Abstract
Purpose
The morning is the most bothersome period for COPD patients. Morning symptom severities in different Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages are not well studied. Furthermore, factors that are associated with morning symptoms, especially the associations with objectively measured physical activity, are also not well described.
Materials and methods
The aim of this cross-sectional observational study was to assess morning symptom severity in GOLD A, B, C and D patients, according to the definitions of the GOLD 2015 statement. Morning symptoms were assessed with the PRO-Morning COPD Symptoms Questionnaire. Differences in morning symptom severity between different COPD stages were assessed with a one-way analysis of variance followed by post hoc analyses. The association between dyspnea severity (assessed with the modified Medical Research Council scale), health status, airflow limitation, lung hyperinflation, anxiety and depression, inflammatory parameters, exacerbations, objectively measured physical activity parameters retrieved from accelerometry and morning symptom severity was evaluated using linear regression analysis.
Results
Eighty patients were included (aged 65.6±8.7 years, forced expiratory volume in 1 second [FEV1] % predicted 55.1±16.9). Mean (±SD) morning symptom score was 19.7 (±11.7). Morning symptom severity was significantly different between COPD stages: mean (±SD) score in GOLD A was 9.7 (±7.2), in GOLD B 19.8 (±10.7), in GOLD C 8.6 (±9.3) and in GOLD D 23.8 (±11.2) (p<0.001). Lower health status, more symptoms, increased anxiety and depression, less physical activity (all p<0.001) and lower FEV1 (p=0.03) were associated with an increased morning symptom severity.
Conclusion
Patients with overall more symptomatic COPD have significant higher morning symptom scores. Morning symptom severity was associated with important clinical outcomes: lower health status, more symptoms, increased anxiety and depression, fewer steps a day, less time in moderate and vigorous physical activity with bouts of at least 10 minutes and lower FEV1. The data suggest that morning symptoms should be carefully assessed in addition to assessment by general COPD-specific questionnaires, especially in those with more symptomatic COPD. More research is needed on potential therapies to improve morning symptoms; this study shows potential targets for intervention.
Introduction
COPD is the fifth leading cause of disability-adjusted life-years worldwide.Citation1 COPD is characterized by airway obstruction, lung emphysema and chronic inflammation of the airways. Airway obstruction is usually progressive over time but variability over short time is low. Still, COPD symptoms vary throughout the day.Citation2 In recent years, it has become apparent that the morning is the most symptomatic part of the day for most COPD patients.Citation3 A recent review showed that the occurrence of morning symptoms in COPD varies widely from 38.9% up to 94.4% of patients and morning symptoms are present in all COPD severity classes.Citation4 However, the prevalence of morning symptoms seems to be heavily dependent on the used questionnaire.Citation5 In addition, few studies assessed the severity of morning symptoms in relation to COPD severity and identified factors that are associated with morning symptoms.Citation2,Citation6,Citation7
One of the important factors in COPD patients is physical activity. People with COPD are more physically inactive compared to their healthy peers.Citation8,Citation9 This is also reflected in the observation that only 26% to 30% of patients with COPD fulfilled the WHO physical activity recommendations.Citation10,Citation11 The lack of sufficient physical activity is associated with more exacerbations, hospitalizations, all-cause mortalityCitation12 and lower quality of life.Citation13 The aetiology of physical inactivity in COPD patients is not completely understood. The lack of activity could be due to the avoidance of pulmonary symptoms, or it can be speculated that physical activity could be a risk factor to develop COPD. Self-reported low physical activity has been shown as a factor that is related with morning symptoms.Citation2 Half of the patients reported that they had made changes in morning routines, even in simple tasks, due to symptoms.Citation14 However, to our knowledge, no studies have been done that evaluate the relationship between objectively measured physical activity by triaxial accelerometry and morning symptoms.
The primary objective of the Morning symptoms in-Depth observAtional Study (MODAS) was to assess the severity of morning symptoms in different Global Initiative for Obstructive Lung Disease (GOLD) stages. The secondary objective was to evaluate the association between dyspnea severity, health status, airflow limitation, lung hyperinflation, anxiety and depression, inflammatory parameters, exacerbations, objectively measured physical activity parameters and morning symptom severity. We hypothesized that patients in more advanced GOLD stages have higher morning symptom scores. Furthermore, we expect that morning symptom severity will be negatively associated with physical activity.
Materials and methods
Study design
The MODAS was a single center, observational, cross-sectional study that was conducted from September 2015 until February 2017 in the Netherlands. The medical ethics committee from the Leiden University Medical Center (LUMC) approved the study (protocol number NL51951.058.15).
Study subjects
Outpatients were recruited from a university medical center (the LUMC), a regional hospital (the Alrijne hospital) and were recruited by distributing flyers in local papers. Interested patients received the study information by letter. Upon agreeing to participate, a visit was scheduled. All patients gave written informed consent.
Patients were eligible if they had a diagnosis of COPD by a physician, met the criteria for GOLD II–IV according to the definitions of the GOLD statementCitation15 and were aged 40 to 80 years, a general symptomatic patient group. Furthermore, they had to be current smokers or ex-smokers with a lifetime tobacco exposure of ≥10 pack years. Patients were excluded if they had a diagnosis of asthma, history of sensitization to allergens or significant other lung disease. In addition, patients were excluded if they had comorbidities that significantly impaired exercise capacity in the opinion of the investigator (eg, severe polyneuropathy, leg amputation). Other exclusion criteria were current malignant diseases or clinical signs of acute heart failure. Patients with mental impairment that could result in noncompliance with the study protocol were also excluded. Also, patients who were currently enrolled in a rehabilitation program or with an exacerbation in the previous 2 months were excluded. An exacerbation was defined as sustained worsening of respiratory symptoms during 48 hours and requiring oral corticosteroid, antibiotic or a combination of this treatment that was initiated by a physician, a visit to the emergency department or hospitalization with or without intensive care visit. Respiratory symptoms included at least one of the Anthonisen criteria (increased dyspnea, sputum volume and sputum purulence).Citation16
Study procedures and outcomes
The study consisted of one visit to the study center. Patients were interviewed by a physician about their employment status, smoking status and COPD exacerbations in the previous year. Furthermore, morning symptoms, dyspnea severity, health status and pulmonary function were assessed and blood was drawn. All pulmonary function tests were performed between 8.30 and 11.00 AM. Immediately following this visit, the patients wore a triaxial accelerometer for 7 consecutive days. To collect information regarding possible adverse events, patients were contacted by telephone after these 7 days.
The occurrence and severity of morning symptoms were assessed with the PRO-Morning COPD Symptoms Questionnaire.Citation17 This questionnaire consists of six questions. Patients rated the severity of dyspnea, sputum production, chest tightness, wheezing and cough in the morning with a Likert scale ranging from 0 to 10 points for each question: 0 points represent no symptoms and 10 points represent symptoms as severe as they can imagine. Limitations in the morning due to COPD were scored with the same Likert scale. Total morning symptom scores ranged from 0 to 60. A total score of 0 indicated no symptoms at all in the morning.
The American College of Sports Medicine (ACSM) and the American Heart Association stated that adults should perform moderate-intensity physical activity for a minimum of 30 minutes (or bouts of at least 10 minutes) for 5 days each week or vigorous-intensity physical activity for a minimum of 20 minutes on 3 days each week.Citation18 To objectively measure physical activity, patients wore a validated triaxial accelerometer (Dynaport MoveMonitor; McRoberts BV, The Hague, the Netherlands)Citation19–Citation21 24 hours a day for 7 consecutive days after baseline visit. Due to non-water resistance, patients were not allowed to wear the accelerometer while taking a shower or a bath. Body posture and types of physical activity, as well the duration and intensity of the activity were measured. Steps, total duration of active and inactive periods, active time in light (1.5–3.0 metabolic equivalent task [MET]), moderate- (3.0–6.0 MET) and vigorous-intensity (6.0–9.0 MET) activities, number of active and inactive periods and number of walking periods ≤10 seconds, 10–20 seconds and >20 seconds were taken as secondary outcomes derived from the accelerometer. Additionally, to take the activity guidelines of the ACSM into account, active time in moderate physical activity (MPA), vigorous physical activity and moderate-to-vigorous physical activity (MVPA) in bouts of at least 10 minutes were measured. Activity was defined as standing, shuffling and walking combined; inactivity was defined as sitting and lying combined. Furthermore, the accelerometer measured the time that it was not worn.
Symptom burden was assessed with the modified Medical Research Council (mMRC) scale with scores ranging from 0 to 5.Citation22 Health status was assessed using the COPD assessment test (CAT).Citation23 Comorbidity was evaluated with the Charlson Comorbidity index (CCI).Citation24 High-sensitive C-reactive protein was measured in serum. Total leukocyte cell counts as well as absolute numbers of eosinophils were assessed. Lung function was assessed post-bronchodilator via spirometry (CareFusion, Masterscreen PFT System) following European Respiratory Society (ERS)/American Thoracic Society (ATS) standards.Citation25 Forced vital capacity (FVC) and forced volume in one second (FEV1) were expressed in absolute and % predicted values, respectively (based on Global Lung Function Initiative 2012).Citation26 Total lung capacity and residual volume (RV) were measured by body box (CareFusion, Masterscreen Body),Citation27 and RV was expressed in % predicted values (based on European Community for Coal and Steel). To assess hyperinflation, the ratio between RV and TLC was calculated. Severity of COPD was reported as GOLD A–D based on symptoms assessed with the mMRC, airway obstruction and exacerbations in the previous yearCitation15 (Supplementary materials).
Statistical analysis
Descriptive data were reported as mean values ± standard deviations (SD) for continuous data with a normal distribution, median with interquartile ranges (IQR) for continuous data with a non-normal distribution or percentages for categorical data. To assess whether there was a normal distribution, histograms were made from each continuous individual variable and the shapes of the histograms were carefully assessed. Differences for age and gender between participants and nonparticipants were analyzed with an independent t-test and a chi-square test, respectively. Differences in morning symptom severity between COPD stages A, B, C and D were assessed with a one-way analysis of variance followed by post hoc analyses.
When the accelerometer was worn less than 22.5 hours a day (94%), the day was excluded from analysis. Mean values derived from the accelerometer were calculated as the mean per patient only from valid days. When there was an adverse event for which a health care provider visit was required during the study period, the patient was excluded from activity analysis since adverse events might have negative effects on physical activity. The association between physical activity, dyspnea severity, health status, anxiety and depression, airflow limitation, lung hyperinflation, laboratory results, the presence of at least one exacerbation in the previous year and morning symptom severity was analyzed with univariable linear regression analysis. We looked at the overall explained variance (R2) of morning symptoms. In addition, regression analyses were adjusted for gender, age, ethnicity, body mass index, current smoking, number of exacerbations in the previous year, long-acting muscarinic antagonist (LAMA) use since LAMAs probably protect against morning symptoms,Citation17 employment status and comorbidity measured with the CCI. Missing data were not replaced. For all analyses, a p-value of <0.05 was considered statistically significant. We used SPSS version 23 for the statistical analysis.
A sensitivity analysis was performed to examine the impact of the CAT, instead of mMRC, to calculate GOLD classes A–D, since previous studies reported that the use of different questionnaires to categorize COPD patients can significantly alter the groups.Citation28,Citation29
Results
Patients
Of the 168 eligible patients who received the patient information letter, 80 patients agreed to participate. No significant differences between participants and nonparticipants were observed for age (mean [±SD] 65.6 [±8.7] and 66.3 [±8.4] years, respectively, p=0.79) and gender (54% and 44.3% males, respectively, p=0.22). No patient dropped out of the study (). shows demographics and baseline characteristics of the participants. Patients had an airflow obstruction with an average FEV1 of 55.1% predicted, and 26% of the patients were current smokers.
Table 1 Baseline characteristics
Morning symptoms
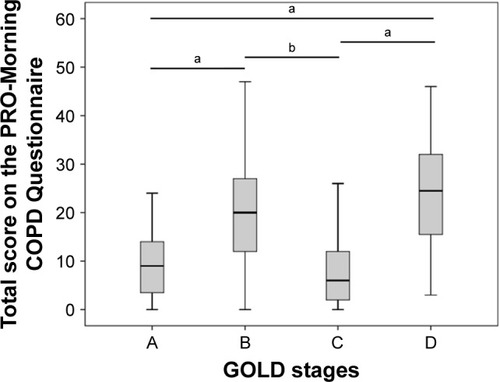
Morning symptoms were present in 96% (77/80) of the patients. Mean (±SD) total severity score for morning symptoms was 17.9 (±11.7) (). The most severe symptoms in the morning were dyspnea, cough and sputum production. Overall, 85.0% of all included patients reported physical activity limitations in the morning due to morning symptoms. Morning symptom scores were significantly different between patients with different COPD stages: mean (±SD) score in GOLD A was 9.7 (±7.2), B 19.8 (±10.7), C 8.6 (±9.3) and D 23.8 (±11.2) (F [df] =10.3 [79], p<0.01) (). Post hoc analyses showed differences between groups A and B (p<0.01), A and D (p<0.01), B and C (p=0.013) and C and D (p<0.01).
Table 2 Occurrence and severity of morning symptoms
Physical activity
Eight patients reported an adverse event during the study period (Table S1) and one patient wore the accelerometer for an insufficient time each day of the study (7.2 to 13.7 hours a day). Ninety-four percent of the measured days from the remaining 71 patients were included in the analysis. The median number of days analyzed per patient was seven. shows outcomes derived from accelerometry. Patients walked an average of 5,754 steps a day, and they spent 11.4 minutes a day in MVPA with bouts of at least 10 minutes.
Table 3 Daily physical activity parameters derived from accelerometry
Relationship between morning symptoms and other parameters
shows the association between baseline patient characteristics and morning symptom severity. When adjusted for confounders, lower health status (estimated regression coefficient =1.194, 95% CI 0.923 to 1.465), higher symptomatic burden (estimated regression coefficient =4.193, 95% CI 2.384 to 6.002), increased anxiety and depression and lower FEV1 were associated with morning symptom severity. Furthermore, physical activity that was objectively measured by accelerometry was associated with morning symptom severity: fewer steps a day (estimated regression coefficient =−0.001, 95% CI −0.002 to −0.000), less time in MVPA with bouts of at least 10 minutes (estimated regression coefficient =−0.135, 95% CI −0.233 to −0.037) and less time in MPA with bouts of at least 10 minutes (estimated regression coefficient =−0.192, 95% CI −0.321 to −0.064) (). The explained variance of all studied patient characteristics was highest for the CAT (62%).
Table 4 Associations between health status, dyspnea severity, anxiety and depression, airflow limitation, lung hyperinflation, inflammatory parameters, exacerbations and morning symptom severity
Table 5 Associations between minutes in active and inactive time, periods in activity and inactivity and morning symptom severity
Sensitivity analysis
When the CAT was used instead of the mMRC scale to classify COPD severity, differences in the morning symptom severity were still present between the different GOLD stages. In addition, there was also a significant difference in morning symptom severity between patients with COPD GOLD B and D (Figure S1).
Discussion
In the present study, morning symptom severity was assessed in COPD GOLD A, B, C and D patients. Furthermore, the association between patient characteristics (with special focus on physical activity) and morning symptom severity was studied. As far as we know, this is the first time the relationship between morning symptom severity and objective measures of the physical activity by triaxial accelerometry has been studied. In the present study, patients with overall more symptomatic COPD had significantly higher morning symptom scores. Lower health status, higher symptomatic burden, increased anxiety and depression, lower FEV1, fewer steps a day and less time in moderate and vigorous physical activity with bouts of at least 10 minutes that was objectively measured by accelerometry were associated with morning symptom severity.
Morning symptom scores were higher in patients with GOLD B and D compared with GOLD A and C stages. However, morning symptoms were not limited to more advanced COPD stages and were also present in patients of group A. These results are in line with the first and so far only study that described the relation between the occurrence of morning symptoms and severity of COPD with GOLD A–D classification.Citation7 In that study, patterns of morning symptom occurrence was more associated with the GOLD A–D categorization compared to GOLD 1–4 classification. GOLD 1–4 classification is based only on airflow limitation, whereas in GOLD A–D a combination of airflow limitation, general symptoms and exacerbations are used to classify the patients. Previous studies have shown associations between morning symptoms and airflow limitation,Citation2,Citation30 general symptomsCitation2,Citation3 and COPD exacerbations in the previous 12 months.Citation30–Citation32 In the current study, FEV1 and symptoms measured with the mMRC were significantly associated with the severity of morning symptoms. Furthermore, unadjusted analyses showed an association between exacerbations in the previous 12 months and morning symptom severity. Since overall symptoms and exacerbations, which are included in the GOLD A–D scheme, are associated with morning symptoms, we believe that GOLD A–D relates more closer to morning symptoms than GOLD 1–4. The sensitivity analyses showed that the differences in morning symptom severity between GOLD A, B, C and D groups were independent of the symptoms scale that was used and no difference between mMRC and CAT was found. Overall, our results are supported by the finding that the occurrence of nighttime symptoms was more closely related to the GOLD A–D classification than the GOLD 1–4 classification.Citation33
In the present study, the occurrence of morning symptoms was higher than that reported in other studies. The use of a different morning symptom questionnaire could be responsible for the higher occurrence in the current study.Citation5 The prevalence of morning symptoms in the present study is in line with a previous trial that studied morning symptoms in more than 3,000 COPD patients.Citation34 In that study, 94.4% of patients with moderate to severe COPD suffered from morning symptoms, measured with Early-Morning Symptoms of COPD Instrument. In the current study, the PRO-Morning COPD Symptoms Questionnaire was used. One previous randomized trial used this questionnaire and found a mean morning symptom severity score of 16.7.Citation17 The MODAS showed a slightly higher mean morning symptom score. This could be due to a slightly different COPD population (lower FEV1, fewer males, less current smokers) or the unknown variance of the morning symptom score. Overall, morning symptoms score are relatively low. For example, analyses on 3,394 COPD GOLD II and III patients have shown a mean morning symptom score of 1.3 (minimum 0, maximum 4).Citation34 Furthermore, we know from previous research that patients underestimated their symptoms and one-third of patients who describe their symptoms as being mild to moderate are not able to leave the house due to breathlessness.Citation35 We think that the PRO-Morning COPD Symptoms Questionnaire can be a suitable tool to assess morning symptoms. Nevertheless, this questionnaire needs to be validated in further studies. Using a validated questionnaire with cutoff scores might help to indicate whether a score of 17.9 could be seen as high or as low impact.
Previous studies that reported the association between morning symptoms and physical activity used self-reported physical activity questionnaires to evaluate physical activity.Citation2,Citation3,Citation6,Citation14,Citation30,Citation34,Citation36,Citation37 However, it is known that self-reported outcomes show discrepancies to objective measured outcomes,Citation38 and therefore we assessed physical activity using an established activity monitor.Citation19,Citation39 Indeed, when analyzing the data retrieved from these monitors we found a relationship between objectively measured physical activity and an increase in morning symptom severity. More specifically, an association between fewer steps a day, less time in MVPA with bouts of at least 10 minutes and less time in MPA with bouts of at least 10 minutes and increase in morning symptom severity was found. Interestingly, when we do not take these bouts into account, time in MPA and MVPA was not associated with morning symptom severity. This means that COPD patients with higher morning symptom scores are able to perform physical activity of moderate and vigorous intensity similar to those with less severe morning symptoms, but they rarely perform this activity longer than 10 minutes at a time. We suggest that these patients do not perform activities for increased amounts of time as an adaptation to, for example, avoid symptoms, and they are therefore not used to being active for more than 10 minutes in moderate or vigorous activity. For light-intensity physical activity, no association with morning symptom severity was found. Sufficient physical activity is important for COPD patients because the lack of it is associated with more exacerbations, hospitalizations, all-cause mortalityCitation12 and lower quality of life.Citation13 These results together underline the importance of morning symptoms and physical activity. Together, they seem to be a new important target for therapy.
The association between lower health status,Citation2,Citation7,Citation30,Citation37,Citation40 more dyspnea,Citation2,Citation3 increased anxiety and depression,Citation2,Citation30,Citation37,Citation40 lower FEV1Citation2,Citation30 and morning symptoms have been described earlier. The association between morning symptoms and overall symptoms might appear to be trivial; however, previous studies have shown that symptoms can vary over the day and therefore more precise assessment of symptoms seems necessary. The present study confirms this; the CAT does not fully cover morning symptoms with an explained variance of 62%. Based on this, we recommend assessing morning symptoms separately from general symptoms in COPD patients, especially in those with more symptomatic COPD.
A strength of this study is the adjustment for multiple confounders in the regression analysis, which was omitted in previous studies.Citation2,Citation37 Furthermore, we believe that this study population is representative of the COPD population since patients were recruited from a variety of sources, namely, a university medical center, a regional hospital and recruitment via flyers. This resulted in a heterogeneous COPD patient group. However, the exclusion of COPD GOLD I patients can be seen as a limitation. We expect that including COPD GOLD I patients would result in a slightly lower mean morning symptom score since morning symptoms are also present in mild COPD.Citation2 However, in this study we decided to focus on more symptomatic patients since it was a cross-sectional study that explored factors that were associated with morning symptoms. One other limitation of this study is that there might be selection bias, since nonparticipants were most likely patients who were not able to come to the study center in the morning. This might have resulted in an underestimation of morning symptoms in the overall COPD population. A limitation for the use of a MoveMonitor was the non-water resistance. For some patients, taking a shower is the most intensive physical activity of the day, and this has not been measured. This resulted in an underestimation of active time. Furthermore, patients were not blinded for the accelerometer. This could have resulted in increased activity since patients felt they were being watched and would not be categorized as “inactive.” However, patients took a comparable amount or fewer steps than reported in previous studies,Citation41,Citation42 suggesting that patients in the MODAS did not adapt their lifestyle to the study. Furthermore, with this observational study design it is not possible to prove causality. Therefore, we are not able to state whether limitation in physical activity is a result of morning symptoms, or if it is the other way around; that is, physical inactivity causes deconditioning which results in an increase of morning symptoms.
Conclusion
Patients with overall more symptomatic COPD have significant higher morning symptom scores. Furthermore, morning symptoms were associated with important clinical outcomes: lower health status, more symptoms, increased anxiety and depression, fewer steps a day, less time in moderate and vigorous physical activity with bouts of at least 10 minutes and lower FEV1. This was the first study that evaluated the relation between morning symptom severity and objective measures of physical activity by triaxial accelerometry. Morning symptoms should be assessed more precisely in addition to assessment by general COPD-specific questionnaires, especially in those with more symptomatic COPD. Therefore, there is need for the validation of a morning symptom questionnaire. In addition, more research is needed on potential therapies to improve morning symptoms, for example, with the improvement of (a combination of) health status, symptoms, anxiety and depression, steps a day, time in moderate and vigorous physical activity with bouts of at least 10 minutes and FEV1.
Acknowledgments
We thank Steven JHA McDowell for critically reading the manuscript. This study was supported by Novartis with an unrestricted research grant.
Supplementary materials
To categorize the patients, the first step was to assess the symptom burden with the modified Medical Research Council (mMRC) scale. A low symptom burden was defined as mMRC 0 to 1 and a high symptom burden as 2 or higher. Moreover, the exacerbation risk was evaluated by airflow obstruction, number of exacerbations in the previous year and number of exacerbations leading to hospital admission in the previous year. A low risk was defined as a forced expiratory volume in 1 second (FEV1) ≥50% and <2 exacerbations a year and no hospital admission due to exacerbation of COPD; a high risk was defined as a FEV1 <50% or ≥2 exacerbations in the previous year or ≥1 hospital admission due to exacerbation of COPD. Group A was defined as a low symptom burden with low risk; group B as high symptom burden with low risk; group C as low symptom burden with high risk and group D as high symptom burden with high risk.
Figure S1 Morning symptom scores in COPD GOLD A, B, C and D patients, categorization with CAT.
Notes: Morning symptoms scores were significantly different between patients with COPD GOLD A 6.3 (±4.4), B 18.4 (±10.1), C 4.0 (±4.7) and D 23.6 (±11.0) (F[df] =13.0 [79], p<0.01); COPD GOLD A (N=12), B (N=29), C (N=5) and D (N=34); ap<0.01, bp<0.05.
Abbreviations: CAT, COPD Assessment Test; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
![Figure S1 Morning symptom scores in COPD GOLD A, B, C and D patients, categorization with CAT.Notes: Morning symptoms scores were significantly different between patients with COPD GOLD A 6.3 (±4.4), B 18.4 (±10.1), C 4.0 (±4.7) and D 23.6 (±11.0) (F[df] =13.0 [79], p<0.01); COPD GOLD A (N=12), B (N=29), C (N=5) and D (N=34); ap<0.01, bp<0.05.Abbreviations: CAT, COPD Assessment Test; GOLD, Global Initiative for Chronic Obstructive Lung Disease.](/cms/asset/30751b92-0718-4afc-ac69-d50a7749c3b1/dcop_a_143387_sf0001_b.jpg)
Table S1 Adverse events during study period
Disclosure
ARvB, MJK and NHC report no conflicts of interest in this work. CT reports grants and personal fees from Novartis during the conduct of the study and personal fees from Boehringer Ingelheim, Astra Zeneca, Teva, and Chiesi outside the submitted work.
References
- MurrayCJBarberRMForemanKJGlobal, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transitionLancet2015386100092145219126321261
- MiravitllesMWorthHSoler CatalunaJJObservational study to characterise 24-hour COPD symptoms and their relationship with patient-reported outcomes: results from the ASSESS studyRespir Res20141512225331383
- PartridgeMRKarlssonNSmallIRPatient insight into the impact of chronic obstructive pulmonary disease in the morning: an internet surveyCurr Med Res Opin20092582043204819569976
- van BuulARKasteleynMJChavannesNHTaubeCAssociation between morning symptoms and physical activity in COPD: a systematic reviewEur Respir Rev20172614316003328049127
- van BuulARKasteleynMJChavannesNHTaubeCMorning symptoms in COPD: a treatable yet often overlooked factorExpert Rev Respir Med201711431132228282500
- KimYJLeeBKJungCYPatient’s perception of symptoms related to morning activity in chronic obstructive pulmonary disease: the SYMBOL StudyKorean J Intern Med201227442643523269884
- TsiligianniIMettingEvan der MolenTChavannesNMorning and night symptoms in primary care COPD patients: a cross-sectional and longitudinal study. An UNLOCK study from the IPCRGNPJ Prim Care Respir Med2016261604027442618
- PittaFTroostersTSpruitMAProbstVSDecramerMGosselinkRCharacteristics of physical activities in daily life in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2005171997297715665324
- TroostersTSciurbaFBattagliaSPhysical inactivity in patients with COPD: a controlled multi-center pilot-studyRespir Med201010471005101120167463
- Donaire-GonzalezDGimeno-SantosEBalcellsEPhysical activity in COPD patients: patterns and boutsEur Respir J2013424993100223258786
- BrawnerCAChurillaJRKeteyianSJPrevalence of physical activity is lower among individuals with chronic diseaseMed Sci Sports Exerc20164861062106726741117
- Garcia-AymerichJLangePBenetMSchnohrPAntoJMRegular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort studyThorax200661977277816738033
- EstebanCQuintanaJMAburtoMImpact of changes in physical activity on health-related quality of life among patients with COPDEur Respir J201036229230020075059
- O’HaganPChavannesNHThe impact of morning symptoms on daily activities in chronic obstructive pulmonary diseaseCurr Med Res Opin201430230131424195740
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal Strategy for the Diagnosis, Management and prevention of COPD; 2017 [updated 2017] Available from: http://www.goldcopd.org/Accessed: May 16, 2017
- AnthonisenNRManfredaJWarrenCPHershfieldESHardingGKNelsonNAAntibiotic therapy in exacerbations of chronic obstructive pulmonary diseaseAnn Intern Med198710621962043492164
- MarinJMBeehKMClemensAEarly bronchodilator action of glycopyrronium versus tiotropium in moderate-to-severe COPD patients: a cross-over blinded randomized study (Symptoms and Pulmonary function in the moRnING)Int J Chron Obstruct Pulmon Dis2016111425143427418815
- HaskellWLLeeIMPateRRPhysical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart AssociationMed Sci Sports Exerc20073981423143417762377
- RabinovichRALouvarisZRasteYValidity of physical activity monitors during daily life in patients with COPDEur Respir J20134251205121523397303
- Van RemoortelHGiavedoniSRasteYValidity of activity monitors in health and chronic disease: a systematic reviewInt J Behav Nutr Phys Act201298422776399
- Van RemoortelHRasteYLouvarisZValidity of six activity monitors in chronic obstructive pulmonary disease: a comparison with indirect calorimetryPloS one201276e3919822745715
- BestallJCPaulEAGarrodRGarnhamRJonesPWWedzichaJAUsefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary diseaseThorax199954758158610377201
- JonesPWHardingGBerryPWiklundIChenWHKline LeidyNDevelopment and first validation of the COPD Assessment TestEur Respir J200934364865419720809
- CharlsonMEPompeiPAlesKLMacKenzieCRA new method of classifying prognostic comorbidity in longitudinal studies: development and validationJ Chronic Dis19874053733833558716
- MillerMRHankinsonJBrusascoVStandardisation of spirometryEur Respir J200526231933816055882
- QuanjerPHStanojevicSColeTJMulti-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equationsEur Respir J20124061324134322743675
- CrieeCPSorichterSSmithHJBody plethysmography – its principles and clinical useRespir Med2011105795997121356587
- CasanovaCMarinJMMartinez-GonzalezCNew GOLD classification: longitudinal data on group assignmentRespir Res201415324417879
- JonesPWNadeauGSmallMAdamekLCharacteristics of a COPD population categorised using the GOLD framework by health status and exacerbationsRespir Med2014108112913524041746
- RocheNSmallMBroomfieldSHigginsVPollardRReal world COPD: association of morning symptoms with clinical and patient reported outcomesCOPD201310667968624127914
- MiravitllesMWorthHSoler-CatalunaJJThe relationship between 24-hour symptoms and copd exacerbations and healthcare resource use: results from an observational study (ASSESS)COPD201613556156826983349
- TsiligianniIMettingEvan der MolenTChavannesNKocksJMorning and night symptoms in primary care COPD patients: a cross-sectional and longitudinal study. An UNLOCK study from the IPCRGNPJ Prim Care Respir Med2016261604027442618
- LangePMarottJLVestboJNordestgaardBGPrevalence of nighttime dyspnoea in COPD and its implications for prognosisEur Respir J20144361590159824488571
- BatemanEDChapmanKRSinghDAclidinium bromide and formoterol fumarate as a fixed-dose combination in COPD: pooled analysis of symptoms and exacerbations from two six-month, multicentre, randomised studies (ACLIFORM and AUGMENT)Respir Res2015169226233481
- RennardSDecramerMCalverleyPMImpact of COPD in North America and Europe in 2000: subjects’ perspective of Confronting COPD International SurveyEur Respir J200220479980512412667
- KesslerRPartridgeMRMiravitllesMSymptom variability in patients with severe COPD: a pan-European cross-sectional studyEur Respir J201137226427221115606
- StephensonJJCaiQMocarskiMTanHDoshiJASullivanSDImpact and factors associated with nighttime and early morning symptoms among patients with chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis20151057758625844033
- ThyregodMBodtgerUCoherence between self-reported and objectively measured physical activity in patients with chronic obstructive lung disease: a systematic reviewInt J Chron Obstruct Pulmon Dis2016112931293827932873
- Van HeesVTSlootmakerSMDe GrootGVan MechelenWVan LummelRCReproducibility of a triaxial seismic accelerometer (DynaPort)Med Sci Sports Exerc200941481081719276852
- Soler-CatalunaJJSauledaJValdesLPrevalence and perception of 24-hour symptom patterns in patients with stable chronic obstructive pulmonary disease in SpainArch Bronconeumol201652630831526774700
- WaschkiBKirstenAMHolzODisease progression and changes in physical activity in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2015192329530626020495
- WatzHWaschkiBBoehmeCClaussenMMeyerTMagnussenHExtrapulmonary effects of chronic obstructive pulmonary disease on physical activity: a cross-sectional studyAm J Respir Crit Care Med2008177774375118048807