Abstract
The goals of COPD therapy are to prevent and control symptoms, reduce the frequency and severity of exacerbations, and improve exercise tolerance. The triple combination therapy of inhaled corticosteroids (ICSs), long-acting beta2 agonists (LABAs), and long-acting muscarinic antagonists (LAMAs) has become an option for maintenance treatment of COPD and as a “step-up” therapy from single or double combination treatments. There is evidence that triple combination ICS/LABA/LAMA with different inhalers improves lung function, symptoms, and health status and reduces exacerbations. A new triple fixed-dose combination of extrafine beclomethasone dipropionate (100 µg/puff)/formoterol fumarate (6 µg/puff)/glycopyrronium bromide (12.5 µg/puff) has been developed as a hydrofluoroalkane pressurized metered dose inhaler. Two large pivotal studies showed that this extrafine fixed ICS/LABA/LAMA triple combination is superior to fixed ICS/LABA combined therapy and also superior to the LAMA tiotropium in terms of lung function and exacerbation prevention in COPD patients at risk of exacerbation. This review considers the new information provided by these clinical trials of extrafine triple therapy and the implications for the clinical management of COPD patients.
Introduction
COPD is one of the leading causes of morbidity and mortality worldwide.Citation1,Citation2 Pharmacological therapy for COPD reduces symptoms, frequency and severity of exacerbations, and improves exercise tolerance and health status.Citation3 Currently, the main treatment options for COPD patients belong to a restricted number of pharmacological classes – that is, bronchodilators (short-acting beta2 agonists [SABAs], long-acting beta2 agonists [LABAs], short-acting muscarinic antagonists [SAMAs], and long-acting muscarinic antagonists [LAMAs]), inhaled corticosteroids (ICSs), and inhibitors of the enzyme phosphodiesterase-4.
Long-acting bronchodilator monotherapy is known to increase lung function, improve patient-reported outcomes (PROs) such as symptoms and quality of life, enhance exercise performance, and reduce exacerbations.Citation4,Citation5 Administering LABA and LAMA concurrently (dual bronchodilator treatment) significantly improves lung function and PROs compared to treatment with a single bronchodilator;Citation6 also, there is evidence for fewer exacerbations when using two long-acting bronchodilators compared to one.Citation7 The scientific rationale behind the additive effects observed when combining bronchodilators includes the different mechanisms of action of beta2 agonists and muscarinic antagonists (stimulation of beta2-adrenergic receptors and inhibition of acetylcholine-induced bronchoconstriction, respectively), and the potential intracellular interactions between these pathways.Citation8,Citation9
A number of studies have shown that long-term treatment with a combination inhaler containing ICS/LABA is more effective than the individual components in improving lung function and PROs and in reducing exacerbation frequency. ICS/LABA combinations are recommended for use in patients at risk of exacerbations, for which the best predictor is the prior history of exacerbations.
For patients who remain symptomatic and/or continue to exacerbate despite treatment with a dual bronchodilator or ICS/LABA combination inhaler, the Global Initiative for Obstructive Lung Disease (GOLD) management strategy recommends step up to triple therapy (ICS plus LABA plus LAMA). In clinical practice, patients also step up to triple from LAMA monotherapy.Citation3 Since the components of triple therapy have different pharmacological mechanisms of action, there is a strong rationale for the use of these drugs together to maximize clinical benefits, including the prevention of exacerbation.Citation10,Citation11
Triple therapy is widely prescribed to COPD patients in clinical practice, commonly using two separate inhalers: an ICS/LABA combination plus a LAMA. A recent review from the UK general practice showed that, from 2004 to 2009, the use of triple therapy increased from 25% to 59% in patients with very severe COPD, with 14% and 19% mild and moderate (based on lung function) COPD patients, respectively, using triple therapy.Citation12 However, while its use has increased, relatively few studies have been conducted to test the efficacy of triple therapy, administered by separate inhalers, compared to ICS/LABA, LABA/LAMA, or LAMA in terms of preventing exacerbations.
This article reviews the available evidence of the efficacy of triple therapy in COPD. We review studies using extemporary triple therapy (ie, therapy delivered through separate inhalers with different posologies), analyzing their strengths and weaknesses. We then focus on new data from recently published large clinical trials using the novel fixed-dose triple combination of extrafine beclomethasone dipropionate (BDP), formoterol fumarate (FF), and glycopyrronium bromide (GB) delivered by a single inhaler. As a general concept, triple therapies that require the use of at least two different inhaler devices increase the risk of inhalation errors and reduce adherence to inhaled treatments. This is particularly evident when the inhalers are of different designs. Thus this single-inhaler triple therapy with BDP/FF/GB simplifies the treatment regimen, with potential to enhance compliance.
Triple therapy using separate inhalers
The key results of randomized controlled clinical trials on the effects of triple therapy (administered as two separate inhalers) in COPD are summarized in . These studies vary considerably in duration, comparator treatments, and type of inhaler devices; nevertheless, there is consistent evidence that triple therapy has significantly greater effects on trough forced expiratory volume in the first second (FEV1) compared to comparator treatments,Citation13–Citation19 with mean differences often being around the level of the minimal clinically important difference (MCID) of 100 mL.Citation20 There is also evidence for a benefit of triple therapy on some PROs; for example, Frith et al showed that, over 12 weeks, triple therapy had a greater effect on health status compared to ICS/LABA treatment.Citation16 However, the benefit of extemporary triple therapy on exacerbation prevention has not been convincingly or consistently demonstrated.
Table 1 Clinical trials with triple therapy in COPD
The Canadian Thoracic Society/Canadian Respiratory Clinical Research Consortium studyCitation18 investigated the effects of triple therapy over 1 year and demonstrated a significant benefit in terms of FEV1 and quality of life compared to LAMA plus LABA, or to LAMA monotherapy. There was no difference between groups on time to first moderate-to-severe exacerbation, but a high dropout rate reduced the statistical power to detect these events.
Welte et al studied triple therapy versus LAMA monotherapy over 3 monthsCitation13 and observed a benefit for triple therapy in terms of exacerbations, although these were atypically reported as either hospitalizations or moderate exacerbations requiring systemic corticosteroids (those requiring antibiotics were not reported).
The FORWARD study compared extrafine BDP/FF versus FF monotherapy and showed the superiority of BDP/FF in reducing the frequency of moderate and severe exacerbations of 28% over FF. A secondary endpoint analysis of the FORWARD study provided supporting evidence for the potential impact of triple therapy on exacerbations.Citation21 Since patients treated with tiotropium were allowed to continue this treatment throughout the study, a pre-specified analysis was performed of triple therapy (BDP/FF plus LAMA) versus LABA plus LAMA in this subgroup of patients (~50% of the randomized population). The results showed a 28% reduction of exacerbations with triple therapy compared with LABA+LAMA, in line with the results of the overall study population. Similarly, 75% of patients in the SPARK trial, comparing single versus dual bronchodilator therapy, were on ICS and the study therefore demonstrates superiority of triple therapy over the combination of ICS and LAMA.Citation7
A retrospective analysis has been performed to evaluate the real-life impact of triple therapy by using the Scottish National Health Service database of patients with COPD from 2001 to 2010.Citation22 The study evaluated the impact of the LAMA tiotropium added to ICS/LABA on all-cause mortality, hospital admissions for respiratory disease, and emergency oral corticosteroid bursts. The analysis included a total of 1,857 patients treated with ICS/LABA plus tiotropium, and 996 treated with ICS/LABA. The adjusted hazard ratio (HR) for ICS/LABA plus tiotropium versus ICS/LABA was 0.65 on all-cause mortality and 0.85 and 0.71 for hospital admissions and courses of oral corticosteroid, respectively. Similarly, a retrospective study of two cohorts of patients with COPD compared a regimen of tiotropium plus ICS/LABA to a historic matched COPD population treated with ICS/LABA.Citation23 The use of triple therapy was associated with a 40% reduced risk of death compared with ICS/LABA. These retrospective real-life analyses provide supportive evidence of the benefits of triple therapy. However, the results of these studies can be influenced by confounding factors and by imbalances between treatment arms and the findings should be interpreted with caution.
Single-inhaler triple therapy – clinical evidence
A novel fixed-dose combination of BDP/FF/GB has been developed as a hydrofluoroalkane (HFA) solution delivered via a pressurized metered dose inhaler (pMDI) with a nominal dose per actuation of 100, 6, and 12.5 µg of BDP, FF, and GB, respectively. Each delivered dose contains 87 µg of BDP, 5 µg of FF, and 9 µg of glycopyrronium (corresponding to 11 µg of GB). BDP/FF/GB fixed-dose combination has an extrafine formulation (ie, the mass median aerodynamic diameter [MMAD] is 1.1 µm for all active ingredients) designated to target both large and small airways. It is administered twice daily as 2 puffs/b.i.d. (total daily dose: 400, 24, and 50 µg expressed as metered dose of BDP, FF, and GB, respectively).
Two pivotal studies have been conducted to evaluate the safety and efficacy of extrafine BDP/FF/GB versus different treatment options for COPD: extrafine BDP/FF/GB has been compared to an ICS/LABA combination in the TRILOGY study, and to a LAMA monotherapy and an extemporary triple combination of ICS/LABA + LAMA in the TRINITY study. The inclusion criteria for both studies were very similar (summarized in ) leading to the enrolment of very similar populations and the duration of randomized treatment was 52 weeks in both studies. This allows for a side-by-side comparison of the results from these studies.
Table 2 Key inclusion criteria of TRILOGYCitation24 and TRINITYCitation25 studies
Study designs
Both studies enrolled COPD patients aged ≥40 years with FEV1 <50% predicted and a documented history of at least one COPD exacerbation in the 12 months prior to screening. Patients had to be symptomatic at screening with a COPD assessment test (CAT) score ≥10. The characteristics of the study populations are shown in .
Table 3 Patients’ characteristics in TRILOGY and TRINITY studies – safety population
TRILOGY was a 52-week, double-blind, randomized, multicenter, two-arm, active-controlled clinical trial to test the superiority of extrafine BDP/FF/GB versus a fixed combination of extrafine BPD plus FF administered via pMDICitation24 (). The three co-primary endpoints were pre-dose FEV1, 2-h post-dose FEV1, and transition dyspnea index (TDI) focal score, all measured at week 26. A total of 1,812 patients were screened, with 1,368 randomized.
Figure 1 (A) TRILOGY study design. (B) TRINITY study design.
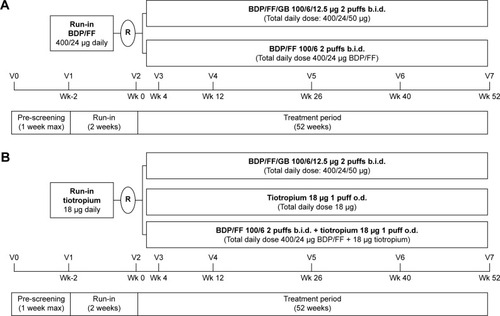
TRINITY was a 52-week double-blind, double-dummy, randomized, multicenter, three-arm parallel-group, active-controlled study to assess the superiority of extrafine BDP/FF/GB versus tiotropium and the non-inferiority of extrafine BDP/FF/GB versus BDP/FF + tiotropium ().Citation25 The primary objective was the comparison with tiotropium in terms of moderate-to-severe COPD exacerbation rate. Pre-specified key secondary objectives evaluated the superiority of fixed triple versus tiotropium and its non-inferiority versus extemporary triple therapy in terms of change from baseline in pre-dose FEV1 at week 52. A total of 3,433 patients were screened, with 2,691 randomized.
Main lung function findings (FEV1)
In TRILOGY, extrafine BDP/FF/GB was superior to BDP/FF for both pre-dose FEV1 (adjusted mean difference 0.081 L [95% CI 0.052; 0.109]; p<0.001) and 2-h post-dose FEV1 (adjusted mean difference 0.117 [95% CI 0.086; 0.147]; p<0.001) at week 26. In TRINITY, extrafine BDP/FF/GB was superior to tiotropium at week 52 for pre-dose FEV1, with an adjusted mean difference of 0.061 L (95% CI 0.037; 0.086), p<0.001. There was no difference between fixed and extemporary triple combination (−0.003 L [95% CI −0.033; 0.027]).
COPD exacerbations
In TRILOGY, the adjusted annual rate of moderate-to-severe exacerbations was 0.41 for the BDP/FF/GB group and 0.53 for the BDP/FF group, with a rate ratio of 0.77 (95% CI 0.65; 0.92; p=0.005), indicating a significant 23% reduction with BDP/FF/GB. In TRINITY, the adjusted exacerbation rate per patient per year was lower with extrafine BDP/FF/GB (0.46) than with tiotropium (0.57); the adjusted rate ratio (95% CI) was 0.80 (95% CI 0.69; 0.92; p=0.0025), indicating a 20% relative reduction.
Both in TRILOGY and TRINITY, the prospective exacerbation rate was higher in patients with two or more exacerbations in the previous year compared to those with one exacerbation (1 exacerbation: 0.37, ≥2 exacerbations: 0.65 in TRILOGY and 1 exacerbation: 0.43, ≥2 exacerbations: 0.59 in TRINITY [data are reported as rates in the BDP/FF/GB arm]). In the subgroups of patients with two or more exacerbations in the previous year, the reduction in the rate of moderate-to-severe exacerbations with triple therapy was larger: 33% and 28% when BDP/FF/GB was compared with BDP/FF in TRILOGY and with tiotropium in TRINITY, respectively (). Notably, extrafine BDP/FF/GB significantly reduced exacerbations by 29% compared to extemporary triple in the subgroup of patients with two or more exacerbations in the previous year ().
Figure 2 Moderate/severe exacerbation rate in COPD patients with two or more moderate/severe exacerbations in the year before study entry.
Abbreviations: BDP, beclomethasone dipropionate; FF, formoterol fumarate; GB, glycopyrronium bromide; ITT, intention-to-treat.
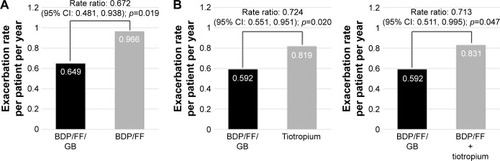
Of note, the percentage of patients treated with BDP/FF/GB who did not experience moderate-to-severe/severe exacerbation during the study was 68.9%/90.5% and 67.4%/93% in TRILOGY and TRINITY, respectively.
A pre-specified analysis was performed in the TRINITY study using the blood eosinophil count prior to randomization to evaluate their predictive value on ICS effect, as previously reported in post-hoc analyses.Citation26 Eosinophil counts ≥2% or ≥0.2×109 cells/L were associated with greater exacerbation rate reductions of 30% (p<0.001) and 36% (p<0.001), respectively, for fixed triple versus tiotropium.
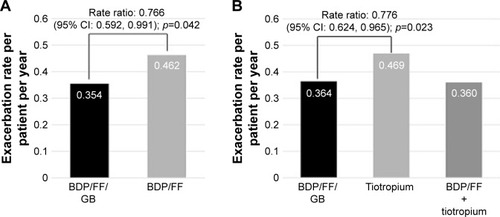
A post-hoc analysis of TRILOGY and TRINITY results was performed on group B patients classified according to the new GOLD 2017 recommendations.Citation3 In TRILOGY study 753 (55%) patients were classified as B patients and in TRINITY 1,324 (49%) were group B. In these GOLD B patients, extrafine BDP/FF/GB significantly reduced moderate/severe exacerbations by 23% versus BDP/FF (adjusted rate ratio 0.77, 95% CI: 0.59; 0.99, p=0.042) and by 22% versus tiotropium in TRINITY (adjusted rate ratio 0.78, 95% CI: 0.62; 0.97, p=0.023) ().
Figure 3 Moderate/severe COPD exacerbation rate in GOLD B patients.
Abbreviations: BDP, beclomethasone dipropionate; FF, formoterol fumarate; GB, glycopyrronium bromide; GOLD, Global Initiative for Obstructive Lung Disease; ITT, intention-to-treat.
Symptoms and health status
TDI focal score was measured only in TRILOGY; there was an increase in both studied groups at all visits, and the adjusted mean difference between treatments (0.21 units [95% CI −0.08; 0.51]) in favor of BDP/FF/GB at 26 weeks was not statistically significant. However, individual responder analysis showed that more patients reported clinically relevant improvements (≥1 unit) in TDI focal score at week 26 with BDP/FF/GB compared to BDP/FF (57.4% versus 51.8% respectively, odds ratio [OR] 1.28; 95% CI 1.03; 1.59; p=0.027).
Clinically relevant improvements from baseline (decrease ≥4 units) in St George’s Respiratory Questionnaire (SGRQ) total score occurred in the BDP/FF/GB group at all visits from week 12 onward in the TRILOGY study, with statistically significant differences between the two groups at weeks 4, 12, and 52. The proportion of SGRQ responders (ie, patients with an improvement of ≥4 points) was 43.2% with BDP/FF/GB and 35.9% with BDP/FF at week 52, showing a statistically significant difference in favor of triple therapy (OR 1.33, 95% CI 1.06; 1.66; p=0.014).
In TRINITY, both fixed and extemporary triple combinations had statistically significant greater improvements in SGRQ total score at week 52 compared with tiotropium (adjusted mean difference [95% CI] of −1.60 [−2.82; 0.38], p=0.010 and −3.18 [−4.66; 1.69], p<0.001, respectively). The percentage of SGRQ responders was statistically significantly greater with both BDP/FF/GB and BDP/FF + tiotropium than with tiotropium at weeks 26 and 52.
Safety
Here, the similar baseline characteristics of patient populations in TRILOGY and TRINITY allowed us to show a pooled analysis of safety data. The majority of patients completed at least 52 weeks of treatment: 71.9% with BDP/FF/GB, 69.3% with BDP/FF, 64.8% with tiotropium, and 69.8% with BDP/FF + tiotropium.
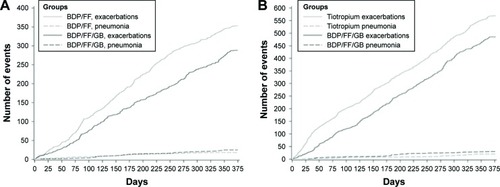
The incidence of pneumonia was low in both the TRILOGY and TRINITY studies (<3% in each group). In the integrated analysis, 51 patients (2.9% of patients treated with fixed triple) reported 55 pneumonias, which represents an event rate of 32.9/1.000 patient-years. Eighteen (2.6%) patients treated with BDP/FF experienced pneumonia with a rate of 28.8/1.000 patient-years, 19 (1.8%) patients treated with tiotropium experienced pneumonia with an event rate of 20.5/1.000 patient-years, and 12 (2.2%) patients treated with BDP/FF + tiotropium had pneumonia with a rate of 25.2/1.000 patient-years. Of note, when a frequency plot was generated considering days in the study versus cumulative number of events (exacerbations versus pneumonia), the number of incident pneumonia resulted minimal compared to that of moderate/severe exacerbations (). In terms of safety, the integrated analysis of both pivotal studies showed a total 2.9% of pneumonia in patients who were treated with fixed triple combination. Of note, the percentage of pneumonia in FLAME study (a similar long-term, 52-week study) was 3.2% in the indacaterol–glycopyrronium group and 4.8% in the salmeterol–fluticasone group.Citation27
Figure 4 Frequency plot considering days in the study versus cumulative number of events (COPD moderate/severe exacerbations and pneumonias) in TRILOGYCitation24 (A) and TRINITYCitation25 (B) studies – ITT population.
The percentage of patients with at least one treatment-emerging adverse event (TEAE) was similar with all treatments (ranging from 54.5% with BDP/FF/GB to 57.8% with tiotropium; Table S1). The percentage of patients with at least one adverse drug reaction was similar between BDP/FF/GB, BDP/FF, and tiotropium (2.9%, 2.1%, and 3.1%, respectively), and higher for BDP/FF + tiotropium (5.0%). The percentage of patients with at least one serious TEAE was slightly higher with BDP/FF (18.1%) than with the other three treatments (13.9% with BDP/FF/GB, 15.2% with tiotropium, and 12.7% with BDP/FF + tiotropium). The percentage of patients with at least one severe TEAE was similar with BDP/FF/GB and tiotropium (9.5% and 9.3%, respectively), but slightly higher with BDP/FF (12.6%) and slightly lower with BDP/FF + tiotropium (6.3%). The percentage of patients with at least one TEAE leading to study medication discontinuation was higher with tiotropium (5.8%) than with BDP/FF (4.9%), BDP/FF/GB (3.9%), or BDP/FF + tiotropium (2.8%).
Across the four treatments, the most common treatment-emergent major adverse cardiovascular events (MACEs) were: stroke and cardiovascular death amongst patients treated with BDP/FF/GB (reported in 0.6% of patients each); acute myocardial infarction amongst patients treated with BDP/FF (reported in 0.6% of patients); heart failure and cardiovascular death amongst patients treated with tiotropium (reported in 0.7% and 0.6% of patients, respectively); cardiovascular death, heart failure, and stroke amongst patients treated with BDP/FF + tiotropium (reported in 0.4% of patients each) (). The MACE rate per 1,000 patients per year was slightly lower with BDP/FF + tiotropium (13.6) than with the other three treatments (range 21.6–25.6). However, the extemporary triple group had the lowest number of patients, and therefore provides a less robust estimate of the MACE frequency, particularly when the overall event rate was low. We conclude, therefore, that there were no significant differences between treatments for MACE frequency.
Table 4 MACEs reported in safety population (integrated analysis)
Single-inhaler triple therapy – what’s new?
Extrafine BDP/FF/GB reduced exacerbation frequency and improved lung function and PROs in two large Phase III studies in patients with severe to very severe COPD. Previous triple therapy studies have shown inconsistent effects on exacerbations, either due to the relatively short duration or insufficient statistical power. These new triple therapy studies were conducted over 1 year, which is a time frame recognized by regulatory agencies and the clinical community as the most appropriate to assess the effect of different therapies on exacerbations, and they were sufficiently powered to observe differences between treatments.
COPD exacerbations are associated with significant morbidity and mortality.Citation3,Citation28 Extrafine BDP/FF/GB was effective in reducing moderate and severe exacerbations by ~20% compared to tiotropium and by 23% compared to BDP/FF. These effect sizes correspond to or exceed the commonly accepted MCID for exacerbationsCitation29 and are larger than those observed when roflumilast (reduction of ~14% versus placebo) was added to the combination therapy of ICS/LABA in COPD patients still suffering from frequent exacerbations.Citation30
Interestingly, in both the triple studies, the effect sizes were larger in patients who had a history of two or more exacerbations compared to one exacerbation in the previous year. Some patients with one exacerbation will experience no exacerbations in the following year.Citation31 Indeed, we observed a lower exacerbation rate in patients with one exacerbation in the previous year compared to those with two or more exacerbations in both studies.
The study designs of both TRILOGY and TRINITY ensured that no patients were stepped down from previous triple therapy. All patients experienced one or more exacerbations in the previous year while being treated with LAMA, LAMA/LABA, ICS/LABA, or ICS and LAMA. The studies, therefore, enrolled patients who in real life are candidates for step-up treatment.
The small airways are of significant importance in COPD, and extrafine aerosols of MMAD 1.1 have been shown to be able to target both larger proximal and smaller distal airways in the lungs.Citation32 A recent analysis from the SPIROMICS study showed an association between small airways disease and the consistent exacerbator phenotype (defined as patient with one exacerbation every year for the 3-year follow-up). The increased computed tomography-defined small airways abnormality was one of the most important variables associated with patients who consistently had acute exacerbations together with previous acute exacerbations and increased CAT score.Citation33 In the TRILOGY study both interventions were delivered by the same inhaler type (pMDI), both formulations were extrafine, and the drugs were inhaled via the same device.
The TRINITY study provides supporting evidence of the benefit of single-inhaler, extrafine triple therapy that led to a reduction of moderate/severe exacerbations by 20% compared to single-inhaler, large-particle monotherapy of LAMA, although the fact that different inhaler devices were used in the free combination should be taken into account in the interpretation.
While there was no overall difference in exacerbation rate for BDP/FF/GB compared to BDP/FF + tiotropium, there was a greater effect with the former treatment in patients with a history of two or more exacerbations in the previous year. This favorable difference is unexpected, as compliance is unlikely to be a major issue in a clinical trial where adherence levels are usually high, although this, of course, is a potential advantage for single-inhaler triple therapy in real life. The SPIROMICS dataCitation33 show that patients who are used to exacerbation have higher small airways impairment than the other patients, providing a possible explanation for the reason why extrafine BDP/FF/GLY was more effective in this population than large-particle formulations.
Previous studies have shown advantages of triple therapy in terms of lung function, and the FEV1 results from TRILOGY and TRINITY provide additional evidence. The MCID for FEV1 in COPD has been estimated at ~100 mL in clinical trials,Citation20,Citation34 and it has been suggested that changes of 5%–10% from baseline can be considered clinically meaningful as well. However, it should be noted that the MCID for a trough FEV1 of 100 mL is generally used for comparisons versus placebo; therefore, a meaningful change when considering the difference between active treatments may be lower (60–90 mL), especially in patients with more severe disease and/or when comparing active treatments.Citation35 Considering the clinical severity of the study populations in TRINITY and TRILOGY, the improvement in pre-dose FEV1 for triple therapy compared to BDP/FF or tiotropium can be considered to be a relevant benefit for these COPD patients.
The magnitude of treatment difference for PROs is often modest in COPD clinical trials comparing active treatments.Citation35 The reasons for this could be related to 1) the duration of follow-up influencing the recall of symptoms and 2) improvements in mean changes for all active treatments resulting in relatively small differences between treatments. As described in the recent Food and Drug Administration (FDA) draft guidance for COPD (May 2016), “activity scales such as TDI that require patients to recall prior symptoms are problematic, because patients’ memories may fade over time, particularly in studies lasting several months”. As for the analysis of SGRQ, the same FDA draft guidance indicates that responder analysis is the preferred primary method for reporting results from SGRQ data. In this regard, when TDI and SGRQ in TRILOGY and TRINITY were analyzed in terms of the percentage of patients responding with a clinically meaningful change, greater benefits of triple therapy versus BDP/FF or tiotropium were demonstrated.
Recent data from the FULFIL study, which compared a single, once-daily, inhaler triple therapy (fluticasone furoate/umeclidinium/vilanterol) with dual therapy (ICS/LABA consisting of budesonide/formoterol) showed a statistically significant reduction in moderate/severe exacerbation rate with triple versus ICS/LABA therapy (35% reduction based on data over only 24-week treatment period).Citation36 There were also significantly greater improvements in lung function and SGRQ with triple therapy compared to ICS/LABA. These results add to the growing body of evidence for the benefits of single-inhaler triple therapy in COPD patients and may further prompt research to clarify advantages or disadvantages of once-daily versus twice-daily dosing in COPD outcome.
Triple therapy – future perspectives
The TRILOGY and TRINITY studies have provided confirming evidence for a clinical benefit of triple therapy over LAMA monotherapy or ICS/LABA treatment, with prevention of exacerbations being a key finding. Triple therapy is commonly used in clinical practice as a step-up treatment for patients who remain symptomatic and/or continue to exacerbate despite LAMA monotherapy or ICS/LABA treatment.
Clinical evidence for the effectiveness of LABA/LAMA combination inhalers has been assessed in recent years. Step up to triple therapy may occur in patients using these dual bronchodilator combinations. We await the results of ongoing clinical trials comparing triple therapy to dual bronchodilator treatments.
There has been much debate about the potential adverse effects of ICS treatment, particularly pneumonia. However, the benefit (reduction of exacerbation) versus the risk (pneumonia) should be always considered,Citation37 and the rate of pneumonia events in the single-inhaler triple therapy studies with BDP/FF/GB was low.
GOLD recommends triple therapy as a step-up option for group D patients. The sub-analysis of TRILOGY and TRINITY of patients with a history of two exacerbations in the previous year (ie, group D patients) certainly supports this recommendation. However, the sub-analysis reported in this review also showed efficacy in reducing exacerbation in patients reporting only one exacerbation in the previous year (GOLD B) while on maintenance treatment including ICS/LABA, LABA/LAMA, or LAMA. While triple therapy is not recommended by GOLD for group B patients, the results of TRINITY and TRILOGY support its use for the subset who continue to exacerbate despite maintenance treatment.Citation38
The TRILOGY and TRINITY studies provide direct evidence in 52-week randomized controlled studies of a superior clinical benefit of fixed-dose triple therapy in comparison to standard of care. The prospective exacerbation rates may be viewed as relatively low, despite recruiting patients with a history of one or more exacerbations in the previous year. Real-world studies can enhance the ability to study patients with more severe disease and those with more exacerbations, and thus provide another avenue to understand the effects of triple therapy.
The single-inhaler triple therapy studies reported here evaluated an extrafine formulation to enhance lung deposition and small airway delivery. It remains to be seen whether the treatment effects reported here are “class effects” that are also seen with other single-inhaler triple therapies. There are relatively few classes of drugs available in COPD, and the single-inhaler triple therapy studies reported here should reassure practicing clinicians that the stepwise approach toward triple therapy is a rational and effective option.
Supplementary material
Table S1 TEAEs reported in ≥1% of patients with any treatment in safety population (integrated analysis)
Disclosure
DS reports grants and personal fees from Almirall, Astra-Zeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Glenmark, Johnson and Johnson, Merck, NAPP, Novartis, Pfizer, Takeda, Teva, Theravance, and Verona and personal fees from Genentech and Skyepharma. OSU has received industry-academic funding from Boehringer Ingelheim, Chiesi, Edmond Pharma, GlaxoSmithKline, Mundipharma International and has received consultancy or speaker fees from Astra Zeneca, Boehringer Ingelheim, Chiesi, Cipla, Edmond Pharma, GlaxoSmithKline, Napp, Novartis, Mundipharma International, Pearl Therapeutics, Roche, Sandoz, Takeda, UCB, Vectura and Zentiva. JV has received honoraria for consulting and presenting from AstraZeneca, Boehringer-Ingelheim, Chiesi, GlaxoSmithKline, and Novartis. AP reports grants, personal fees, non-financial support, and others from Chiesi, AstraZeneca, GlaxoSmith-Kline, Boehringer Ingelheim, Merck Sharp & Dohme, Pfizer, Takeda, Mundipharma and TEVA, personal fees and non-financial support from Menarini, Novartis and Zambon, and grants from Sanofi. MC received honoraria for consultancy from Chiesi Farmaceutici SpA.
MSp, SP, and MSc are full-time employees of Chiesi Farmaceutici SpA. The authors report no other conflicts of interest in this work.
References
- LopezADShibuyaKRaoCChronic obstructive pulmonary disease: current burden and future projectionsEur Respir J200627239741216452599
- MathersCDLoncarDProjections of global mortality and burden of disease from 2002 to 2030PLoS Med2006311e44217132052
- VogelmeierCFCrinerGJMartinezFJGlobal strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summaryAm J Respir Crit Care Med2017195555758228128970
- KewKMDiasSCatesCJLong-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysisCochrane Database Syst Rev20143CD010844
- OharJADonohueJFMono and combination therapy of long-acting bronchodilators and inhaled corticosteroids in advanced COPDSemin Respir Crit Care Med201031332133320496301
- VinckenWAumannJChenHHenleyMMcBryanDGoyalPEfficacy and safety of coadministration of once-daily indacaterol and glycopyrronium versus indacaterol alone in COPD patients: the GLOW6 studyInt J Chron Obstruct Pulmon Dis2014921522824596459
- WedzichaJADecramerMFickerJHAnalysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group studyLancet Respir Med20131319920924429126
- CazzolaMMolimardMThe scientific rationale for combining long-acting beta2-agonists and muscarinic antagonists in COPDPulm Pharmacol Ther201023425726720381630
- CalverleyPMBurgePSSpencerSAndersonJAJonesPWBronchodilator reversibility testing in chronic obstructive pulmonary diseaseThorax200358865966412885978
- CazzolaMDi MarcoFSantusPThe pharmacodynamic effects of single inhaled doses of formoterol, tiotropium and their combination in patients with COPDPulm Pharmacol Ther2004171353914643169
- van NoordJAAumannJLJanssensEComparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPDEur Respir J200526221422216055868
- JamesGDDonaldsonGCWedzichaJANazarethITrends in management and outcomes of COPD patients in primary care, 2000–2009: a retrospective cohort studyNPJ Prim Care Respir Med2014241401524990313
- WelteTMiravitllesMHernandezPEfficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2009180874175019644045
- SinghDBrooksJHaganGCahnAO’ConnorBJSuperiority of “triple” therapy with salmeterol/fluticasone propionate and tiotropium bromide versus individual components in moderate to severe COPDThorax200863759259818245142
- CazzolaMAndòFSantusPA pilot study to assess the effects of combining fluticasone propionate/salmeterol and tiotropium on the airflow obstruction of patients with severe-to-very severe COPDPulm Pharmacol Ther200720555656116914336
- FrithPAThompsonPJRatnavadivelRGlisten Study GroupGlycopyrronium once-daily significantly improves lung function and health status when combined with salmeterol/fluticasone in patients with COPD: the GLISTEN study, a randomised controlled trialThorax201570651952725841237
- SilerTMKerwinESousaARDonaldAAliRChurchAEfficacy and safety of umeclidinium added to fluticasone furoate/vilanterol in chronic obstructive pulmonary disease: Results of two randomized studiesRespir Med201510991155116326117292
- AaronSDVandemheenKLFergussonDCanadian Thoracic Society/Canadian RespiratoryClinical Research ConsortiumTiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trialAnn Intern Med2007146854555517310045
- SousaARRileyJHChurchAZhuCQPunekarYSFahyWAThe effect of umeclidinium added to inhaled corticosteroid/long-acting β2-agonist in patients with symptomatic COPD: a randomised, double-blind, parallel-group studyNPJ Prim Care Respir Med2016261603127334739
- DonohueJFMinimal clinically important differences in COPD lung functionCOPD20052111112417136971
- WedzichaJASinghDVestboJExtrafine beclomethasone/formoterol in severe COPD patients with history of exacerbationsRespir Med201410881153116224953015
- ShortPMWilliamsonPAElderDHJLipworthSIWSchembriSLipworthBJThe impact of tiotropium on mortality and exacerbations when added to inhaled corticosteroids and long-acting beta2 agonist therapy in COPDChest20121411818621799028
- LeeTAWilkeCJooMOutcomes associated with tiotropium use in patients with chronic obstructive pulmonary diseaseArch Intern Med2009169151403141019667304
- SinghDPapiACorradiMSingle inhaler triple therapy versus inhaled corticosteroid plus long-acting beta2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trialLancet20163881004896397327598678
- VestboJPapiACorradiMSingle inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trialLancet2017389100821919192928385353
- SiddiquiSHGuasconiAVestboJBlood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2015192452352526051430
- WedzichaJABanerjiDChapmanKRIndacaterol-glycopyrronium versus salmeterol-fluticasone for COPDN Engl J Med2016374232222223427181606
- Lopez-CamposJLAgustiAHeterogeneity of chronic obstructive pulmonary disease exacerbations: a two-axes classification proposalLancet Respir Med20153972973426165134
- ChapmanKRBergeronCBhutaniMDo we know the minimal clinically important difference (MCID) for COPD exacerbations?COPD201310224324923514218
- WedzichaJACalverleyPMRabeKFRoflumilast: a review of its use in the treatment of COPDInt J Chron Obstruct Pulmon Dis201611819026792988
- HurstJRVestboJAnzuetoAEvaluation of COPD longitudinally to identify predictive surrogate endpoints (ECLIPSE) investigators. Susceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- UsmaniOSTreating the small airwaysRespiration20128444145323154684
- HanMKQuibreraPMCarrettaEEFrequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohortLancet Respir Med20175861962628668356
- CazzolaMMacNeeWMartinezFJOutcomes for COPD pharmacological trials: from lung function to biomarkersEur Respir J200831241646918238951
- SinghDNew combination bronchodilators for chronic obstructive pulmonary disease: current evidence and future perspectivesBr J Clin Pharmacol201579569570825377687
- LipsonDABarnacleHBirkRFULFIL trial: once-daily triple therapy in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2017196443844628375647
- LiapikouAToumbisMTorresAManaging the safety of inhaled corticosteroids in COPD and the risk of pneumoniaExpert Opin Drug Saf20151481237124726113207
- FabbriLMRoversiSBeghèBTriple therapy for symptomatic patients with COPDLancet2017389100821864186528385354
