Abstract
Background
COPD is the fourth leading cause of death in the world. It is a common, progressive, treatable and preventable disease. The exacerbation of COPD is associated with the peripheral muscle force, forced expiratory volume in 1 second (FEV1), the quality of life and mortality. Many studies indicated that the mucoactive medicines could reduce the exacerbations of COPD. This study summarized the efficacy of carbocisteine as a treatment for COPD.
Methods
We searched the randomized controlled trials (RCTs) following electronic bibliographic databases: MedLine, Embase, Cochrane Library and Web of Science. We additionally searched gray literature database: OpenSIGLE. We also additionally searched the clinical trial registers: ClinicalTrials.gov register and International Clinical Trials Registry Platform Search Portal. We used RCTs to assess the efficacy of the treatments. We included studies of adults (older than 18 years) with COPD. We excluded studies that were published as protocol or written in non-English language (Number 42016047078).
Findings
Our findings included data from four studies involving 1,357 patients. There was a decrease in the risk of the rate of total number of exacerbations with carbocisteine compared with placebo (−0.43; 95% confidence interval [CI] −0.57, −0.29, P<0.01). Carbocisteine could also improve the quality of life (−6.29; 95% CI −9.30, −3.27) and reduce the number of patients with at least one exacerbation (0.86; 95% CI 0.78, 0.95) compared with placebo. There was no significant difference in the FEV1 and adverse effects and the rate of hospitalization.
Interpretation
Long-term use of carbocisteine (500 mg TID) may be associated with lower exacerbation rates, the smaller number of patients with at least one exacerbation and higher quality of life of patients with COPD.
Introduction
COPD, the fourth leading cause of death in the world, is a common, progressive, treatable and preventable disease.Citation1 According to the studies made before, the prevalence of COPD was ~7%–11%. The prevalence of the male was higher than that of female.Citation2 It is characterized by predominantly fixed airway obstruction through a variety of processes. The pathogenesis involves many components, including the hypersecretion of mucus, oxidative stress and inflammation in the airway and lung.Citation3 COPD had effects on not only the lung but also the other organs. In the later phase, the patients usually had the symptoms of low weight, malnutrition and depression. It also can induce pulmonary heart disease, pulmonary encephalopathy and so on. COPD leads to many health and financial problems, so it is important to make appropriate strategies to prevent and treat COPD.
COPD exacerbations are defined as an acute worsening of respiratory symptoms that result in additional therapy (Global Initiative for Chronic Obstructive Lung Disease [GOLD] 2017).Citation4 The low frequency of COPD exacerbations was associated with the improvement in peripheral muscle force, forced expiratory volume in 1 second (FEV1) and the quality of life.Citation5–Citation7 The frequency of severe exacerbations lead to the increased mortality.Citation8 Bacterium, virus or sputum eosinophilia was related to exacerbations of COPD (59%, 29% and 28%, respectively).Citation9 Therefore, there is a high need to develop an optimal strategy that can control the frequencies of exacerbations. There were many methods to control the exacerbations of COPD, such as long-term use of long-acting inhaled anticholinergics, inhaled beta2-agonists and inhaled corticosteroids (ICSs). The long-term use of mucoactive medicines was still a controversial treatment for COPD because of the heterogeneity detected in each study.Citation10 Although the effects on selected patients differ when different doses are used, it is necessary to find an appropriate dose.
Carbocisteine (S-carboxymethylcysteine or SCMC) is a mucoactive drug that is widely used in Europe and Asia. It is both available in SCMC and its lysine salt. The half-life of carbocisteine is 1.33 hours.Citation11 The dose of carbocisteine for COPD is various, ranges from 750 mg twice daily to 4.5 g once daily. Carbocisteine has efficacy on antioxidation, anti-inflammation and mucolysis. The antioxidation and anti-inflammation of carbocisteine are effective to preserve alpha-1-antitrypsin activity, which is inactivated by oxidative stress. The inactivation of alpha-1-antitrypsin is associated with the extensive tissue injury, which can be observed in chronic emphysema.Citation12 The antioxidation and anti-inflammation of carbocisteine may play an important role in the long-term usage in COPD. In this study, we investigated the efficacy of carbocisteine on stable COPD.
Methods
We followed the Preferred Reporting Item for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for meta-analyses and systematic reviews.Citation13 The statement was registered at International Prospective Register of Systematic Reviews (PROSPERO; Number 42016047078; https://www.crd.york.ac.uk/PROSPERO).
We included randomized, placebo-controlled studies of oral carbocisteine for a period of at least 3 months. Our findings included studies of adults (older than 18 years) with COPD. COPD was defined by the criteria of the GOLD or the World Health Organization. We excluded studies that were published as protocol or written in non-English language.
We searched the following electronic bibliographic databases: MedLine, Embase, Cochrane Library and Web of Science. We additional searched gray literature database OpenSIGLE and the clinical trial registers such as ClinicalTrials.gov register and International Clinical Trials Registry Platform Search Portal. The search for randomized, controlled trials published up to September 1, 2016. The search strategy is shown in the “Supplementary materials” section.
Two authors checked the relevant studies independently. Trials were selected from identified studies, based on previously agreed inclusion criteria. Study characteristics and outcomes were collected by two authors. Two authors independently extracted data from included studies and entered results into the RevMan5.3 software. We used RevMan5.3 for this analysis.
Two authors independently assessed the risk of bias in included studies, as recommended by the International Cochrane Collaboration. Disagreements between the authors over the risk of bias in particular studies were resolved by discussion.
The primary outcomes included exacerbation rates (total number). COPD exacerbations are defined as an acute worsening of respiratory symptoms that result in additional therapy.Citation4
The secondary outcomes were 1) measurement of lung function, including FEV1; 2) the rates of hospitalization and mortality; 3) the number of patients with at least one exacerbation; 4) the quality of life (St George’s Respiratory Questionnaire) and 5) the adverse effects.
We pooled the results using mean differences (MDs) for continuous outcomes and risk ratios for binary outcomes and calculated 95% confidence intervals and two-sided P-values for each outcome. Heterogeneity between the studies in effect measures was assessed using both the χ2 test and the I2 statistic. We used subgroup meta-analyses to explore heterogeneity in effect estimates according to dose, duration and race. We also assessed evidence of publication bias.
Results
We identified 22 randomized clinical trials of carbocisteine. In all, four studies met the inclusion criteria and were included in the meta-analysis, with a total of 1,357 patients. In the four studies, only one study (Zheng et alCitation17) was of high quality. The four trials were all published before 2016. The mean trial duration was 10.4 months (range 6–12 months). The dose of carbocisteine for the four trials was 1,500 mg daily (one trial [Allegra et alCitation14] used carbocisteine lysine 2,700 mg daily, equivalent to carbocisteine 1,500 mg daily). A summary of the study selection process is shown in .
All trials had a randomized, double-blind, parallel-group design. Details of studies are included in .
Table 1 Major study characteristics from the four studies in the meta-analysis
We wrote to authors of four studies (Allegra et al,Citation14 Tatsumi et al,Citation15 Yasuda et al,Citation16 Zheng et alCitation17) for more information.Citation14–Citation17 We received more data from one study (Allegra et alCitation14). The “Supplementary materials” section describes trial quality metrics and the list of include/exclude criteria of studies.
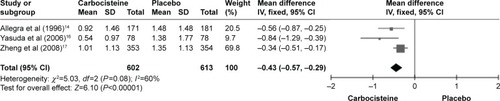
Exacerbation rates (total number)
A significant decrease was seen in the rate of exacerbations in the carbocisteine group compared with the placebo group (three studies: n=1,215; MD −0.43; 95% confidence interval [CI] −0.57, −0.29, I2=60%, P<0.00001; ).
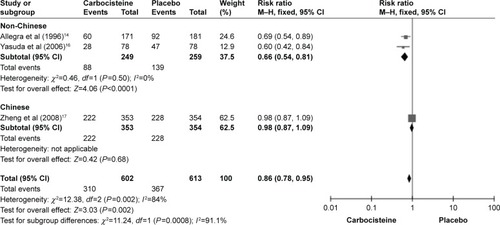
Number of patients with at least one exacerbation
There were three studies (Allegra et al,Citation14 Yasuda et alCitation16 and Zheng et alCitation17) that reported the outcome of the number of patients with at least one exacerbation. One study (Zheng et alCitation17) showed no difference in the risk of the outcome. Each of the others showed a significantly reduced number of patients with at least one exacerbation. A significant difference was noted in the number of patients with at least one exacerbation compared with placebo (three studies: n=1,215; relative risk [RR] 0.86; 95% CI 0.78, 0.95, I2=84%, Supplementary materials, P=0.002). The number needed to treat (NNT) was 12.
Quality of life (St George’s Respiratory Questionnaire)
There were two studies (Tatsumi et alCitation15 and Zheng et alCitation17) that reported this outcome. All the outcomes were evaluated by St George’s Respiratory Questionnaire. A significant decrease was seen in the rate of exacerbations in the carbocisteine group compared with the placebo group (two studies: n=849; MD −6.29; 95% CI −9.30, −3.27, I2=61%, Supplementary materials, P<0.0001; total St George’s Respiratory Questionnaire.
Adverse effects
There were three studies (Allegra et al,Citation14 Tatsumi et alCitation15 and Zheng et alCitation17) that reported this outcome. The most common adverse events reported were gastrointestinal problems. No significant difference was seen with carbocisteine compared with placebo (three studies: n=1,201; RR 1.02; 95% CI 0.73, 1.43, I2=61%, Supplementary materials, P=0.75). In addition, no significant difference was noted in the adverse effects of gastrointestinal problems (three studies: n=1,201; RR 1.38; 95% CI 0.71, 2.66, I2=77%, Supplementary materials, P=0.34). No fatal adverse effects were found in the four studies.
FEV1
Only one study (Tatsumi et alCitation15) reported the values of the FEV1 after treatment. No significant difference was noted in it (one study: n=142; MD −0.18; 95% CI −0.01, 0.37, Supplementary materials, P=0.07).
Rates of hospitalization
Only one study (Allegra et alCitation14) involved the rate of hospitalization. No significant difference was seen in it (one study: n=352; RR 9.52; 95% CI 0.52, 175.57, Supplementary materials, P=0.13).
Rates of mortality
There were two studies (Zheng et alCitation17 and Yasuda et alCitation16) that reported the rate of mortality. However, no one died in the two studies, so we could not evaluate it.
Subgroup analysis
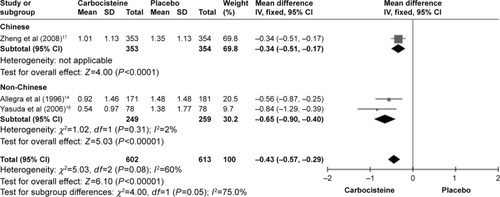
Considering outcome of the rate of exacerbations, the subgroup with the population of Chinese and non-Chinese is described in . The subgroup with the population of non-Chinese showed a large reduction in the carbocisteine group (two studies: n=508, MD =−0.34; 95% CI −0.90, −0.40, I2=2%, P<0.00001; ) compared to placebo. The subgroup with the population of Chinese showed no difference in the risk of the outcome (one study: n=707, MD =−0.34; 95% CI −0.51, −0.17, P<0.0001; ).
Figure 3 Forest plot of comparison. The subgroup analysis of rate of total number of exacerbations.
We used subgroup analysis with different formulations, durations and the definition of COPD. There was substantial heterogeneity in subgroup analysis (Supplementary materials). We planned to use subgroup analysis with different concomitant treatments, exacerbation rates and airway obstruction, but failed because of the lack of data.
In the outcome of the number of patients with at least one exacerbation, the subgroup with the population of non-Chinese showed an obvious decrease in heterogeneity (I2=0%) and a significant difference was seen in the carbocysteine carbocisteine group (two studies: n=508; RR =0.66; 95% CI 0.54, 0.81, I2=0%, P<0.0001; ) compared to placebo. The NNT was 6. In the subgroup with the population of Chinese, no difference in the risk of the outcome (one study: n=707; RR =0.98; 95% CI 0.87, 1.09, P=0.68; ) was noted.
Discussion
Our results showed that compared with the placebo, the long-term use of carbocisteine reduced 0.43 exacerbations per participant per year (95% CI −0.57, −0.29). A substantial heterogeneity was detected in the outcome (I2=60%). The subgroup with the population of non-Chinese showed an obvious decrease in heterogeneity (I2=2%) and a noticeable reduction in the exacerbation rate (−0.65; 95% CI −0.90, −0.40; ). The subgroup with the population of Chinese showed a moderate reduction (−0.34; 95% CI −0.51, −0.17; ).
Carbocisteine significantly reduced the number of patients with at least one exacerbation (0.86; 95% CI 0.78, 0.95). The NNT was 12. A substantial heterogeneity was detected in the outcome (I2=84%). We additional used a subgroup analysis with this outcome. The subgroup with the population of non-Chinese showed an obvious decrease in heterogeneity. The NNT was 6. The subgroup with the population of Chinese noted no difference in the risk of the outcome.
The heterogeneity might be explained by the race of Chinese and non-Chinese. It might indicate that carbocisteine had a little response in Chinese. However, there is no other study that supported this hypothesis. A similar finding was detected in another Chinese, randomized, double-blind, placebo-controlled study that included 964 participants. It used a similar mucoactive medicine (N-acetylcysteine 600 mg bid) in COPD.Citation18 In this study, no significant difference was seen in the number of patients with at least one exacerbation (odds ratio [OR] 0.92; 95% CI 0.69, 1.22, P=0.56). However, there were two meta-analyses that showed that N-acetylcysteine could reduce the number of patients with at least one exacerbation compared to placebo.Citation19,Citation20 Although the indirect evidence supported the hypothesis, it still needs more head-to-head study to confirm.
A randomized controlled trial (RCT) of carbocisteine (Zheng et alCitation17)Citation18 showed no significant interaction between carbocisteine treatment and COPD stages. It also showed no significant interaction between treatment and concomitant ICSs or naive ICs.
It was shown that carbocisteine could improve the quality of life (−6.29; 95% CI −9.30, −3.27, I2=61%; SGRQ). It was higher than the clinical minimally important difference, which was 4 units.Citation21 However, the result should be used with caution, because of the substantial heterogeneity.
As we know, carbocisteine had anti-inflammatory and anti-oxidant effects. Some studies proved that it could reduce the exacerbations through directly scavenging reactive oxygen species and could protect against the development of cigarette smoke extract-induced emphysema.Citation22,Citation26 In animal studies, carbocisteine had benefits of decreasing airway Haemophilus influenzae load by the expectorant action.Citation25 Other studies also showed that carbocisteine inhibits the infection of rhinovirus and seasonal influenza virus.Citation23,Citation24 In ex-smoker patients with COPD, carbocisteine had efficacy in reducing exhaled interleukin-6 and interleukin-8 concentrations, which improved significantly the ability of clinical variables to predict mortality in patients with COPD.Citation27,Citation28
Limitations of this analysis are as follows. First, a substantial heterogeneity was detected in some outcomes. The conclusion needs to be used with caution. Second, we excluded the studies with non-English, and the four studies reported benefitted outcomes. Publication bias may be seen in this review. It may cause overestimate outcomes. Third, all the data were collected from different RCTs. The trials had their own participants and outcomes, but the participants and outcomes were defined similarly in each trial.
No prior meta-analyses have evaluated the long-term use of carbocisteine. Our findings supported that long-term use of carbocisteine may be associated with reduced exacerbation rates and decreased number of patients with at least one exacerbation in patients with COPD. It also may improve the quality of life.
Author contributions
Zhenliang Xiao initiated and designed the study. Zheng Zeng, Xiaoling Huang and Dan Yang participated in study design. Zheng Zeng drafted the manuscript. All authors read and approved the final manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
We would like to acknowledge the contributions of Professor Phillippa Poole, who gave us more information on the study by Allegra et al.Citation14
Disclosure
The authors report no conflicts of interest in this work.
References
- WedzichaJASeemungalTACOPD exacerbations: defining their cause and preventionLancet2007370958978679617765528
- LandisSHMuellerovaHManninoDMContinuing to Confront COPD International Patient Survey: methods, COPD prevalence, and disease burden in 2012–2013Int J Chron Obstruct Pulmon Dis2014959724944511
- BarnesPJInflammatory mechanisms in patients with chronic obstructive pulmonary diseaseJ Allergy Clin Immunol20161381162727373322
- GOLD 2017 Global Strategy for the Diagnosis, Management and Prevention of COPD Available from: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/Accessed 16 Nov 2016
- SpruitMAGosselinkRTroostersTMuscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-IThorax200358975212947130
- MartinALJessicaMKyleFThe association of lung function and St. George’s respiratory questionnaire with exacerbations in COPD: a systematic literature review and regression analysisRespir Res201617111526739476
- SeemungalTARDonaldsonGCPaulEAEffect of exacerbation on quality of life in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19981575 pt 114189603117
- Soler-CataluñaJJMartínez-GarcíaMARomán SánchezPSalcedoENavarroMOchandoRSevere acute exacerbations and mortality in patients with chronic obstructive pulmonary diseaseThorax2005601192593116055622
- BafadhelMMckennaSTerrySAcute exacerbations of COPD: identification of biological clusters and their biomarkersAm J Respir Crit Care Med2011184666267121680942
- PoolePChongJCatesCJMucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary diseaseCochrane Database Syst Rev2015107CD001287
- BragaPCBorsaMDeALPharmacokinetic behavior of S-carboxymethylcysteine-Lys in patients with chronic bronchitisClin Ther1982464807093981
- StockleyRAAlpha-1-antitrypsin and the pathogenesis of emphysemaLung1987165161773104702
- MoherDLiberatiATetzlaffJPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementZhong Xi Yi Jie He Xue Bao200979889896
- AllegraLCordaroCIGrassiCPrevention of acute exacerbations of chronic obstructive bronchitis with carbocysteine lysine salt monohydrate: a multicenter, double-blind, placebo-controlled trialRespiration19966331741808739489
- TatsumiKFukuchiYPEACE Study GroupCarbocisteine improves quality of life in patients with chronic obstructive pulmonary diseaseJ Am Geriatr Soc2007551118841886
- YasudaHYamayaMSasakiTCarbocisteine reduces frequency of common colds and exacerbations in patients with chronic obstructive pulmonary diseaseJ Am Geriatr Soc200654237838016460403
- ZhengJPKangJHuangSGEffect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): a randomised placebo-controlled studyLancet200837196292013201818555912
- ZhengJPWenFQBaiCXTwice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trialLancet Respir Med20142318719424621680
- SutherlandERCrapoJDBowlerRPN-acetylcysteine and exacerbations of chronic obstructive pulmonary diseaseCOPD20063419520217361500
- FowdarKChenHHeZThe effect of N-acetylcysteine on exacerbations of chronic obstructive pulmonary disease: a meta-analysis and systematic reviewHeart Lung2017462120128
- JonesPWInterpreting thresholds for a clinically significant change in health status in asthma and COPDEur Respir J200219339811936514
- NogawaHIshibashiYOgawaACarbocisteine can scavenge reactive oxygen species in vitroRespirology2009141535919144049
- YasudaHYamayaMSasakiTCarbocisteine inhibits rhinovirus infection in human tracheal epithelial cellsEur Respir J2006281515816510461
- YamayaMNishimuraHShinyaKInhibitory effects of carbocisteine on type A seasonal influenza virus infection in human airway epithelial cellsAm J Physiol Lung Cell Mol Physiol20102992160168
- SunLTangLXuYWangSLiYKangJThe effect and mechanism of action of carbocysteine on airway bacterial load in rats chronically exposed to cigarette smokeRespirology20101571064107120807377
- MasayukiHYundenDYanCCarbocisteine protects against emphysema induced by cigarette smoke extract in ratsChest201113951101110820847042
- CarpagnanoGERestaOFoschino-BarbaroMPExhaled Interleukine-6 and 8-isoprostane in chronic obstructive pulmonary disease: effect of carbocysteine lysine salt monohydrate (SCMC-Lys)Eur J Pharmacol2004505316917515556150
- CelliBRLocantoreNYatesJInflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2012185101065107222427534