volume varies from breath-to-breath, affecting the prevalence of EFLT.Citation8,Citation15 Although the prevalence of EFLT increased as the FEV1% predicted declined, not all patients with very severe airway obstruction were found to have EFLT. In previous studies on elderly and COPD with the NEP technique, higher prevalence of EFLT have been reported than in the present study.Citation11,Citation35,Citation36 Different inclusion criteria and differences in sensitivity between the NEP and the FOT technique, together with the possibility that in borderline patients the application of a NEP per se could lead to development of EFLT, are likely explanations. The wide reference range of FEV1 inevitably may also lead to an overestimation of the suspected lung function decline in many patients.Citation23
As previously noted, EFLT varies between breaths in many patients with COPD and varies with repeat testing, likely reflecting differences in the end-expiratory lung volume on different test days.Citation13 Physiological variability in these measurements might require the repetition of this test in different periods to increase the sensitivity of our test. Despite this limitation, important relationships related to the presence of tidal flow limitation were seen, even when the data were controlled for severity of airflow obstruction measured by FEV1. The data reflect results from the largest recruiting center in the ECLIPSE study, in which patients were carefully characterized using standardized methodologies including oscillatory mechanics.Citation18,Citation37
Conclusion
Consistent differences in clinically relevant outcomes were found, such as exercise capability, exacerbations, hospitalizations, and death in COPD patients with baseline beyond the ULN. This is below the established threshold for EFLT. Our data support previous studies showing that patients with EFLT have worse lung mechanics than is evident from FEV1 measurement alone.Citation11,Citation38 The present study suggests that evidence of EFLT, measured during tidal breathing in an effort-independent fashion, can identify a subgroup of COPD patients with worse clinical outcomes.
Author contributions
The corresponding author BBA wrote the manuscript, had access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. PMAC was in the ECLIPSE Scientific Committee and PSB in the ECLIPSE Steering Committee. They both contributed to the development of the research design. PSB, JAH, and TMLE also contributed in the data collection. All authors, including RLJ and RD, contributed to the data analysis, the clinical interpretation of the data, and to reviewing the final submission.
Acknowledgments
The study was sponsored by GlaxoSmithKline. The sponsor had no role in the design of the study, collection and analysis of the data, nor in the preparation of the manuscript.
The work was performed at Haukeland University Hospital. The authors thank all participants who took part in the study, all members of the Bergen Respiratory Research Group who contributed to the data collection, and offer special thanks to Lene Svendsen, Rita Oppedal, Tina Endresen-Vinsjevik, and Eli Nordeide who performed/supervised the pulmonary function tests.
Supplementary materials
Figure S1 Scatterplot of plotted against FEV1 % predicted in healthy controls (N=229). Mean represented by the solid line. The dashed line represents the 97.5th percentile, the upper limit of normal (ULN).
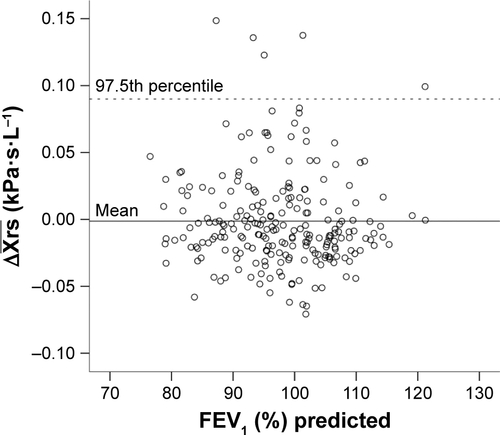
Table S1 Baseline characteristics in men and women (N=425)
Disclosure
The authors report no conflicts of interest in this work.
References
- LozanoRNaghaviMForemanKGlobal and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010Lancet201238098592095212823245604
- From the Global Strategy for the Diagnosis, Management and Prevention of COPDGlobal Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 Available from: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/Accessed April 18, 2017
- SorianoJBAlfagemeIAlmagroPDistribution and prognostic validity of the new Global Initiative for Chronic Obstructive Lung Disease grading classificationChest2013143369470223187891
- FranciosiLGPageCPCelliBRMarkers of disease severity in chronic obstructive pulmonary diseasePulm Pharmacol Ther200619318919916019244
- GelbAFHoggJCMüllerNLContribution of emphysema and small airways in COPDChest199610923533598620705
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 20112011 Available from: www.goldcopd.orgAccessed January 2, 2012
- HyattREThe interrelationships of pressure, flow, and volume during various respiratory maneuvers in normal and emphysematous subjectsAm Rev Respir Dis19618367668313717137
- O’DonnellDEVentilatory limitations in chronic obstructive pulmonary diseaseMed Sci Sports Exerc2001337 SupplS647S65511462073
- CalverleyPMKoulourisNGFlow limitation and dynamic hyperinflation: key concepts in modern respiratory physiologyEur Respir J200525118619915640341
- DiazOVillafrancaCGhezzoHRole of inspiratory capacity on exercise tolerance in COPD patients with and without tidal expiratory flow limitation at restEur Respir J200016226927510968502
- EltayaraLBecklakeMRVoltaCAMilic-EmiliJRelationship between chronic dyspnea and expiratory flow limitation in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19961546 Pt 1172617348970362
- O’DonnellDEWebbKAExertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflationAm Rev Respir Dis19931485135113578239175
- AarliBBCalverleyPMJensenRLEaganTMBakkePSHardieJAVariability of within-breath reactance in COPD patients and its association with dyspnoeaEur Respir J201545362563425359342
- DellacàRLDuffyNPompilioPPExpiratory flow limitation detected by forced oscillation and negative expiratory pressureEur Respir J200729236337417079262
- DellacàRLSantusPAlivertiADetection of expiratory flow limitation in COPD using the forced oscillation techniqueEur Respir J200423223224014979497
- O’DonnellDELavenezianaPDyspnea and activity limitation in COPD: mechanical factorsCOPD20074322523617729066
- JetmalaniKTimminsSBrownNJExpiratory flow limitation relates to symptoms during COPD exacerbations requiring hospital admissionInt J Chron Obstruct Pulmon Dis20151093994525999709
- VestboJAndersonWCoxsonHOECLIPSE investigatorsEvaluation of COPD longitudinally to identify predictive surrogate end-points (ECLIPSE)Eur Respir J200831486987318216052
- MahlerDAWellsCKEvaluation of clinical methods for rating dyspneaChest19889335805863342669
- FerrisBGEpidemiology Standardization Project (American Thoracic Society)Am Rev Respir Dis19781186 Pt 21120
- Standardization of spirometry, 1994 update. American Thoracic SocietyAm J Respir Crit Care Med19951523110711367663792
- OostveenEMacLeodDLorinoHERS Task Force on Respiratory Impedance MeasurementsThe forced oscillation technique in clinical practice: methodology, recommendations and future developmentsEur Respir J20032261026104114680096
- GulsvikATostesonTBakkePHumerfeltSWeissSTSpeizerFEExpiratory and inspiratory forced vital capacity and one-second forced volume in asymptomatic never-smokers in NorwayClin Physiol200121664866011722472
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function LaboratoriesATS statement: guidelines for the six-minute walk testAm J Respir Crit Care Med2002166111111712091180
- PedersenAGEllingsenCLData quality in the Causes of Death RegistryTidsskr Nor Laegeforen2015135876877025947599
- KeeneONCalverleyPMJonesPWVestboJAndersonJAStatistical analysis of exacerbation rates in COPD: TRISTAN and ISOLDE revisitedEur Respir J2008321172418591336
- PolkeyMISpruitMAEdwardsLDSix-minute-walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalizationAm J Respir Crit Care Med2013187438238623262518
- GuenetteJAWebbKAO’DonnellDEDoes dynamic hyperinflation contribute to dyspnoea during exercise in patients with COPD?Eur Respir J201240232232922183485
- MarinJMCarrizoSJGasconMSanchezAGallegoBCelliBRInspiratory capacity, dynamic hyperinflation, breathlessness, and exercise performance during the 6-minute-walk test in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200116361395139911371407
- SpruitMAPolkeyMICelliBEvaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study investigatorsPredicting outcomes from 6-minute walk distance in chronic obstructive pulmonary diseaseJ Am Med Dir Assoc201213329129721778120
- SinghSJPuhanMAAndrianopoulosVAn official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory diseaseEur Respir J20144461447147825359356
- HurstJRVestboJAnzuetoAEvaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) InvestigatorsSusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- JenkinsCRJonesPWCalverleyPMEfficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo-controlled TORCH studyRespir Res2009105919566934
- ManninoDMThornDSwensenAHolguinFPrevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPDEur Respir J200832496296918579551
- de BisschopCMartyMLTessierJFBarberger-GateauPDartiguesJFGuénardHExpiratory flow limitation and obstruction in the elderlyEur Respir J200526459460116204588
- KoulourisNGValtaPLavoieAA simple method to detect expiratory flow limitation during spontaneous breathingEur Respir J1995823063137758567
- CrimCCelliBEdwardsLDECLIPSE investigatorsRespiratory system impedance with impulse oscillometry in healthy and COPD subjects: ECLIPSE baseline resultsRespir Med201110571069107821481577
- O’DonnellDEBertleyJCChauLKWebbKAQualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanismsAm J Respir Crit Care Med199715511091159001298
- GlaxoSmithKlineEvaluation of COPD (Chronic Obstructive Pulmonary Disease) to Longitudinally Identify Predictive Surrogate Endpoints (ECLIPSE) Available from: https://clinicaltrials.gov/show/NCT0292552?. NLM identifier: NCT0292552Accessed April 4, 2017