Abstract
Introduction
The efficacy of long-term noninvasive positive pressure ventilation (NPPV) in stable hypercapnic COPD patients with respiratory failure remains unclear. The aim of this meta-analysis was to critically assess the efficacy of long-term NPPV on mortality, acute exacerbation, exercise capacity, symptoms and significant physiological parameters (lung function, respiratory muscle function and gas exchange).
Patients and methods
We performed an electronic literature search using the PubMed, Cochrane Library, Embase, OVID and Chinese Biomedical Literature Database in May 2017. Studies comparing treatment effects of NPPV with oxygen therapy in stable hypercapnic COPD patients with respiratory failure were conducted, and at least one of the following parameters were reviewed: frequency of acute exacerbation, mortality, lung function, respiratory muscle function, gas exchange, exercise capacity.
Results
Seven studies with 810 subjects were identified. The partial pressure of arterial carbon dioxide (PaCO2) significantly decreased in patients who received long-term NPPV (weighted mean difference [WMD] −3.73, 95% CI: −5.83 to −1.64, P=0.0005). No significant difference was found in mortality, partial pressure of arterial oxygen (PaO2), frequency of acute exacerbation, lung function, respiratory muscle function and exercise capacity. The subgroup analysis showed that NPPV significantly improved the survival of patients when it was targeted at greatly reducing hypercapnia (WMD 0.35, 95% CI: 0.19 to 0.64, P=0.0006).
Conclusion
The results indicate that long-term NPPV decreases the PaCO2 of stable hypercapnic COPD patients with respiratory failure and improves mortality with the aim of reducing PaCO2.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
COPD is the fourth fatal cause worldwide;Citation1 it has been predicted by the World Health Organization that COPD would become the third by 2030.Citation2 Airflow limitation and alveolar hypoventilation can damage the arterial oxygen exchange function of COPD patients.Citation3 As disease gets worse, hypoxemia and hypercapnia caused by progressing peripheral airway obstruction contribute to the decreasing pulmonary gas exchange ability, destruction of pulmonary parenchyma and abnormality of pulmonary vessels, which increases the frequency of acute exacerbation. Patients with chronic respiratory failure and hypercapnia have severe dyspnea, lower quality of life and even increased mortality.Citation4
Long-term oxygen therapy (LTOT) has already been demonstrated to improve hypoxemia, dyspnea, life quality and survival.Citation5 Noninvasive positive pressure ventilation (NPPV) could improve gas exchange function by increasing tidal volume and producing positive airway pressure;Citation6 relax and relieve respiratory muscle fatigueCitation7 and improve respiratory center’s sensitivity to CO2.Citation8 Besides, NPPV could be associated with lower hospitalization rates.Citation9
Despite the abovementioned positive elements, randomized controlled trials (RCTs) have not achieved an agreement about applying long-term NPPV to treating stable COPD patients with respiratory failure so far. In 2014, a meta-analysis by Struik et alCitation10 failed to evaluate the mortality, the frequency of acute exacerbation and the hospitalization rate due to the lack of experiment support. In recent years, several new large RCTs have evaluated the potential efficacy of long-term NPPV on stable COPD patients with respiratory failure, especially in mortality and acute exacerbation.Citation11,Citation12
We conducted a meta-analysis for the update of current literature. The aim of this meta-analysis was to evaluate the effect of long-term NPPV on stable hypercapnic COPD patients with respiratory failure in mortality, acute exacerbation, exercise capacity, symptoms and significant physiological parameters (lung function, respiratory muscle function and gas exchange).
Patients and methods
Literature search
We conducted a comprehensive search based on the Cochrane Handbook (version 5.1.0) by searching the PubMed, Embase, Cochrane Library, OVID and Chinese Biomedical Literature Database for RCTs in humans through May 2017. The following words were used as key words: chronic obstructive pulmonary disease OR COPD AND noninvasive positive pressure ventilation OR NPPV OR NIV OR NIPPV AND routine treatment OR long-term oxygen therapy OR LTOT. Additionally, we performed a review of the reference lists of recognized studies, and when necessary a supplementary search of relevant studies was conducted.
Selection of studies and data collection
Our findings included studies that met the following criteria: 1) RCT of adults (>18 years); 2) stable COPD patients diagnosed according to Global Initiative for Chronic Obstructive Lung Disease 2017: the diagnosis of COPD was based on appropriate symptoms (such as dyspnea, chronic cough and sputum production), significant exposures to noxious stimuli (such as tobacco and pollution) and the presence of a post-bronchodilator forced expiratory volume in 1 second to forced vital capacity ratio (FEV1/FVC) <0.70;Citation13 3) associated with chronic type II respiratory failure (diagnostic criteria: partial pressure of arterial oxygen [PaO2] <60 mmHg and partial pressure of arterial carbon dioxide [PaCO2] >50 mmHg);Citation14 4) NPPV was used at least 5 hours per day for at least 3 months in the intervention group, while control group received the same management except NPPV and 5) measurement of hospital admission, mortality, lung function, respiratory muscle function, gas exchange and exercise capacity.
A prespecified protocol was used to extract data from included studies by two reviewers independently. In this process, the following study characteristics were collected: study type, first author, location, publication date, study design, sample size and outcomes. Differences in opinion between the two reviewers were settled by discussion and team consensus.
Risk of bias assessment
A methodological quality and risk of bias assessment of included studies was performed by the two reviewers independently. An approach based on the Cochrane Risk of Bias Tool that evaluated studies as high, low or unclear risk was used.Citation15 Divergences between the two reviewers in particular studies were settled by discussion.
Statistical analysis
We used Review Manager (version 5.3.5) to pool the results and assess the treatment efficacy. A random efficacy model was used in the case of significant heterogeneity between the studies. A relative risk (RR) with 95% confidence interval (95% CI) was computed for binary variables and for consecutive variables; a weighted mean difference (WMD) with 95% CI was calculated.
Both the Cochrane Q test and I2 statistic were used to assess heterogeneity across study outcomes.Citation16 For Cochrane Q test, significant heterogeneity was considered with a P-value of <0.1. For I2 statistic, I2<25%, 25%≤I2<50% or I2≥50% corresponds to low, moderate or high significance of heterogeneity, respectively.Citation17
Results
Study inclusion and characteristics
The process of study selection is shown in . A total of 1,004 studies were retrieved through database search. Among them, 211 duplicates and 780 irrelevant studies were removed. After screening the full-text articles, six studies were excluded: five studies for using NPPV in a short time (time <4 hours per day or <3 months)Citation18–Citation22 and one study for enrolling patients who were not hypercapnic.Citation23
Figure 1 Flowchart of the study selection process.
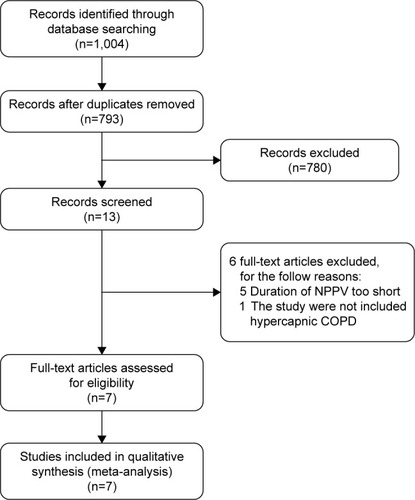
Finally, seven studies with 810 subjects were used for this meta-analysis,Citation5,Citation11,Citation12,Citation24–Citation27 and the basic characteristics of included studies are listed in .
Table 1 Main characteristics of included studies
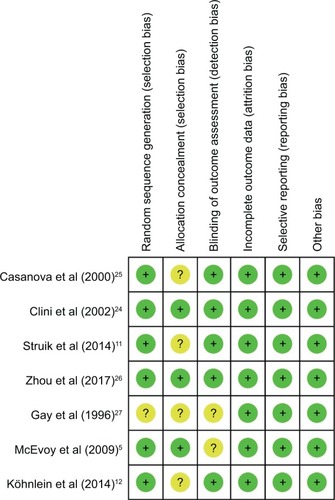
Risk of bias assessment
As shown in , two studies were judged to be at low risk of bias,Citation24,Citation26 while the other five studies were unclear. Six studies used the random sequence generation to generate allocation sequences.Citation5,Citation11,Citation12,Citation24–Citation26 Three studies used appropriate allocation and concealment methods,Citation5,Citation25,Citation26 but the other four studies did not state specific methods. Because NPPV could not be blinded, the researchers and patients were aware of which kind of therapy was chosen, no studies performed blinding.
Mortality
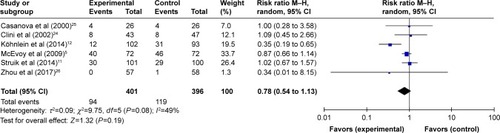
Six studies (797 subjects) reporting mortalityCitation5,Citation11,Citation12,Citation24–Citation26 were pooled and summarized in . In patients treated with NPPV, mortality was not lowered significantly (95% CI: 0.54 to 1.13, P=0.19), and a moderate degree of heterogeneity was present (I2=49%).
Acute exacerbation
Five studies evaluated the frequency of acute exacerbation including the hospitalization and/or intensive care unit (ICU) admission. Among them, four studies showed that there was no significant difference in hospitalization rate and/or ICU admission rate between experiment group and control group.Citation5,Citation11,Citation24,Citation25 However, Köhnlein et alCitation12 found that NPPV reduced hospitalization significantly in the intervention group (average hospitalization frequency =2.2 times/person) compared with the control group (average hospitalization frequency =3.1 times/person) after a follow-up for 1 year. However, because the indices and methods for evaluating acute exacerbation were not the same and some literature did not show sufficient data, the data of acute exacerbation have not been combined and analyzed.
Gas exchange
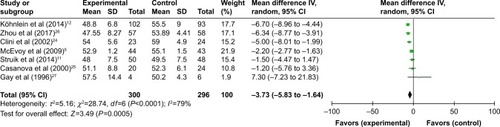
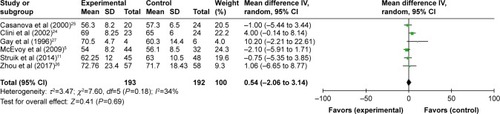
All seven studies reported gas exchange data,Citation5,Citation11,Citation12,Citation24–Citation27 and in patients treated with NPPV, PaCO2 significantly decreased (WMD −3.73, 95% CI: −5.83 to −1.64, P=0.0005) (). NPPV could not improve PaO2 statistically (WMD 0.30, 95% CI: −1.78 to 2.37, P=0.78) ().
Lung function and respiratory muscle strength
This research evaluated lung functional indexes of FEV1, FEV1 total predicted value (FEV1% pred), FVC and maximal inspiratory pressure (PImax). Combined analysis showed that NPPV could not improve lung function and strength of respiratory muscle ().
Table 2 Meta-analysis of lung function and respiratory muscle strength
Exercise capacity
Although 6-minute walk distance (6MWD) was used in four studies to evaluate the exercise capacity, data could not be combined due to the different data types used in these studies. One study showed a significant improvement in the NPPV group; the minimal clinical improvement in 6MWD of 54 m was reached in 21 (36.8%) patients in the NPPV group versus 10 (17.2%) patients in the control group (P=0.02).Citation26 However, the other three studies showed no significant improvement in the NPPV group.Citation12,Citation24,Citation27
Dyspnea
Due to the different measurement scales used in different studies, data could not be combined. Casanova et alCitation25 showed a reduction in dyspnea at 3 months on two dyspnea scales (modified medical research council dyspnea scale [mMRC], P=0.035 and Borg scale, P=0.039), and this improvement was maintained at 6 months on the Borg scale (P=0.033) in the NPPV group. Clini et alCitation24 reported that the improvement of dyspnea was more significant after 2 years (P=0.013) than the first year (P=0.048) in the NPPV group. The studies by Struik et alCitation11 and Zhou et alCitation26 showed no significant difference in dyspnea.
Subgroup analysis
According to the subgroup analysis of different NPPV techniques, the results showed that there was no decrease in mortality when NPPV did not aim at improving PaCO2 (RR =0.92, 95% CI: 0.74 to 1.15, P=0.47) (). However, within the subset of 310 patients in the studies by Köhnlein et alCitation12 and Zhou et al,Citation26 patients who received NPPV mainly aimed at decreasing PaCO2 demonstrated significant improvement in mortality compared with those who underwent standard oxygen therapy only (RR =0.35, 95% CI: 0.19 to 0.64, P=0.0006) ().
Table 3 Subgroup analysis
There was substantial heterogeneity in PaCO2 (I2=79%). We found that the heterogeneity of PaCO2 was significantly decreased in both groups (I2=25%/0%) and significant heterogeneity existed between two groups (I2=93.3%). No significant improvement was found in PaO2 in both groups (P=0.79/P=0.71).
Discussion
This meta-analysis of seven RCTs including 810 patients with stable hypercapnic COPD and respiratory failure comparing the efficacy of long-term NPPV and LTOT showed that long-term NPPV significantly decreased the PaCO2 of COPD patients with chronic type II respiratory failure; however, no significant difference was found in mortality, frequency of acute exacerbation, PaO2, lung function, respiratory muscle function and exercise capacity.
The subgroup analysis showed that different ventilation parameters had an impact on the effect of NPPV. The efficacy in improving mortality was not obvious with parameters that were only set to improve respiratory muscle function and reduce dyspnea symptoms. However, the subgroup analysis of combined data from the two studies revealed that patients in the NPPV group mainly aimed at reducing PaCO2 had a lower risk of mortality than those in the control group.Citation12,Citation26
Several studies have shown that chronic hypercapnia was an important symbol of poor prognosis in COPD patients.Citation28,Citation29 Long-term survival analysis showed that mortality would increase by 30%–40% in 2 years if patients have both chronic respiratory failure and hypercapnia.Citation30 According to a long-term observation on stable COPD patients carried out by Leger et al,Citation31 stable COPD patients with long-term survival had a significantly lower PaCO2 than the early death.
In addition, respiratory muscle dysfunction is an important feature of chronic respiratory failure.Citation32 It was said that hypercapnia would decline the strength and endurance of diaphragm.Citation33 At the same time, an unsatisfactory position in length–tension curve caused by chronic hyperventilation would affect the contraction of diaphragm, which would lead to respiratory muscle fatigue and respiratory pump failure. Therefore, some scholars believed that NPPV could benefit the patients with stable hypercapnic COPD by reducing the burden of respiratory muscle, relieving the fatigue and improving the gas exchange.Citation34,Citation35 However, most of the previous RCTs could not reduce PaCO2 significantly probably due to the low intermittent positive airway pressure (IPAP) of 10–20 cmH2O in NPPV. Current studies showed that IPAP in NPPV reaching 30 cmH2O could obviously improve the level of arterial blood gas in patients with stable hypercapnic COPD.Citation6,Citation35,Citation36 A long-term study carried out by Windisch et alCitation6 showed that NPPV targeted at greatly reducing hypercapnia (average IPAP =28 cmH2O) could significantly improve the levels of arterial blood gas and lung function of COPD patients with chronic respiratory failure and reduce the mortality to 86% in 2 years. Consistent with the abovementioned opinion, we found that high IPAP settings benefited patients more. In the subgroup analysis, one group’s primary end point was to reduce PaCO2 with an average IPAP of 19.7 cmH2O, and the mortality decreased significantly; the other group’s primary end point was not to reduce PaCO2 with an average IPAP of 13.62 cmH2O, and no significant improvement was found in mortality.
It was reported that hypercapnia was a risk factor to increase the frequency of acute exacerbation of COPD patients.Citation37 A 6-month follow-up of 1,016 in-patients with both COPD and hypercapnia was carried out by Connors et al,Citation38 which revealed that half of them needed rehospitalization and 7% of patients even needed it over three times. Köhnlein et alCitation12 supported the abovementioned opinion by comparing the NPPV with oxygen therapy on COPD patients with hypercapnia, in which the aim of NPPV was to reduce PaCO2 to 20% of the basic value or 48.1 mmHg, and it revealed that NPPV could significantly lower the frequency of hospitalization compared with standard oxygen therapy.
Dyspnea and decreasing ventilation function limited the exercise capacity of patients with COPD. However, relevant studies showed that injury of lung function was the explanation for not only decreasing exercise capacity but also peripheral and respiratory muscle dysfunction. Gosselink et alCitation39 tested the respiratory and peripheral muscle force of 40 COPD patients and found that COPD patients have reduced respiratory muscle strength (mean 64% of control subjects’ value) and peripheral muscle strength (mean 75% of control subjects’ value) compared to normal subjects. By searching the literature, we noticed that hypercapnia was an important contributing cause of muscle dysfunction.Citation40 The study of Zhou et al,Citation26 which aimed to reduce PaCO2, showed that NPPV was helpful in improving exercise capacity, yet we failed to combine the data due to different data types. Therefore, more high-quality studies are needed to confirm the effect of NPPV in improving exercise capacity of COPD patients.
As different syndrome evaluating scales were used in inclusive trials, including the Borg scale,Citation25 the Baseline and Transitional Dyspnea IndexCitation5 and the MRC scale,Citation11,Citation24,Citation25 we were unable to comment on the symptom of dyspnea.
A meta-analysis by Struik et alCitation10 in 2014 found no evidence to support the application of routine NPPV in stable COPD patients, yet the subgroup analysis showed a significant decrease of PaCO2 in patients with higher IPAP levels (IPAP ≥18 cmH2O) as well as in hypercapnic patients (baseline PaCO2 ≥55 mmHg). Comparing with this meta-analysis, we analyzed the data of updated studies, aiming to assess the efficacy of long-term NPPV on stable hypercapnic COPD patients. We noticed that three recent studiesCitation11,Citation12,Citation25 changed the results greatly and provided our research more sense. In addition, it was identified that all of the three studies set ventilatory pressures at a high level, of which the IPAP/expiratory positive airway pressures (EPAPs) were set at 21.6/4.8,Citation11 19.2/4.8Citation12 and 17.8/4.2 cmH2O,Citation25 which might significantly improve the level of arterial blood gas and lung function in COPD patients. Importantly, the study by Zhou et al was the first reported randomized clinical trial to use NPPV ventilators equipped with built-in software to monitor the ventilator parameters in home setting in stable hypercapnic COPD patients, which found that using NPPV ventilator equipped with built-in software decreased the rate of abnormalities. In other words, using NPPV ventilator equipped with built-in software facilitated the improvement of compliance and quality control of NPPV use so as to improve the accuracy of data.
Our findings supported that NPPV decreased PaCO2, and when primary end point was aimed at greatly reducing hypercapnia, NPPV improved the survival of patients. However, consistent with Struik et al, no significance was found in PaO2, frequency of acute exacerbation, lung function, respiratory muscle function and exercise capacity.
There were several limitations in this meta-analysis: 1) the quality of trials was inconsistent, for example, two studies were judged to be at low risk of bias, whereas more than two uncertain or improper methodologies were found in one study; 2) the differences in the types of data and evaluation methods that were reported limited the application of meta-analysis in some indices, such as exercise capacity, acute exacerbation and dyspnea, which may be of interest. Future studies that include the abovementioned outcomes will enable conclusions to be drawn. However, further studies are desperately required to identify the improvements by NPPV on stable hypercapnic COPD patients.
Conclusion
This meta-analysis suggests that NPPV decreases the PaCO2 of stable hypercapnic COPD patients with respiratory failure and improves mortality with the aim of reducing PaCO2.
Author contributions
All authors contributed towards data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by Open Project of State Key Laboratory of Respiratory Disease (SKLRD2016OP019), the Medical Scientific Research Foundation of Guangdong Province (A2016399), the Clinical Research training program of Southern Medical University (LC2016PY032), and the Guangdong Provincial College Students’ Innovation and Entrepreneurship Training Program (201612121054).
Disclosure
The authors report no conflicts of interest in this work.
References
- OjoOLaganALRajendranVPathological changes in the COPD lung mesenchyme – novel lessons learned from in vitro and in vivo studiesPulm Pharmacol Ther201429212112824747433
- DecramerMJanssensWMiravitllesMChronic obstructive pulmonary diseaseLancet201237998231341135122314182
- TanWCMahayiddinAACharoenratanakulSGlobal Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (UPDATE 2014) Global Initiative for Chronic Obstructive Lung Disease 2014 GOLDRespirology200510191715691232
- SlenterRHSprootenRTKotzDWesselingGWoutersEFRohdeGGPredictors of 1-year mortality at hospital admission for acute exacer-bations of chronic obstructive pulmonary diseaseRespiration2013851152623037178
- McEvoyRDPierceRJHillmanDNocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trialThorax200964756156619213769
- WindischWKostiSDreherMVirchowJCJrSorichterSOutcome of patients with stable COPD receiving controlled noninvasive positive pressure ventilation aimed at a maximal reduction of PaCo2Chest2005128265766216100151
- AnonymousClinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation – a consensus conference reportChest1999116252153410453883
- NickolAHNicholasHHopkinsonNSMechanisms of improvement of respiratory failure in patients with COPD treated with NIVInt J Chron Obstruct Pulmon Dis20083345346218990974
- TuggeyJMPlantPKElliottMWDomiciliary non-invasive ventilation for recurrent acidotic exacerbations of COPD: an economic analysisThorax2003581086787114514940
- StruikFMLacasseYGoldsteinRSKerstjensHAWijkstraPJNocturnal noninvasive positive pressure ventilation in stable COPD: a systematic review and individual patient data meta-analysisRespir Med2014108232933724157199
- StruikFMSprootenRTKerstjensHANocturnal non-invasive ventilation in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure: a randomised, controlled, parallel-group studyThorax201469982683424781217
- KöhnleinTWindischWKohlerDNon-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trialLancet Respir Med20142969870525066329
- VogelmeierCFCrinerGJMartinezFJGlobal strategy for the diagnosis, management and prevention of chronic obstructive lung disease 2017 reportAm J Resp Crit Care20171955557582
- OanaSMukherjiJAcute and chronic respiratory failureHandb Clin Neurol201411927328824365302
- HigginsJPAltmanDGGotzschePCThe Cochrane Collaboration’s tool for assessing risk of bias in randomised trialsBMJ2011343d592822008217
- LauJIoannidisJPSchmidCHQuantitative synthesis in systematic reviewsAnn Intern Med199712798208269382404
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- SuCLChiangLLChiangTYYuCTKuoHPLinHCDomiciliary positive expiratory pressure improves pulmonary function and exercise capacity in patients with chronic obstructive pulmonary diseaseJ Formos Med Assoc2007106320421117389164
- GurgunATuncelSKarapolatHUluerHThe effect of adding noninvasive ventilation to supplemental oxygen during exercise training in severe COPD: a randomized controlled studyChest20151484909A
- DíazOBéginPTorrealbaBJoverELisboaCEffects of noninvasive ventilation on lung hyperinflation in stable hypercapnic COPDEur Respir J20022061490149812503709
- DiazOBéginPAndresenMPhysiological and clinical effects of diurnal noninvasive ventilation in hypercapnic COPDEur Respir J20052661016102316319330
- van’t HulAGosselinkRHollanderPPostmusPKwakkelGTraining with inspiratory pressure support in patients with severe COPDEur Respir J2006271657216387937
- SinDDWongEMayersIEffects of nocturnal noninvasive mechanical ventilation on heart rate variability of patients with advanced COPDChest2007131115616317218570
- CliniESturaniCRossiAThe Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patientsEur Respir J200220352953812358325
- CasanovaCCelliBRTostLLong-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPDChest200011861582159011115443
- ZhouLLiXGuanLHome noninvasive positive pressure ventilation with built-in software in stable hypercapnic COPD: a short-term prospective, multicenter, randomized, controlled trialInt J Chron Obstruct Pulmon Dis2017121828031706
- GayPCHubmayrRDStroetzRWEfficacy of nocturnal nasal ventilation in stable, severe chronic obstructive pulmonary disease during a 3-month controlled trialMayo Clin Proc19967165335428642881
- MatkovicZHuertaASolerNPredictors of adverse outcome in patients hospitalised for exacerbation of chronic obstructive pulmonary diseaseRespiration2012841172622327370
- YangHXiangPZhangEIs hypercapnia associated with poor prognosis in chronic obstructive pulmonary disease? A long-term follow-up cohort studyBMJ Open2015512e008909
- GoreJMBrophyCJGreenstoneMAHow well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancerThorax200055121000100611083884
- LegerPBedicamJMCornetteANasal intermittent positive pressure ventilation. Long-term follow-up in patients with severe chronic respiratory insufficiencyChest199410511001058275718
- KlimathianakiMVaporidiKGeorgopoulosDRespiratory muscle dysfunction in COPD: from muscles to cellCurr Drug Targets201112447848821194407
- JuanGCalverleyPTalamoCSchnaderJRoussosCEffect of carbon dioxide on diaphragmatic function in human beingsN Engl J Med1984310148748796422298
- WedzichaJAMuirJNoninvasive ventilation in chronic obstructive pulmonary disease, bronchiectasis and cystic fibrosisEur Respir J200220377778412358358
- SchucherBZerbstJBaumannHJDie nichtinvasive Beatmung bei Patienten mit stabiler, schwergradiger chronisch-obstruktiver Bronchitis und Lungenemphysem (COPD). [Noninvasive mechanical ventilation in patients with stable severe COPD]Pneumologie2004586428434 German15216436
- WindischWVogelMSorichterSNormocapnia during nIPPV in chronic hypercapnic COPD reduces subsequent spontaneous PaCO2Respir Med200296857257912195837
- AlmagroPBarreiroBOchoa De EchagüenARisk factors for hospital readmission in patients with chronic obstructive pulmonary diseaseRespiration200673331131716155352
- ConnorsAFJrDawsonNVThomasCOutcomes following acute exacerbation of severe chronic obstructive lung disease the SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments)Am J Respir Crit Care Med19961544 pt 19599678887592
- GosselinkRTroostersTDecramerMDistribution of muscle weakness in patients with stable chronic obstructive pulmonary diseaseJ Cardiopulm Rehabil200020635336011144041
- DecramerMDe BenedettoFDel PonteAMarinariSSystemic effects of COPDResp Med20059912S3S10