Abstract
Smoking-related interstitial lung disease (ILD) consists of a heterogeneous group of disorders that are considered a distinct entity. The 2013 American Thoracic Society and European Respiratory Society recommendations classified respiratory bronchiolitis (RB)/RB-ILD and desquamative interstitial pneumonia (DIP) as smoking-related idiopathic interstitial pneumonias (IIPs). The overlapping histopathological and radiological patterns of smoking-related IIPs must be considered. Overlap patterns of smoking-related IIPs are not easily classified as a single disorder. The initial radiological manifestation and follow-up changes are heterogeneous, even when diagnosed pathologically as RB or DIP. Therefore, a clinical–radiological–pathological consensus is important in the diagnosis of smoking-related IIPs, and long-term evaluation is essential to monitor the morphological changes in these patients. In this article, we reviewed the clinical, radiological, and pathological findings, and also the changes in radiological manifestations of smoking-related IIPs over time.
Introduction
Tobacco smoke is a toxic and carcinogenic mixture composed of >5,000 types of chemicals that causes many pulmonary and systemic effects in human beings.Citation1,Citation2 Inhalation of the toxic particles associated with cigarette smoking and the subsequent immune response may lead to a variety of pathological manifestations.Citation3 Cardiovascular disease, chronic obstructive pulmonary disease (COPD), and lung cancer are the most frequent causes of smoking-related deaths; however, many patients also suffer from smoking-related interstitial lung disease (ILD).Citation2,Citation4 Recently, smoking has also been implicated in the pathogenesis of ILDs, such as desquamative interstitial pneumonia (DIP), respiratory bronchiolitis (RB)/RB-ILD, Langerhans cell histiocytosis (LCH), and idiopathic pulmonary fibrosis (IPF).Citation5 However, the relationship between cigarette smoking and ILD has not yet been established.Citation1,Citation6 Smoking-related ILD consists of a heterogeneous group of disorders that can overlap with one another or coexist with other sequelae of cigarette smoking, such as emphysema.Citation3,Citation4
In particular, RB/RB-ILD and DIP, which were classified as smoking-related idiopathic interstitial pneumonias (IIPs) in the revision of the 2013 American Thoracic Society/European Respiratory Society (ATS/ERS) IIP statement, represent a histological spectrum of macrophage accumulation depending on the extent and distribution of this accumulation.Citation7–Citation9 RB/RB-ILD and DIP remain separately classified because of their differences in clinical entities, radiological manifestations and response to treatment.Citation9 However, it is not surprising that the histopathological patterns and imaging findings may overlap between RB/RB-ILD and DIP; a prospective diagnosis can be difficult.Citation3,Citation4,Citation10 Few studies have examined the early or long-term follow-up manifestations on chest computed tomography (CT) in patients with RB/RB-ILD or DIP. In this article, we reviewed the clinical, radiological, and pathological findings and also the changes in radiological manifestations over time of smoking-related IIPs.
Smoking-related idiopathic interstitial pneumonias (IIPs)
RB and RB-ILD
RB is a universal inflammatory reaction of the respiratory bronchioles that always occurs in current smokers and is known as smoker’s bronchiolitis.Citation9,Citation11,Citation12 RB associated with clusters of pigmented alveolar macrophages was first described in 1974.Citation13 Exposure to cigarette smoke causes an increase in the number of alveolar macrophages that are created in an attempt to remove any inhaled particles and changes the macrophage phenotype to enhance resolution of inflammation and tissue remodeling.Citation3,Citation6,Citation14,Citation15 RB occurs because of increased macrophage numbers and is regarded as a normal physiological response to inhaled cigarette smoke.Citation6,Citation12 The prominent histological feature of RB is a bronchiolocentric accumulation of yellow–brown pigmented macrophages (smoker’s macrophages) in the respiratory bronchioles and adjacent alveoli.Citation8,Citation12,Citation16 Most patients with RB are asymptomatic, but a small proportion of smokers with RB develop clinical, physiological, and imaging features of ILD, which is termed RB-ILD.Citation12,Citation17 In 1987, Myers et alCitation18 described six patients with clinical and radiological features of chronic ILD whose open lung biopsies showed RB and fibrosis that extended from the peribronchiolar regions into the adjacent alveolar septa. RB-ILD is a rare disorder characterized by dyspnea and cough and represents a pulmonary function abnormality encountered in heavy smokers in the third to sixth decades of life with a 30 pack-year history at the time of diagnosis.Citation12 Pulmonary function tests (PFTs) reveal a mixed obstructive–restrictive defect with a mild to moderate decrease in the diffusing capacity of the lung for carbon monoxide (DLco).Citation12,Citation19,Citation20 The severity of impairment of PFT is a feature that distinguishes RB-ILD from RB.Citation8
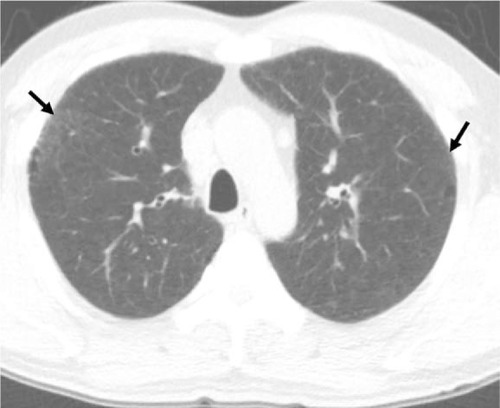
In RB/RB-ILD, the chest radiograph is usually normal and has a limited diagnostic value.Citation21 Bronchial wall thickening and ground-glass opacity (GGO) are frequent on chest radiograph in patients with RB/RB-ILD.Citation22 Given that macrophages are distributed in the respiratory bronchioles, centrilobular nodules are a computed tomographic feature associated with the bronchiolocentric histology of RB/RB-ILD.Citation3,Citation23 The most common CT findings of RB/RB-ILD are poorly defined centrilobular nodules with an upper zone distribution, patchy GGOs, which are correlated with macrophage accumulation in the alveolar spaces, and mild interlobular septal thickening ().Citation3,Citation17,Citation22,Citation24,Citation25 Ancillary CT findings are bronchial wall thickening and emphysema due to cigarette smoking.Citation17,Citation22,Citation24 The differential diagnoses of RB/RB-ILD include subacute hypersensitivity pneumonitis (HP), DIP and nonspecific interstitial pneumonia (NSIP).Citation17 Because patients with HP are usually nonsmokers and cigarette smoke is considered to be protective against HP, a history of smoking or the presence of emphysema on CT is an important clue to help distinguish RB from HP.Citation3,Citation26 GGO of RB/RB-ILD is less extensive and more poorly defined and tends to predominate in the upper lobes more frequently than that typically observed in DIP.Citation17
Figure 1 A 45-year-old male smoker with pathologically diagnosed RB.
Abbreviations: CT, computed tomography; RB, respiratory bronchiolitis.
DIP
DIP is exceedingly rare, and its true prevalence and incidence are unknown.Citation27 This condition was first recognized by Liebow et alCitation28 in 1965 and was described as histopathological findings of uniform air space filling by “large cells” that were interpreted as the accumulation of desquamated cells from the alveolar wall.Citation29 However, this view has long been discarded and it is now recognized that these cells are macrophages.Citation30,Citation31 RB/RB-ILD and DIP are the abnormal accumulation of macrophages in the air spaces, and the key features that differentiate between the two are the distribution and extent of macrophage accumulation ().Citation32 The hallmark histological feature of DIP is extensive, diffuse and uniform airspace filling by smoker’s macrophages.Citation29,Citation30 The relationship between cigarette smoke and DIP has been proven, and 40%–90% of patients affected by DIP are smokers.Citation4,Citation33,Citation34 However, DIP is not always associated with cigarette smoke and is also reported to occur as a reactive phenomenon in a variety of other conditions, including drug reactions, leukemia, asbestosis, pneumoconiosis, and connective tissue diseases such as rheumatoid arthritis, lupus, and scleroderma.Citation35–Citation43 The average age at symptom onset is during the fourth and sixth decades, and patients present with slowly progressive breathlessness during exercise and a dry cough.Citation4,Citation29,Citation39 PFTs reveal restrictive pattern with a decreased DLco.Citation8
Table 1 Summary of key features
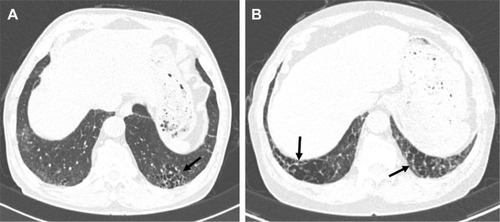
Chest radiographs are often normal or show GGO or a reticulonodular pattern in the lower zone, but these patterns are nonspecific for the detection of DIP.Citation24,Citation28,Citation33,Citation44 CT finding mainly seen in DIP is diffuse GGOs with subpleural and lower zone predominance, which correlate histologically with the partial replacement of alveolar air by macrophages ().Citation4,Citation29,Citation45,Citation46 Hartman et alCitation45 reported that in 22 patients with biopsy-proven DIP, GGOs that involved the mid and lower zones were seen in all cases. Unlike other inhalational lung diseases, DIP tends to be predominant in the lower lung zone; the reason for this pathogenesis is unclear.Citation3 Other findings in patients with DIP are irregular linear opacities, traction bronchiectasis, cystic spaces, and emphysema.Citation45 Cystic spaces may be present in areas of GGOs, and the small cystic air spaces seen in DIP have been shown to be histologically correlated with dilated alveolar ducts and bronchiolectasis.Citation47 It is important to recognize that the CT features of DIP are nonspecific. In one study, confident diagnoses by experienced thoracic radiologists were correct in 59% of cases of DIP.Citation48 DIP differs from NSIP by its absence of fibrotic features, such as traction bronchiectasis and volume loss.Citation17 A history of cigarette smoking and an increase in alveolar macrophages in bronchoalveolar lavage analysis may also be helpful in the diagnosis of DIP.Citation8,Citation17 The ATS/ERS IIP statement mentioned that DIP and RB have to be distinguished from airspace enlargement with fibrosis (AEF). AEF is not regarded as distinct from IIP and is a smoking-related phenomenon.Citation9,Citation17 AEF demonstrates the presence of more interstitial fibrosis admixed with emphysema ().Citation17,Citation49,Citation50
Figure 2 A 57-year-old male smoker with pathologically diagnosed DIP.
Notes: The axial CT image shows GGO in a subpleural and lower distribution. Thin-walled cysts (black arrows) are seen within the region of the GGOs. Image courtesy of Samsung Medical Center.
Abbreviations: CT, computed tomography; DIP, desquamative interstitial pneumonia; GGO, ground-glass opacity.
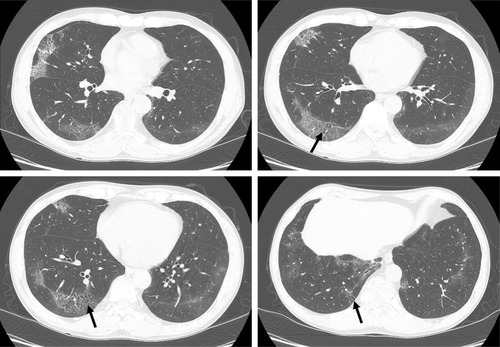
Figure 3 CT features of AEF.
Abbreviations: AEF, airspace enlargement with fibrosis; CT, computed tomography.
Difficulties in establishing the diagnosis of smoking-related IIPs
Pathologists may often encounter a mixture of histopathological patterns that will suggest more than one disease and therefore may find it difficult to make a single pathological diagnosis.Citation31,Citation32 Overlapping patterns of ILDs are not easily classified as a single disorder.Citation51 Smoking-related IIPs are good examples of ILD overlap.Citation51 RB/RB-ILD may share some histopathological and radiological patterns with DIP.Citation3,Citation4,Citation10 Although the distinction between RB/RB-ILD and DIP is determined by the distribution and extent of macrophage accumulation, this is subjective and may vary depending on the site of biopsy.Citation3 Studies have shown that the histopathological pattern of RB/RB-ILD may overlap with the pattern of DIP.Citation10,Citation34 Despite histological overlap between RB/RB-ILD and DIP, the radiological manifestations of RB/RB-ILD and DIP are often different.Citation3 Therefore, imaging distinctions can help to distinguish the two diseases. Key findings of RB/RB-ILD are poorly defined centrilobular nodules and patchy GGOs with an upper zone distribution, whereas in DIP, bilateral GGOs have a lower zone distribution; cystic lesions may be present in areas of GGO.Citation3,Citation17 However, it is not surprising that imaging findings can overlap between RB/RB-ILD and DIP, and a prospective diagnosis can be difficult.Citation3,Citation4 A study evaluating the radiological pattern of 40 biopsy-proven RB, RB-ILD, or DIP patients described their radiological overlap; some overlap of radiological findings between RB/RB-ILD and DIP exists, and the radiological features of these disorders represent a different degree of severity of small airway and parenchymal reactions to cigarette smoke.Citation46
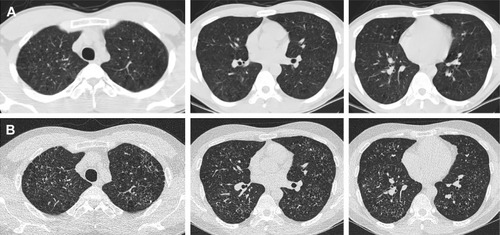
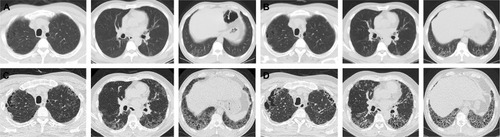
The interrelationships between the various smoking-related diffuse ILDs are now emerging.Citation52 There are coexistent radiological–histopathological patterns, or one entity is transformed into another apparently distinct entity.Citation51 LCH is an uncommon ILD that is characterized by the proliferation of Langerhans-type cells, and it occurs almost exclusively in smokers or ex-smokers.Citation53,Citation54 Because of the strong association with smoking, emphysema and RB-/DIP-like changes frequently coexist in patients with LCH (); therefore, the radiological and histopathological differentiation of LCH from these disorders may be difficult.Citation29,Citation32 In one study, RB-/DIP-like changes were present in all biopsy specimens taken from 14 patients with LCH, and they were correlated with cumulative exposure to cigarette smoke.Citation55 Smoking-related IIPs are difficult to distinguish from other ILDs and sometimes co-occur. The coexistence of DIP and NSIP is frequent.Citation8 Furthermore, ~30% of patients with DIP are reported to be indistinguishable from NSIP.Citation56 Given that fibrotic NSIP has a temporally uniform pattern of interstitial fibrosis, this result is similar to the findings of DIP histopathology in which the macrophages have been removed from the alveoli.Citation57
Figure 4 A 26-year-old male ex-smoker with pathologically diagnosed RB.
Abbreviations: CT, computed tomography; RB, respiratory bronchiolitis.
In the diagnosis of smoking-related IIPs, the surgical or radiological finding may be evident, but the diagnosis may be difficult because of histological and radiological overlapping or coexistence of other ILDs. A representative area of the disease or a coexistent fibrotic process at the time of biopsy may not have been included in the biopsy. It is possible to diagnose correctly smoking-related IIPs as comprehensively evaluating pathology, imaging, and clinical manifestations. Therefore, the role of multidisciplinary teams in the diagnosis of smoking-related IIPs is important.
Natural history and longitudinal behavior of smoking-related IIPs
There is a relative lack of large prospective studies describing radiological findings or longitudinal changes in cigarette smokers with biopsy-proven RB or DIP.Citation17 Remy-Jardin et alCitation58 reported that GGO, emphysema, and centrilobular nodules increased from 28% to 42%, 26% to 40%, and 33% to 35%, respectively, in current smokers during a mean follow-up period of 5.5 years. In a 3-year follow-up study, centrilobular nodules increased in 7.1% of patients with an RB pattern and were stable in 85.7%.Citation59 In another study, centrilobular nodules and GGO improved in all patients with biopsy-proven RB-ILD who quit smoking, and the reticular patterns, emphysema, and traction bronchiolectasis remained unchanged during the follow-up period.Citation60 RB can be found in ex-smokers many years after smoking cessation.Citation11 In addition, some patients with RB-ILD remain unchanged or experience a deteriorated condition after smoking cessation and corticosteroid therapy.Citation10,Citation20,Citation60 The degree of improvement in RB features on CT may depend on the duration of smoking cessation and the degree of inflammation and fibrosis.Citation60 The extent of GGO and cystic lesions seen on CT in most patients with DIP remains unchanged or improves with corticosteroid therapy.Citation47,Citation61 In a long-term CT follow-up study of patients with DIP, an increase in the frequency of thin-walled cysts within the GGOs was observed, and the new appearance of honeycombing was noted in five of 14 patients. This study indicated that some patients with DIP may progress to fibrosis with honeycombing changes on CT despite a favorable response to corticosteroid therapy.Citation62
RB is a very common finding in cigarette smokers, with a prevalence ranging from 57% to 100%.Citation4,Citation29 In current or ex-smokers, it is possible that RB can coexist with other ILDs (). Initial radiological manifestations and long-term changes were heterogeneous, even when diagnosed pathologically as RB or DIP. A different study reported that fibrotic NSIP or usual interstitial pneumonia (UIP) patterns may be seen in patients with pathologically proven DIP on high-resolution CT several years after a biopsy has been carried out.Citation63 In one case, a surgical lung biopsy revealed DIP and the histopathology at the time of lung transplantation revealed a pattern of NSIP.Citation64 It is therefore possible that DIP may evolve to fibrotic NSIP or UIP. However, diagnosis of DIP at biopsy might have represented sampling error that areas with a coexistent fibrotic process at the time of biopsy may not have been included in the biopsy.Citation63 Therefore, a longitudinal study with larger populations of patients with smoking-related IIPs is needed to confirm this. Although pathologically confirmed as smoking-related IIPs, radiological patterns of a variety of ILDs may also be seen by considering serial radiological examinations (). Long-term follow-up of images is important because smoking-related IIPs may overlap and can coexist with other ILDs or there may be a sampling error at the time of biopsy.
Figure 5 A 66-year-old male ex-smoker with lung biopsy-proven RB-ILD.
Notes: There were bronchiolocentric GGOs and traction bronchiectasis in both lungs that represented airway-centered interstitial fibrosis. Image courtesy of Samsung Medical Center.
Abbreviations: GGO, ground-glass opacity; ILD, interstitial lung disease; RB, respiratory bronchiolitis.
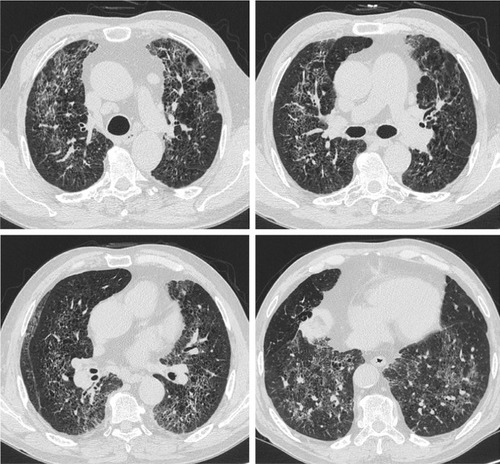
Figure 6 A 44-year-old male smoker with pathologically diagnosed RB-ILD.
Abbreviations: CT, computed tomography; GGO, ground-glass opacity; ILD, interstitial lung disease; RB, respiratory bronchiolitis; UIP, usual interstitial pneumonia.
Conclusion
Histological, imaging, and clinical differentiation of smoking-related IIPs in smokers is not black and white. The histopathological differentiation of RB/DIP and other ILDs may be challenging. Initial radiological manifestations and long-term changes were heterogeneous, even when diagnosed as pathologically RB/RB-ILD or DIP. The mainstay of treatment in patients with RB/RB-ILD is smoking cessation, and treatment in patients with DIP consists of corticosteroid. In addition, clinical deterioration may be seen in two-third of patients with untreated DIP. DIP or RB/RB-ILD patients with coexistent UIP or NSIP may have a worse prognosis. Therefore, it is important to distinguish the smoking-related IIPs accurately. Since the overlap of features makes it impossible to assign a single specific diagnostic entity, a clinical–radiological–pathological discussion in smokers is important for making the diagnosis of smoking-related IIPs; long-term radiological evaluation is essential for monitoring morphological changes that may occur during follow-up in these patients.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The use of images was approved by the Institutional Review Board of Samsung Medical Center, and informed consent was waived.
Disclosure
The authors report no conflicts of interest in this work.
References
- CaminatiAHarariSSmoking-related interstitial pneumonias and pulmonary Langerhans cell histiocytosisProc Am Thorac Soc20063429930616738193
- TalhoutRSchulzTFlorekEvan BenthemJWesterPOpperhuizenAHazardous compounds in tobacco smokeInt J Environ Res Public Health20118261362821556207
- KligermanSFranksTJGalvinJRClinical-radiologic-pathologic correlation of smoking-related diffuse parenchymal lung diseaseRadiol Clin North Am20165461047106327719975
- MadanRMatalonSViveroMSpectrum of smoking-related lung diseases: imaging review and updateJ Thorac Imaging2016312789126479130
- VassalloRRyuJHSmoking-related interstitial lung diseasesClin Chest Med201233116517822365253
- MargaritopoulosGAVasarmidiEJacobJWellsAUAntoniouKMSmoking and interstitial lung diseasesEur Respir Rev20152413742843526324804
- NeurohrCBehrJChanges in the current classification of IIP: a critical reviewRespirology201520569970426011188
- MargaritopoulosGAHarariSCaminatiAAntoniouKMSmoking-related idiopathic interstitial pneumonia: a reviewRespirology20162115764
- TravisWDCostabelUHansellDMATS/ERS Committee on Idiopathic Interstitial PneumoniasAn official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumoniasAm J Respir Crit Care Med2013188673374824032382
- MoonJdu BoisRMColbyTVHansellDMNicholsonAGClinical significance of respiratory bronchiolitis on open lung biopsy and its relationship to smoking related interstitial lung diseaseThorax199954111009101410525560
- FraigMShreeshaUSaviciDKatzensteinALRespiratory bronchiolitis: a clinicopathologic study in current smokers, ex-smokers, and never-smokersAm J Surg Pathol200226564765311979095
- SieminskaAKuziemskiKRespiratory bronchiolitis-interstitial lung diseaseOrphanet J Rare Dis2014910625011486
- NiewoehnerDEKleinermanJRiceDBPathologic changes in the peripheral airways of young cigarette smokersN Engl J Med1974291157557584414996
- MehtaHNazzalKSadikotRTCigarette smoking and innate immunityInflamm Res2008571149750319109742
- WallaceWAGilloolyMLambDIntra-alveolar macrophage numbers in current smokers and non-smokers: a morphometric study of tissue sectionsThorax19924764374401496503
- RaoRNGoodmanLRTomashefskiJFJrSmoking-related interstitial lung diseaseAnn Diagn Pathol200812644545718995211
- SverzellatiNLynchDAHansellDMJohkohTKingTEJrTravisWDAmerican Thoracic Society-European Respiratory Society classification of the idiopathic interstitial pneumonias: advances in knowledge since 2002Radiographics20153571849187126452110
- MyersJLVealCFJrShinMSKatzensteinALRespiratory bronchiolitis causing interstitial lung disease. A clinicopathologic study of six casesAm Rev Respir Dis198713548808843565934
- PortnoyJVeraldiKLSchwarzMIRespiratory bronchiolitis-interstitial lung disease: long-term outcomeChest2007131366467117356078
- RyuJHMyersJLCapizziSADouglasWWVassalloRDeckerPADesquamative interstitial pneumonia and respiratory bronchiolitis-associated interstitial lung diseaseChest2005127117818415653981
- DaviesGWellsAUdu BoisRMRespiratory bronchiolitis associated with interstitial lung disease and desquamative interstitial pneumoniaClin Chest Med2004254717726vi15564017
- ParkJSBrownKKTuderRMHaleVAKingTEJrLynchDARespiratory bronchiolitis-associated interstitial lung disease: radiologic features with clinical and pathologic correlationJ Comput Assist Tomogr2002261132011801899
- AttiliAKKazerooniEAGrossBHFlahertyKRMyersJLMartinezFJSmoking-related interstitial lung disease: radiologic-clinical-pathologic correlationRadiographics200828513831396 discussion 1396–138818794314
- PalmucciSRoccasalvaFPuglisiSClinical and radiological features of idiopathic interstitial pneumonias (IIPs): a pictorial reviewInsights Imaging20145334736424844883
- Remy-JardinMRemyJGosselinBBecetteVEdmeJLLung parenchymal changes secondary to cigarette smoking: pathologic-CT correlationsRadiology199318636436518430168
- LacasseYSelmanMCostabelUHP Study GroupClinical diagnosis of hypersensitivity pneumonitisAm J Respir Crit Care Med2003168895295812842854
- VassalloRDiffuse lung diseases in cigarette smokersSemin Respir Crit Care Med201233553354223001806
- LiebowAASteerABillingsleyJGDesquamative interstitial pneumoniaAm J Med19653936940414338290
- MartenKHansellDMImaging of macrophage-related lung diseasesEur Radiol200515472774115633061
- FarrGHHarleyRAHennigarGRDesquamative interstitial pneumonia. An electron microscopic studyAm J Pathol19706033473705459746
- WalshSLNairADesaiSRInterstitial lung disease related to smoking: imaging considerationsCurr Opin Pulm Med201521440741625978626
- DesaiSRRyanSMColbyTVSmoking-related interstitial lung diseases: histopathological and imaging perspectivesClin Radiol200358425926812662946
- CarringtonCBGaenslerEACoutuREFitzGeraldMXGuptaRGNatural history and treated course of usual and desquamative interstitial pneumoniaN Engl J Med197829815801809634315
- YousemSAColbyTVGaenslerEARespiratory bronchiolitis-associated interstitial lung disease and its relationship to desquamative interstitial pneumoniaMayo Clin Proc19896411137313802593722
- AbrahamJLHertzbergMAInorganic particulates associated with desquamative interstitial pneumoniaChest1981801 suppl67707249745
- BoneRCWolfeJSobonyaREDesquamative interstitial pneumonia following chronic nitrofurantoin therapyChest1976692 suppl2962971248310
- CorrinBPriceABElectron microscopic studies in desquamative interstitial pneumonia associated with asbestosThorax19722733243315039447
- EsmaeilbeigiFJuvetSHwangDMittooSDesquamative interstitial pneumonitis in a patient with systemic lupus erythematosusCan Respir J2012191505222332137
- GodbertBWisslerMPVignaudJMDesquamative interstitial pneumonia: an analytic review with an emphasis on aetiologyEur Respir Rev20132212811712323728865
- GoldsteinJDGodleskiJJHermanPGDesquamative interstitial pneumonitis associated with monomyelocytic leukemiaChest19828133213256948665
- IshiiHIwataASakamotoNMizunoeSMukaeHKadotaJDesquamative interstitial pneumonia (DIP) in a patient with rheumatoid arthritis: is DIP associated with autoimmune disorders?Intern Med2009481082783019443979
- LougheedMDRoosJOWaddellWRMuntPWDesquamative interstitial pneumonitis and diffuse alveolar damage in textile workers. Potential role of mycotoxinsChest19951085119612007587416
- SwartzJSChatterjeeSParambilJGDesquamative interstitial pneumonia as the initial manifestation of systemic sclerosisJ Clin Rheumatol201016628428620808169
- PatchefskyASIsraelHLHochWSGordonGDesquamative interstitial pneumonia: relationship to interstitial fibrosisThorax19732866806934595813
- HartmanTEPrimackSLSwensenSJHansellDMcGuinnessGMullerNLDesquamative interstitial pneumonia: thin-section CT findings in 22 patientsRadiology199318737877908497631
- HeynemanLEWardSLynchDARemy-JardinMJohkohTMullerNLRespiratory bronchiolitis, respiratory bronchiolitis-associated interstitial lung disease, and desquamative interstitial pneumonia: different entities or part of the spectrum of the same disease process?AJR Am J Roentgenol199917361617162210584810
- AkiraMYamamotoSHaraHSakataniMUedaESerial computed tomographic evaluation in desquamative interstitial pneumoniaThorax19975243333379196515
- JohkohTMullerNLCartierYIdiopathic interstitial pneumonias: diagnostic accuracy of thin-section CT in 129 patientsRadiology1999211255556010228542
- KatzensteinALMukhopadhyaySZanardiCDexterEClinically occult interstitial fibrosis in smokers: classification and significance of a surprisingly common finding in lobectomy specimensHum Pathol201041331632520004953
- KawabataYHoshiEMuraiKSmoking-related changes in the background lung of specimens resected for lung cancer: a semiquantitative study with correlation to postoperative courseHistopathology200853670771419102010
- WalshSLHansellDMDiffuse interstitial lung disease: overlaps and uncertaintiesEur Radiol20102081859186720204644
- WellsAUNicholsonAGHansellDMChallenges in pulmonary fibrosis. 4: smoking-induced diffuse interstitial lung diseasesThorax2007621090491017909189
- HarmonCMBrownNLangerhans cell histiocytosis: a clinicopathologic review and molecular pathogenetic updateArch Pathol Lab Med2015139101211121426414464
- RodenACYiESPulmonary Langerhans cell histiocytosis: an update from the pathologists’ perspectiveArch Pathol Lab Med2016140323024026927717
- VassalloRJensenEAColbyTVThe overlap between respiratory bronchiolitis and desquamative interstitial pneumonia in pulmonary Langerhans cell histiocytosis: high-resolution CT, histologic, and functional correlationsChest200312441199120514555547
- SawataTBandoMNakayamaMMatoNYamasawaHSugiyamaYInfluence of smoking in interstitial pneumonia presenting with a non-specific interstitial pneumonia patternIntern Med201655202939294427746429
- HansellDMNicholsonAGSmoking-related diffuse parenchymal lung disease: HRCT-pathologic correlationSemin Respir Crit Care Med200324437739216088559
- Remy-JardinMEdmeJLBoulenguezCRemyJMastoraISobaszekALongitudinal follow-up study of smoker’s lung with thin-section CT in correlation with pulmonary function testsRadiology2002222126127011756735
- SverzellatiNGuerciLRandiGInterstitial lung diseases in a lung cancer screening trialEur Respir J201138239240021233262
- NakanishiMDemuraYMizunoSChanges in HRCT findings in patients with respiratory bronchiolitis-associated interstitial lung disease after smoking cessationEur Respir J200729345346117135233
- HartmanTEPrimackSLKangEYDisease progression in usual interstitial pneumonia compared with desquamative interstitial pneumonia. Assessment with serial CTChest199611023783828697837
- KawabataYTakemuraTHebisawaADesquamative Interstitial Pneumonia Study GroupDesquamative interstitial pneumonia may progress to lung fibrosis as characterized radiologicallyRespirology20121781214122122805187
- CraigPJWellsAUDoffmanSDesquamative interstitial pneumonia, respiratory bronchiolitis and their relationship to smokingHistopathology200445327528215330806
- TazelaarHDWrightJLChurgADesquamative interstitial pneumoniaHistopathology201158450951620854463
