Abstract
Background and objectives
Exacerbations are important outcomes in COPD both from a clinical and an economic perspective. Most studies investigating predictors of exacerbations were performed in COPD patients participating in pharmacological clinical trials who usually have moderate to severe airflow obstruction. This study was aimed to investigate whether predictors of COPD exacerbations depend on the COPD population studied.
Methods
A network of COPD health economic modelers used data from five COPD data sources – two population-based studies (COPDGene® and The Obstructive Lung Disease in Norrbotten), one primary care study (RECODE), and two studies in secondary care (Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoint and UPLIFT) – to estimate and validate several prediction models for total and severe exacerbations (= hospitalization). The models differed in terms of predictors (depending on availability) and type of model.
Results
FEV1% predicted and previous exacerbations were significant predictors of total exacerbations in all five data sources. Disease-specific quality of life and gender were predictors in four out of four and three out of five data sources, respectively. Age was significant only in the two studies including secondary care patients. Other significant predictors of total exacerbations available in one database were: presence of cough and wheeze, pack-years, 6-min walking distance, inhaled corticosteroid use, and oxygen saturation. Predictors of severe exacerbations were in general the same as for total exacerbations, but in addition low body mass index, cardiovascular disease, and emphysema were significant predictors of hospitalization for an exacerbation in secondary care patients.
Conclusions
FEV1% predicted, previous exacerbations, and disease-specific quality of life were predictors of exacerbations in patients regardless of their COPD severity, while age, low body mass index, cardiovascular disease, and emphysema seem to be predictors in secondary care patients only.
Introduction
It is well known that the progression of COPD may be accompanied by exacerbations, that is, an acute worsening of symptoms. Exacerbations are associated with an accelerated decline in lung function,Citation1,Citation2 increase in mortality,Citation3,Citation4 significant impairment of health-related quality of life,Citation5–Citation7 and increased health care utilization and associated costs.Citation8–Citation10 Consequently, reducing exacerbations is one of the most important treatment goals in COPD from both a clinical and an economic perspective.Citation11,Citation12 Because not all patients experience exacerbations, identification of patients who are at high risk of exacerbations is important to use treatment options in an efficient way.
Health economic decision models for COPD are used for evaluating long-term effectiveness and cost-effectiveness of treatment options for COPD. Accurate and precise estimation of the exacerbation risk in these models is very important because exacerbations are associated with high health care costs and therefore strongly influence the cost outcomes of the models. However, most of the currently available cost-effectiveness models include an exacerbation risk specified by degree of airflow obstructionCitation13 only and they are not able to distinguish high-risk patients based on other relevant predictors, such as previous exacerbations.Citation14 Therefore, the focus of the Fourth Annual Meeting of the International COPD Health Economic Modelling Network in 2015 was to develop prediction models for exacerbations including several relevant patient and disease characteristics as predictors.
Most of the previously published studies investigating predictors of exacerbations were done in COPD patients participating in pharmacological clinical trials who usually have moderate to severe airflow obstruction.Citation14–Citation19 A few studies were done in primary care patients with less severe COPD.Citation20–Citation22 Because the studies included different candidate predictors or used different definitions for predictors, it is difficult to conclude whether predictors of exacerbations differ between patient populations with varying disease severity.
The aim of the current study was to estimate prediction models for the total number of exacerbations and severe exacerbations using five large sources of patient-level data and compare the estimated models between patient populations.
Methods
Procedure
Since 2011, a worldwide network of people involved in health economic modeling for COPD (ie, COPD modeling teams, employees of pharmaceutical companies interested in COPD modeling, clinicians, health economists, and epidemiologists) gathered together for three one-day meetings with the aim to discuss and compare the different available COPD models and share best practices about COPD modeling.Citation13,Citation23 In May 2015 participants in this COPD modeling network were contacted to explore their interest in participating in a modeling exercise for the fourth COPD modeling meeting. To participate in this so-called modeling challenge, participants needed to have access to a database with patient-level data with the following characteristics: 1) a minimum of about 500 patients, 2) follow-up of at least 1 year, 3) moderate and severe exacerbations measured, and 4) several demographic and clinical patient characteristics available. For the first part of the challenge, participants were asked to estimate several pre-specified prediction models including one or multiple predictors and using different statistical methods. In the second part of the challenge, the estimated prediction models were validated. A structured Microsoft Excel file was used to collect the results in a uniform way before the meeting. During the meeting the results were presented and discussed.
Sources of data
Data from five different data sources were used for the modeling challenge: two population-based studies – the COPDGene® study and the Obstructive Lung Disease in Norrbotten (OLIN) study – one study in primary care patients – the RECODE trial – and two studies including secondary care patients – the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study and the UPLIFT trial.Citation24–Citation28 The COPDGene study is a multi-institutional study of past and current smokers to identify the genetic factors that control the development and progression of COPD. The primary analysis cohort dataset consists of about 10,300 smokers in the general population. For this modeling challenge a subgroup of 3,756 patients with a diagnosis of COPD were included, who were on average followed for 4.7 years.Citation24 The OLIN study is a population-based screening study for COPD in the general population in northern Sweden. The prevalence of more severe patients is therefore low, reflective of actual prevalence. For the current study, the data of patients with a COPD diagnosis enrolled in 2005 and their follow-up data for 2006 were used. Observations of severe exacerbations were scarce, and therefore no separate analysis for severe exacerbations was performed.Citation25 The RECODE trial was a 2-year cluster-randomized trial in which 20 primary care teams were randomized to an intervention group of general practitioner practices that implemented an integrated care program for COPD and 20 teams were randomized to a usual care group.Citation26 The ECLIPSE study was a non-interventional, longitudinal prospective 3-year study in COPD patients aged 40–75 years with a baseline post-bronchodilator forced expiratory volume in 1 s (FEV1)% predicted <80%, baseline post-bronchodilator FEV1/forced vital capacity (FVC) <0.7, and a smoking history of at least 10 pack-years.Citation27 The UPLIFT trial was a 4-year randomized controlled trial comparing tiotropium versus placebo in patients with a diagnosis of COPD (FEV1/FVC <70%), age ≥40 years, >10 pack-years, and an FEV1% predicted <70%.Citation28 The study protocols of all five studies were approved by the relevant ethics and review boards of the participating centers.
Exacerbations
Participants were asked to use the following definition for an exacerbation: a moderate exacerbation was defined as an increase in symptoms requiring a visit to a health care provider and a course of antibiotics and/or oral steroids. A severe exacerbation was defined as an exacerbation requiring hospitalization.
Predictions models
Prediction models were estimated separately for total and severe exacerbations. For each data source, a randomly selected 67% of the patient population was used to estimate the prediction models, while the remaining 33% was set aside for validation purposes.
Five different prediction models for the annual exacerbation rate were estimated using negative binomial regressionCitation29 with the natural logarithm of the total time at risk in years as offset variable and the number of exacerbations as outcome. The time at risk was defined as total time in the study, which was shorter than the duration of the study for patients who dropped out or died before the end of the study. The estimated regression coefficients for the predictors were transformed into incidence rate ratio (IRR) by taking the exponent of the estimated coefficient. All variables included as predictors were measured at baseline. The five different prediction models varied in the number of predictors and the type of model used. The first two prediction models were pre-specified and included the same predictors for all data sources. For the first model, groups were asked to estimate a model including the predictors FEV1% predicted at baseline and treatment for the two trials, that is, RECODE and UPLIFT. For the second model, groups were asked to include the same patient and clinical parameters as in a previously published prediction model by Briggs et al,Citation16 that is, sex, age, FEV1% predicted, total number of (severe) exacerbations in the year prior to baseline (depending on the outcome used), disease-specific quality of life, body mass index (BMI) <20 kg/m2, history of cardiovascular disease and treatment, if applicable. For model 3, groups were asked to include all patient and clinical parameters from model 2 plus other possible relevant variables available in the data source to be chosen by the modeling team, for example, dyspnea, COPD duration, and other comorbidities. The latter predictors were different for the different data sources. In the fourth model, the impact of using a different statistical method was explored. Model 4 included the same predictors as model 3 but used a zero-inflated binomial regression model with an indicator of previous exacerbations at baseline in the zero-model. With model 5, the impact of two different definitions for previous exacerbations was assessed using multilevel negative binomial regression. Model 5 included the same predictors as model 3 and had two variants: one using exacerbations prior to baseline as predictor (model 5A) and one using exacerbations in the previous period as predictor (model 5B; using the exacerbation rate in Year 1 to predict the rate in Year 2, the rate in Year 2 to predict the rate in Year 3, etc.).
Validation
For the validation part, participants were asked to use the remaining 33% of the population in their data source. First the mean observed exacerbation rate was calculated using the observation time of each patient as a weight, that is, patients with a longer follow-up have a higher weight than patients with a short follow-up. In addition, the predicted exacerbation rate for each patient based on each of the five different models was calculated by filling in the estimated regression equations for each individual patient. Thereafter, the mean predicted exacerbation rate over all patients was determined. Finally, absolute errors were calculated as the absolute difference between the individual observed and predicted exacerbation rates for each patient. The mean absolute error (MAE) between the observed and predicted rates was calculated using the observation time of the individual patient as a weight. If the MAE is small, the observed and predicted exacerbation rates are fairly similar. If the MAE is large, the predicted rate for patients is substantially different from the observed rate.
Results
Characteristics of the patients included in the five different participating data sources are shown in . For the ECLIPSE and UPLIFT studies, the mean FEV1% predicted was about 48%, and more than half of the patients had severe to very severe airflow obstruction. Patients in the population-based OLIN study had the highest mean FEV1% predicted (76%). In this data source, only 7% of the patients were classified as having severe or very severe airflow obstruction. Mean FEV1% predicted of the population-based COPDGene study and the primary care-based RECODE study were 57% and 68%, respectively.
Table 1 Baseline characteristicsTable Footnote# of the patients in the five data sources, data are mean or %
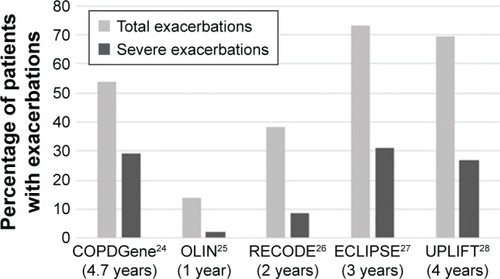
shows the percentage of patients with at least one exacerbation during follow-up. The lowest percentages were found for the OLIN study with a follow-up duration of 1 year. Percentages in ECLIPSE were highest, although UPLIFT had a longer follow-up.
Figure 1 Percentage of patients with at least one (severe) exacerbation during follow-up with the duration of follow-up presented in brackets.
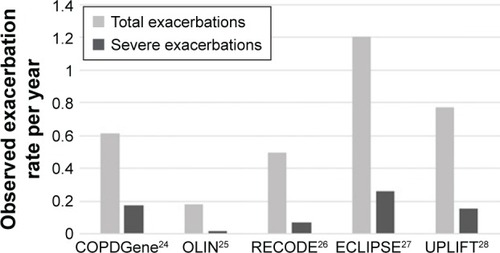
shows the mean exacerbation rates during follow-up for all five data sources. The number of exacerbations per patient-year ranged from 0.18 in the OLIN study to 1.20 in the ECLIPSE study. The number of severe exacerbations per patient-year varied between 0.02 for OLIN and 0.26 for ECLIPSE.
Figure 2 Mean annual total and severe exacerbation rates during follow-up. (Rates are calculated as the sum of exacerbations over all patients divided by the sum of follow-up time to correct for patients with a short follow-up time.)
Results of prediction model 1 including only FEV1% predicted and treatment, if applicable, showed that for all five data sources FEV1% was a significant predictor of total and severe exacerbations. Model 2, that is, with addition of sex, age, exacerbations prior to baseline, disease-specific quality of life, low BMI, and history of cardiovascular disease showed that besides FEV1% predicted, exacerbations prior to baseline and disease-specific quality of life were important predictors of exacerbations. Results of model 3, which also allowed inclusion of other data source-specific variables besides the fixed set of predictors of model 2, are shown in for total exacerbations and for severe exacerbations. Both tables show IRRs, while more detailed information on coefficients, standard errors, and p-values for the different predictors are presented in the Supplementary materials (Tables S1–S5).
Table 2 Prediction models for total exacerbations including a fixed set of predictors and other database-specific predictors (results from multivariate analysis)
Table 3 Prediction models for severe exacerbations including a fixed set of predictors and other database-specific predictors (results from multivariate analysis)
FEV1% predicted and exacerbations prior to baseline were the most important significant predictors of total exacerbations in all five data sources (p-values <0.001) (). Disease-specific quality of life, that is, St George’s Respiratory Questionnaire (SGRQ) total score was also an important significant predictor in all four databases for which it was available. For the other significant predictors, there was more variation in p-values. Sex was a predictor in three out of five databases with female patients having higher rates. Age was significant only in the two studies including secondary care patients, ECLIPSE and UPLIFT. Results for current smoking in two studies showed that current smokers had a lower incidence rate for exacerbations than former smokers, while two other studies found a non-significant higher rate for current smokers. Other predictors found to be predictive of a higher exacerbation rate available in a single data source were: increasing number of pack-years, presence of cough and wheeze, 6-min walking test, and inhaled corticosteroid (ICS) use at baseline. An increase in resting oxygen was found to be associated with a decrease in exacerbation rate in COPDGene.
Predictors of the number of severe exacerbations were in general the same as of the total number of exacerbations, but in addition low BMI, history of cardiovascular disease, presence of emphysema, and current smoking were found to be predictors in secondary care COPD patients (ECLIPSE and/or UPLIFT). Contrary to the total number of exacerbations, sex was a significant predictor of the number of severe exacerbations in only one database (COPDGene).
Results for model 4 using a zero-inflated negative binomial regression model instead of regular negative binomial regression showed that using this type of model is relevant only when the proportion of patients without any exacerbations is very high. Only for the OLIN study in which 86% of patients had no exacerbations during follow-up, the coefficient for the zero-inflated model parameter was significant. Using exacerbations in the previous period as predictor instead of using exacerbations in the year prior to baseline (model 5) did not seem to improve the model fit much. Only for UPLIFT, the study with the longest follow-up period, a slight improvement in the model fit was observed.
Validation results for the different models are shown in . In general, for total number of exacerbations the mean annual predicted rates were somewhat higher than the mean observed rates, but the MAEs of the models were large. Compared to the model including FEV1 only (model 1), the MAEs for the models including more patient characteristics tended to be slightly lower indicating that the models with more patient characteristics resulted in slightly better predictions on the individual level. For severe exacerbations, the mean annual predicted rates were higher than the mean observed rates for all models in all databases. Contrary to the results for total exacerbations, the MAEs did not seem to decrease when more patient and clinical parameters were added to the models.
Table 4 Model validation results for total and severe exacerbations: weighted mean observed annual exacerbation rate, mean predicted annual exacerbation rates, and weighted MAEs
Discussion
This study aimed to estimate prediction models for total and severe exacerbations using different COPD patient populations to explore whether predictors of exacerbations are different in patient populations with a different disease severity. Results showed that FEV1% predicted and previous exacerbations were significant predictors in all data sources regardless of the severity of the COPD population. The importance of previous exacerbations as predictor of future exacerbations was already well known from studies mainly performed in secondary care patients with severe COPD.Citation14–Citation17,Citation19 The current study showed that the number of previous exacerbations is also a good predictor in patients with less severe airflow obstruction. Even if more than 40% of the patients were classified as having mild obstruction (OLIN studies), previous exacerbations were found to be a strong predictor. The SGRQ total score was also found to be an important significant predictor of exacerbations in four out of four data sources, which was also in line with three previously performed studies in severe COPD patients.Citation14,Citation16,Citation17 One study in COPD patients with less severe airflow obstruction reported an association between exacerbation risk and responses to the clinical COPD questionnaire.Citation30 Previous studies already showed that patients with frequent exacerbations have a lower health-related quality of life, but in the current study health status was found to be an independent predictor of new exacerbations after adjusting for previous exacerbations. Additional analyses using the UPLIFT and RECODE data showed that especially the SGRQ symptom sub-score and the SGRQ impact sub-score were predictive of future exacerbation risk, while the SGRQ activity sub-score was not found to be a significant predictor.
Female gender was found to be a predictor of total exacerbations in three out of five data sources. In the UPLIFT trial, female gender was borderline significant (p=0.07). For severe exacerbations, only one data source found a significant impact of gender. Seven out of nine studies found in the literature confirmed the finding that female patients have a higher number of total exacerbations.Citation15–Citation17,Citation19,Citation21,Citation22,Citation31 Little is known about the reason why women seem to have more exacerbations. It may be related to physiological or treatment-related factors,Citation32 but it might also be explained by the health care-based definition used to identify exacerbations and the fact that women tend to seek health care more often.Citation33 In the current study, age was a predictor only in the two studies including patients with severe airflow obstruction. This finding seems to be in line with the literature that showed an association between age and exacerbations in five out of seven studies including patients with severe airflow obstruction,Citation14–Citation19,Citation31 while this association was found in only one out of five studies including less severe patients.Citation20–Citation22,Citation30,Citation34 In none of the currently explored data sources, history of cardiovascular disease was a predictor of total exacerbations, while several studies in patients with less severe airflow obstruction did find an association.Citation20–Citation22,Citation34 Predictors of severe exacerbations were in general the same as of total exacerbations. Due to the definition used, predictors of severe exacerbations actually need to be interpreted as predictors of hospitalization for an exacerbation. The results showed that patients with mainly severe airflow obstruction and additional risk factors, that is, history of cardiovascular disease, low BMI, current smoking, and presence of emphysema, are more likely to be hospitalized, which is as expected.
Model validation results showed that the models predicted exacerbation rates quite well on an average level, because the mean predicted rates were comparable although somewhat higher than the mean observed rate. However, the MAEs of the models were large indicating that the models were predicting less well on an individual level. MAEs seem to decrease from model 1 to model 3, showing that adding more patient and clinical parameters seemed to improve predictions on the individual level slightly.
Based on the results of the prediction models, it is difficult to come up with interventions or treatment advice to reduce the risk of an exacerbation. Some factors, such as gender and age, cannot be influenced, while others such as FEV1 and SGRQ total score can be influenced by treatment. They are well recognized as important treatment goals, but it is a challenge to change them substantially within a short time period. Therefore, results of the current study mainly create awareness about which type of patients are more likely to experience exacerbations and hence should be closely monitored. It further stresses on the need for new, more targeted predictors of exacerbations like biomarkers, which were unfortunately not present in the available databases.
A strength of the current study is that exactly the same methods were used in five different data sources. By using the same type of model with a partially fixed set of predictors and ensuring variables were defined in the same way in all data sources, the comparability of the results between the different data sources, and hence populations, was improved as much as possible.
A limitation of the study is that there are slight differences in the way exacerbations are defined in the five available databases. All databases included treatment with antibiotics or oral corticosteroids in the definition. But in some databases patients needed to have an increase in respiratory symptoms lasting for a pre-specified number of days, while in other databases an unscheduled visit to a health care provider was a requirement. Next to that, the studies were performed in different countries with differences in treatment patterns and access to health care. These differences in the definitions and health care settings mainly affected the observed exacerbation rates and most likely not the analyses of predictors. Heterogeneity in the definition of a severe exacerbation might have had more impact on the results. Because the different studies were performed in different health care settings, the likelihood to be hospitalized for an exacerbation may also vary substantially between patients. This may partly explain why the predictors of total and severe exacerbations are very similar. Despite this, we found some new predictors of severe exacerbations especially in secondary care patients.
Although a large number of different predictors of exacerbations were included in the different regression models, not all potential relevant predictors were included in the analyses. Especially, information on biomarkers is lacking in the current study. This was mainly because the majority of studies were performed almost 10 years ago, when data on biomarkers were not yet collected as often as they are nowadays. Using older data might have had an impact on the absolute exacerbation rates observed in the different databases. Because several new treatment options became available in the last decade, exacerbation rates in more recent trials might be lower. However, a lower rate is unlikely to greatly influence the variables that were found to be predictors.
In conclusion, FEV1% predicted, previous exacerbations, and disease-specific quality of life were identified as predictors of the total number of exacerbations in COPD patients regardless of their COPD severity. In secondary care patients age was found to be a predictor of total exacerbations, and low BMI, history of cardiovascular disease, and presence of emphysema were predictors of hospitalization for an exacerbation.
Acknowledgments
The COPDGene project is supported by award numbers R01 HL089897, R01 HL089856, and K01 HL125858 from the National Heart, Lung, and Blood Institute. The ECLIPSE study was supported by GlaxoSmithKline (SCO104960/NCT0292552). Financial support for the OLIN study was received mainly from The Swedish Heart & Lung Foundation (20050428, 20090244, and 20150488), The Swedish Research Council (80586701), ALF (216371) a regional agreement between Umeå University and Norrbotten County Council (NLL-574941), Norrbotten County Council, the Swedish Asthma-Allergy Foundation, and Visare Norr. The RECODE study has been funded by a Dutch Healthcare insurance company (Stichting Achmea Gezondheidszorg) and the Netherlands Organisation for Health Research and Development (Zon-MW) (project number 171002203). The UPLIFT trial was funded by Boehringer Ingelheim. The current study was financially supported by Boehringer Ingelheim International, GlaxoSmithKline, the Netherlands, and Novartis International.
Supplementary materials
Table S1 Prediction models for total number of exacerbations and number of severe exacerbations using COPDGene data: coefficients (SE) and p-valuesTable Footnote*
Table S2 Prediction models for total number of exacerbations using OLIN data: coefficients (SE) and p-valuesTable Footnote*
Table S3 Prediction models for total number of exacerbations and number of severe exacerbations using RECODE data: coefficients (SE) and p-valuesTable Footnote*
Table S4 Prediction models for total number of exacerbations and number of severe exacerbations using ECLIPSE data: coefficients (SE) and p-valuesTable Footnote*
Table S5 Prediction models for total number of exacerbations and number of severe exacerbations using UPLIFT data: coefficients (SE) and p-valuesTable Footnote*
References
- ReganEAHokansonJEMurphyJRGenetic epidemiology of COPD (COPDGene) study designCOPD201071324320214461
- MontnemeryPAdelrothEHeumanKPrevalence of obstructive lung diseases and respiratory symptoms in southern SwedenRespir Med199892121337134510197227
- KruisALBolandMRSchoonveldeCHRECODE: design and baseline results of a cluster randomized trial on cost-effectiveness of integrated COPD management in primary careBMC Pulm Med20131311723522095
- VestboJAndersonWCoxsonHOECLIPSE investigatorsEvaluation of COPD longitudinally to identify predictive surrogate end-points (ECLIPSE)Eur Respir J200831486987318216052
- DecramerMCelliBTashkinDPClinical trial design considerations in assessing long-term functional impacts of tiotropium in COPD: the UPLIFT trialCOPD20041230331217136995
Disclosure
The authors report no conflicts of interest in this work.
References
- DonaldsonGCSeemungalTABhowmikAWedzichaJARelationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax2002571084785212324669
- CelliBRThomasNEAndersonJAEffect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH studyAm J Respir Crit Care Med2008178433233818511702
- Soler-CatalunaJJMartinez-GarciaMARoman SanchezPSalcedoENavarroMOchandoRSevere acute exacerbations and mortality in patients with chronic obstructive pulmonary diseaseThorax2005601192593116055622
- HoogendoornMHoogenveenRTRutten-van MolkenMPVestboJFeenstraTLCase-fatality of COPD exacerbations: a meta-analysis and statistical modeling approachEur Respir J201137350851520595157
- MiravitllesMFerrerMPontAZalacainREffect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up studyThorax200459538739515115864
- SeemungalTADonaldsonGCPaulEABestallJCJeffriesDJWedzichaJAEffect of exacerbation on quality of life in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19981575141814229603117
- SpencerSCalverleyPMBurgePSJonesPWImpact of preventing exacerbations on deterioration of health status in COPDEur Respir J200423569870215176682
- AnderssonFBorgSJanssonSAThe costs of exacerbations in chronic obstructive pulmonary disease (COPD)Respir Med200296970070812243316
- OostenbrinkJBRutten-van MolkenMPResource use and risk factors in high-cost exacerbations of COPDRespir Med200498988389115338802
- O’ReillyJFWilliamsAERiceLHealth status impairment and costs associated with COPD exacerbation managed in hospitalInt J Clin Pract20076171112112017577296
- Global Initiative for chronic Obstructive Lung Disease (GOLD)Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease2013
- CrinerGJBourbeauJDiekemperRLPrevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society GuidelineChest2015147489494225321320
- HoogendoornMFeenstraTLAsukaiYPatient heterogeneity in health economic decision models for chronic obstructive pulmonary disease: are current models suitable to evaluate personalized medicine?Value Health201619680081027712708
- HurstJRVestboJAnzuetoAEvaluation of COPD longitudinally to identify predictive surrogate endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- BeehKMGlaabTStowasserSCharacterisation of exacerbation risk and exacerbator phenotypes in the POET-COPD trialRespir Res201314111624168767
- BriggsASpencerMWangHManninoDSinDDDevelopment and validation of a prognostic index for health outcomes in chronic obstructive pulmonary diseaseArch Intern Med20081681717918195198
- MakeBJErikssonGCalverleyPMA score to predict short-term risk of COPD exacerbations (SCOPEX)Int J Chron Obstruct Pulmon Dis20151020120925670896
- NiewoehnerDELokhnyginaYRiceKRisk indexes for exacerbations and hospitalizations due to COPDChest20071311202817218552
- JenkinsCRCelliBAndersonJASeasonality and determinants of moderate and severe COPD exacerbations in the TORCH studyEur Respir J2012391384521737561
- BertensLCReitsmaJBMoonsKGDevelopment and validation of a model to predict the risk of exacerbations in chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis2013849349924143086
- MullerovaHShuklaAHawkinsAQuintJRisk factors for acute exacerbations of COPD in a primary care population: a retrospective observational cohort studyBMJ Open2014412e006171
- McGarveyLLeeAJRobertsJGruffydd-JonesKMcKnightEHaughneyJCharacterisation of the frequent exacerbator phenotype in COPD patients in a large UK primary care populationRespir Med2015109222823725613107
- HoogendoornMFeenstraTLAsukaiYCost-effectiveness models for chronic obstructive pulmonary disease: cross-model comparison of hypothetical treatment scenariosValue Health201417552553625128045
- ReganEAHokansonJEMurphyJRGenetic epidemiology of COPD (COPDGene) study designCOPD201071324320214461
- MontnemeryPAdelrothEHeumanKPrevalence of obstructive lung diseases and respiratory symptoms in southern SwedenRespir Med199892121337134510197227
- KruisALBolandMRSchoonveldeCHRECODE: design and baseline results of a cluster randomized trial on cost-effectiveness of integrated COPD management in primary careBMC Pulm Med20131311723522095
- VestboJAndersonWCoxsonHOECLIPSE investigatorsEvaluation of COPD longitudinally to identify predictive surrogate end-points (ECLIPSE)Eur Respir J200831486987318216052
- DecramerMCelliBTashkinDPClinical trial design considerations in assessing long-term functional impacts of tiotropium in COPD: the UPLIFT trialCOPD20041230331217136995
- KeeneONCalverleyPMJonesPWVestboJAndersonJAStatistical analysis of exacerbation rates in COPD: TRISTAN and ISOLDE revisitedEur Respir J2008321172418591336
- Al-aniSSpigtMHofsetPMelbyeHPredictors of exacerbations of asthma and COPD during one year in primary careFam Pract201330662162824115012
- HuseboGRBakkePSAanerudMPredictors of exacerbations in chronic obstructive pulmonary disease: results from the Bergen COPD cohort studyPLoS One2014910e10972125279458
- KilicHKokturkNSariGCakirMDo females behave differently in COPD exacerbation?Int J Chron Obstruct Pulmon Dis20151082383025977604
- OwensGMGender differences in health care expenditures, resource utilization, and quality of careJ Manag Care Pharm20081432618439060
- KerkhofMFreemanDJonesRChisholmAPriceDBEffectiveness GroupPredicting frequent COPD exacerbations using primary care dataInt J Chron Obstruct Pulmon Dis2015102439245026609229
