Abstract
Near-patient testing (NPT) allows clinical decisions to be made in a rapid and convenient manner and is often cost effective. In COPD the peripheral blood eosinophil count has been demonstrated to have utility in providing prognostic information and predicting response to treatment during an acute exacerbation. For this potential to be achieved having a reliable NPT of blood eosinophil count would be extremely useful. Therefore, we investigated the use of the HemoCue® WBC Diff System and evaluated its sensitivity and specificity in healthy, asthmatic and COPD subjects. This method requires a simple skin prick of blood and was compared to standard venepuncture laboratory analysis. The HemoCue® WBC Diff System measured the peripheral blood eosinophil count in healthy, asthma and COPD subjects with very close correlation to the eosinophil count as measured by standard venepuncture. The correlations were unaffected by disease status. This method for the measurement of the peripheral blood eosinophil count has the potential to provide rapid near-patient results and thus influence the speed of management decisions in the treatment of airway diseases.
Introduction
Modern medical practice is often constrained due to an environment of time and resource limitation, and mechanisms to counteract this are actively sought; rapid and accurate bedside diagnostic tools, such as near-patient tests (NPTs) are one such mechanism.Citation1 NPTs provide rapid results and can guide treatment decisions;Citation2 having been established in clinical care guidelines for the management of chronic kidney diseaseCitation3 and diabetes.Citation4 In respiratory medicine, current NPTs include the oxygen saturation probe, the peak flow meter, exhaled nitric oxide and the spirometer. Although able to provide an immediate result, these current respiratory NPTs are limited in their ability to identify treatment response.Citation5,Citation6 Changes in serial peak flow readings are able to confirm a history of occupational asthmaCitation7 and worsened outcomes following an acute exacerbation,Citation8 but provide no information regarding inflammatory stateCitation9 or airways treatable trait.Citation10 The sputum and peripheral blood eosinophil count (PBEC) has been shown to be a useful measure in determining corticosteroid response in airways disease.Citation11–Citation15 Exhaled nitric oxide is a good surrogate of eosinophilic airway inflammation but is affected by smoking.Citation16 The HemoCue® WBC Diff System (HemoCue AB, Ängelholm, Sweden) is a portable NPT, offering a 5-point differential full blood count within 2 minutes from a single drop of blood.Citation17 This technology has been evaluated by one group in 76 patients with severe asthma (Heffler et alCitation18) and they found there to be a good correlation between the HemoCue system and standard laboratory measurements of blood eosinophils. It is unknown how this NPT method is in patients with COPD and also in healthy adults. We thus evaluated the HemoCue WBC Diff System NPT against matched automated laboratory results to assess its sensitivity and specificity in measuring PBEC in healthy adults and patients with airways disease.
Methods
A comparison of peripheral blood leukocyte counts from the HemoCue WBC Diff NPT device against corresponding laboratory automated results (Abbott Architect ci8200; Abbott Laboratories, Abbott Park, IL, USA) was conducted from healthy controls and subjects with asthma and COPD. Whole venous blood (5 mL in ethylene-diamine-tetra-acetic acid [EDTA] prepared tube) was taken via venepuncture and delivered to the local pathology laboratory at the Oxford University Hospitals NHS Foundation Trust. Following venepuncture a pin-prick of blood was also taken (one drop, approximately 10 μL) and analyzed with the results being reported immediately. Allergy status was determined by a self-reported history of atopy. Correlations and intra-class correlation coefficients (ICCs) were calculated to measure relationships between the NPT and automated laboratory results for eosinophil, neutrophil and total leukocyte cell counts (cells ×109/L). Sensitivity and specificity for identifying percentage PBEC were also calculated. Bland–Altman plots were generated to assess proportional bias. In a separate subgroup of subjects with COPD (n=20), up to three drops of blood were measured for assessment of variation with repeated sampling. The lower limit of detection of PBEC for the NPT and laboratory automated analyzer was 0.1 and 0.03×109 cells/L. Ethical approval was obtained from the Leicestershire, Northamptonshire and Rutland Ethics Committee (08/H0406/189) and all volunteers provided written informed consent.
Results
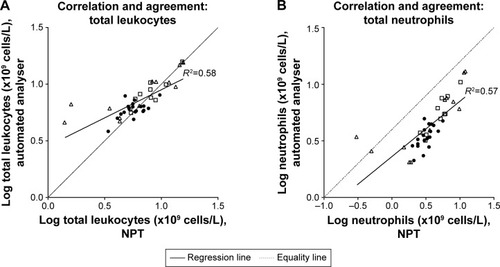
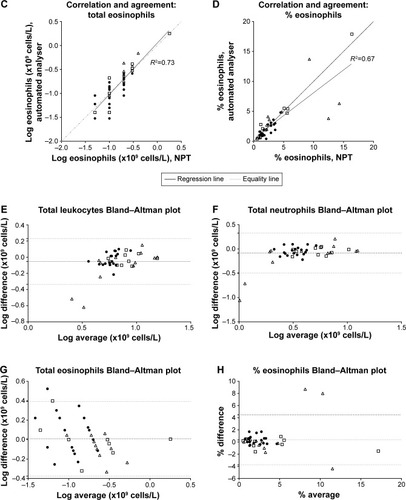
In the comparison study, 41 subjects (20 men) were recruited, with a mean age (range) of 47 (23–79) years. This consisted of 19 healthy controls, 12 asthma and 10 COPD subjects. A self-reported history of atopy occurred in 11 (30%). The geometric means (95% confidence interval [CI]) for all subjects for total leukocytes, neutrophils and eosinophils measured using the NPT device were 6.3 (5.3–7.3), 3.5 (2.8–4.4) and 0.2 (0.1–0.2) ×109 cells/L respectively and for automated laboratory results were 7.1 (6.4–7.9), 4.3 (3.7–4.9) and 0.2 (0.1–0.2) ×109 cells/L respectively. For percentage eosinophils, the mean (SD) for the NPT device and automated results were 3.4 (3.6) and 3.1 (3.3) respectively. Paired analysis demonstrated no differences of PBEC between the NPT and automated laboratory analyzer for absolute and %PBEC (p=0.78 and p=0.29, respectively). Strong correlations and agreement, presented in , were seen for all subjects (total eosinophils r=0.85; total neutrophils r=0.75; and total leukocytes, r=0.76). The mean ICC (95% CI) for percentage PBEC was 0.89 (0.74–0.95) and total PBEC was 0.98 (0.95–0.99) respectively. This indicates minimal variability between the results using NPT and automated analyzer. Atopy did not affect these results. In subjects with asthma or COPD, the NPT identified a blood eosinophil level of above or below 2%, determined by the automated laboratory analyzer as the gold standard, correctly in 20 (91%) with a sensitivity and specificity of 94% and 83% respectively. At high levels of % eosinophils proportional bias was noted (). In the repeated-sampling sub-study of COPD subjects, the intra-class coefficient (95% CI) of total leukocytes, total neutrophils and total eosinophils was 0.95 (0.82–0.98), 0.90 (0.74–0.97) and 0.90 (0.73–0.96) respectively, with a Cronbach Alpha reliability statistic of >0.95 for each measure.
Figure 1 (A–D) Correlation and agreement plots for total leukocyte, neutrophil and eosinophil cell counts (×109 cells/L) between NPT (HemoCue® WBC Diff System; HemoCue AB, Ängelholm, Sweden) and automated laboratory analyser (Abbott Architect ci8200; Abbott Laboratories, Abbott Park, IL, USA). ● Healthy volunteer controls; □ COPD; Δ asthma. (E–H) Bland–Altman plots for total leukocyte, neutrophil and eosinophil cell counts (×109 cells/L), difference and average between NPT (HemoCue® WBC Diff System) and automated laboratory analyzer (Abbott Architect ci8200). Horizontal lines set at bias and upper and lower 95% CI of the bias. ● Healthy volunteer controls; □ COPD; Δ asthma.
Conclusion
The NPT HemoCue WBC Diff device, using a pin-prick of blood sample, is comparable to an automated Abbott Architect ci8200 laboratory analyzer when measuring PBEC levels and total cell counts in whole blood in both healthy volunteers and individuals with airways disease. This result was not affected by a self-reported history of allergy, is minimally variable and has excellent sensitivity in correctly identifying a percentage PBEC level ≥2%. There was excellent agreement and correlation of the PBEC measured in the NPT, with very close margins of agreement. Proportional bias at higher levels of % eosinophil counts were seen, however, if this NPT was to be used for quantification of a binary outcome threshold of 2% to make treatment decisions regarding corticosteroidsCitation12 then this proportional bias at higher levels of PBEC is not clinically significant. Repeated sampling, in patients with COPD, was also found to be excellent. We suggest that this NPT can be used to measure eosinophil counts in patients with asthma and COPD.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to acknowledge all the volunteers for their assistance in this study. Mona Bafadhel is funded by a National Institute of Health Research (NIHR) Post-doctoral Fellowship (PDF-2013-06-052). This paper presents independent research funded by the National Institute of Health Research and the views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Disclosure
MB and REKR have received honoraria from AstraZeneca, Boehringer Ingelheim, Chiesi, Novartis and Pfizer, and report no other conflicts of interest in this work. KH, CMC, CB, JHD, and HPJ report no conflicts of interest in this work.
References
- CrookMANear patient testing and pathology in the new millenniumJ Clin Pathol2000531273010767852
- FitzmauriceDAHobbsFMurrayETHolderRLAllanTFRosePEOral anticoagulation management in primary care with the use of computerized decision support and near-patient testing: A randomized, controlled trialArch Intern Med2000160152343234810927732
- National Institute of Clinical ExcellenceChronic kidney disease in adults: assessment and management2014 Available from: https://www.nice.org.uk/Guidance/cg182Accessed August 17, 2017
- National Insitute of Clinical ExcellenceType 2 diabetes in adults: management2015 Available from: https://www.nice.org.uk/guidance/ng28Accessed August 17, 2017
- JensenLAOnyskiwJEPrasadNGNMeta-analysis of arterial oxygen saturation monitoring by pulse oximetry in adultsHeart Lung19982763874089835670
- IzbickiGAbboudSJordanPPerruchoudAPBolligerCTA comparison of a new transtelephonic portable spirometer with a laboratory spirometerEurRespir1999141209213
- BurgeCBMooreVCPantinCFRobertsonASBurgePSDiagnosis of occupational asthma from time point differences in serial PEF measurementsThorax2009641032103619850961
- BirringSSHeartinEWilliamsTJBrightlingCEPavordIDPeak expiratory flow sequence in acute exacerbations of asthmaBMJ20013227297128111375231
- HaldarPPavordIDShawDECluster analysis and clinical asthma phenotypesAm J Respir Crit Care Med2008178321822418480428
- AgustiABelEThomasMTreatable traits: toward precision medicine of chronic airway diseasesEur Respir J201647241041926828055
- GreenRHBrightlingCEMcKennaSAsthma exacerbations and sputum eosinophil counts: a randomised controlled trialLancet200236093471715172112480423
- BafadhelMMcKennaSTerrySBlood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trialAm J Respir Crit Care Med20121861485522447964
- BafadhelMDaviesLCalverleyPMAaronSDBrightlingCEPavordIDBlood eosinophil guided prednisolone therapy for exacerbations of COPD: a further analysisEur Respir J201444378979124925917
- WatzHTetzlaffKWoutersEFBlood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trialLancet Resp Med201645390398
- SivaRGreenRHBrightlingCEEosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trialEur Respir J200729590691317301099
- BarnesPJDweikRAGelbAFExhaled nitric oxide in pulmonary diseases: a comprehensive reviewChest2010138368269220822990
- Osei-BimpongAJuryCMcLeanRLewisSMPoint-of-care method for total white cell count: an evaluation of the HemoCue WBC deviceIn J Lab Hematol2009316657664
- HefflerETerranovaGChessariCPoint-of-care blood eosinophil count in a severe asthma clinic settingAnn Allergy Asthma Immunol20171191162028668237
