Abstract
Background
Emphysema is a distinct feature for classifying COPD, and smoking history (≥10 pack-years) is one of several newly proposed criteria for asthma–COPD overlap (ACO). We studied whether or not a smoking history (≥10 pack-years) and emphysema are useful markers for classifying ACO and differentiating it from asthma with chronic airflow obstruction (CAO).
Methods
We retrospectively studied the mortalities and frequencies of exacerbation in 256 consecutive patients with ACO (161 with emphysema and 95 without emphysema) who had ≥10 pack-years smoking history, 64 asthma patients with CAO but less of a smoking history (<10 pack-years) and 537 consecutive patients with COPD (452 with emphysema and 85 without emphysema) from 2000 to 2016. In the patients with emergent admission, the causes were classified into COPD exacerbation, asthma attack, and others.
Results
No asthma patients with CAO had emphysema according to computed tomography findings. The prognoses were significantly better in patients with asthma and CAO than in those with ACO and COPD and better in those with ACO than in those with COPD. In both ACO and COPD patients, the prognoses were better in patients without emphysema than in those with it (P=0.027 and P=0.023, respectively). The frequencies of emergent admission were higher in COPD patients than in ACO patients, and higher in patients with emphysema than in patients without emphysema. ACO/emphysema (+) patients experienced more frequent admission due to COPD exacerbation (P<0.001), while ACO/emphysema (−) patients experienced more frequent admission due to asthma attack (P=0.014).
Conclusion
A smoking history (≥10 pack-years) was found to be a useful marker for differentiating ACO and asthma with CAO, and emphysema was a useful marker for classifying ACO. These markers are useful for predicting the overall survival and frequency of exacerbation.
Introduction
Among the patients with chronic airflow obstruction (CAO), concurrent asthma and COPD is common with a reported prevalence rate between 15% and 55% among COPD patients.Citation1–Citation3 This wide range of prevalence rates largely stems from the diagnostic criteria for overlap syndrome. A joint project of the Global Initiative for Asthma (GINA) and Global Initiative for Chronic Obstructive Lung Disease (GOLD) addressed the diagnostic approach for asthma–COPD overlap (ACO), previously known as asthma–COPD overlap syndrome (ACOS).Citation4 However, the GINA/GOLD guidelines addressed only the clinical description, not the definition, and in real-world practice, it is difficult to differentiate whether CAO in patients with asthma is the result of airway remodeling from asthma or the effect of concurrent smoking.
The original definition of ACOS reported by Gibson and Simpson was “increased variability of airflow and incompletely reversible airflow limitation”,Citation5 implying that all cases of asthma with CAO are ACOS. Recently, a global expert panel discussion proposed new consensus criteria for ACO including a smoking history of ≥10 pack-years as a major criterion.Citation6 According to the proposed criteria, asthma patients with CAO but less of a smoking history are excluded from ACO in areas where air pollution is not a potential etiology of COPD. However, whether or not a smoking history of ≥10 pack-years is a suitable marker for the diagnosis of ACO has yet to be examined.
Until inhaled corticosteroids (ICS) became widely used, the coexistence of uncontrolled asthma and COPD was associated with more frequent exacerbations,Citation7 a worse prognosisCitation8,Citation9 and a greater decline of FEV1 than COPD without asthma.Citation10,Citation11 However, recent studies have suggested that ACO includes mild-to-moderate disease with a better prognosis than COPD,Citation12,Citation13 and the GINA/GOLD guidelineCitation4 states that exacerbation in ACO can be reduced by treatment.
Starting from 2000, we observed all consecutive patients with CAO who were able to undergo full examinations by specialists for COPD, asthma and other airway diseases. From 2000 to 2011, we initially recruited 1,960 consecutive patients with not-fully-reversible airflow obstruction.Citation12 CAO was confirmed by spirometry every 1 or 2 years. For a differential diagnosis, all patients were evaluated by high-resolution computed tomography (HRCT) at the start of the observation. In this study, we further followed up their clinical records until June 2016, and investigated whether or not a smoking history of ≥10 pack-years is a useful marker for differentiating ACO and asthma with CAO and whether computed tomography (CT)-diagnosed emphysema is a useful marker for subclassifying ACO in terms of mortality and exacerbation. As a reference, we also compared the mortality and exacerbation between ACO and COPD.
Methods
Study subjects
We reviewed 857 consecutive patients ≥40 years of age with CAO (537 patients with COPD, 256 patients with ACO and 64 asthma patients with CAO) who presented to the Saitama Cardiovascular and Respiratory Center (a tertiary referral center with 155 beds for respiratory disease) in Saitama, Japan. CAO was defined as post-bronchodilator FEV1/FVC <0.7 throughout the observation period.
Study design
This was a retrospective observational cohort study in which clinical data were collected from medical records. ACO was defined according to the global expert panel consensus criteriaCitation6 with mild modification including all three major criteria: 1) CAO in individuals ≥40 years of age, 2) a smoking history of ≥10 pack-years, 3) a documented history of asthma and at least one minor criterion: (1) a documented history of atopy or allergic rhinitis, 2) a bronchodilator response of FEV1 ≥200 mL and 12% from baseline values using salbutamol, and 3) peripheral blood eosinophil count >300 cells/μL). In this study, the asthma history at any age was accepted because asthma features are not specific and the first asthma event is often difficult to define.Citation4
COPD was diagnosed as previously described:Citation12,Citation14 1) a smoking history of ≥10 pack-years, 2) CAO in individuals ≥40 years of age and 3) other respiratory diseases excluded.
The entry criteria of asthma with CAO were as follows: 1) CAO in individuals ≥40 years of age and 2) a documented history of asthma and at least one minor criterion (a documented history of atopy or allergic rhinitis, or a bronchodilator response of FEV1 ≥200 mL and 12% from baseline values using salbutamol or peripheral blood eosinophil count >300 cells/μL).
HRCT was performed at the start of the observation, and the presence of emphysema was determined by two radiologists. In a previous study,Citation12 the observation period ended at March 1, 2012, but the subjects continued to be monitored and recorded. In this study, the cohort was followed up until June 30, 2016. The group of asthma patients with CAO in a previous studyCitation12 was newly classified as patients with ACO and asthma with CAO with or without 10-pack-year smoking history. The patients who could not be followed up for >1 year were excluded in order to evaluate the annual exacerbation rate. Subjective symptoms, prescription history, laboratory data and visiting history were monitored. This study was approved by the institutional review board of Saitama Cardiovascular and Respiratory Center (No 2012001). General prior consent to review their medical records was obtained from patients at the first visit to the hospital. No further consent was required for this study.
Assessment of exacerbation
The histories of exacerbation were obtained from the medical records. Total exacerbation events were defined as the worsening or new onset of symptoms such as dyspnea, cough, sputum, fever, or wheezing that required a prescription change.Citation15 Such symptoms can be observed by respiratory infections, worsening of asthma or other causes, such as heart failure, and precise differential diagnoses were sometimes difficult in ambulatory practice. However, in patients who required emergent admission, their history, physical examination findings, results of laboratory tests, HRCT findings and response to the treatments were well preserved in the medical record. The cause of admission recorded by the doctor in charge was reevaluated and ultimately classified by two study pulmonologists. Radiology-proven pneumonia was not excluded from causes of COPD exacerbation in emergent admission because bronchopneumonia is often detected by HRCT even if the chest radiology findings are almost normal.
In ACO patients and asthma patients with CAO, admission with severe wheezing that required oral or parenteral corticosteroids was designated as an asthma attack, and admission with symptoms of airway infection, such as fever, absence of severe wheezing and increased serum C-reactive protein >1 mg/dL requiring antibiotics, was designated as COPD exacerbation according to the study criteria.
Statistical analyses
The baseline characteristics are represented as the mean ± SD, median (interquartile range) or number (%), as appropriate. Differences among the groups were analyzed using the Mann–Whitney U test or chi-squared test. Subgroups were compared via an analysis of variance.
The survival in each subgroup was estimated by Kaplan–Meier analysis. Mortality rates were compared with a log-rank test and the Cox proportional hazards model. Time was defined from the initial examination until death. Censoring took place when participants were lost to follow-up or were still alive on June 30, 2016. A P-value of <0.05 was considered statistically significant. All data were analyzed using SPSS version 22 (IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics
Demographic data of patients with ACO, COPD and asthma with CAO are shown in and . The median observation periods were 6.6, 8.3 and 9.2 years, respectively. Patients with ACO had a higher female ratio but a similar post-bronchodilator FEV1 compared to COPD patients. All of the asthma patients with CAO (<10-pack-year smoking history) had post-bronchodilator FEV1/FVC <0.7 throughout the observation period. Most of them had a long asthma history (14.1±12.1 years) at the start of observation. None of the patients with asthma with CAO had CT-diagnosed emphysema.
Table 1 Characteristics of the subjects
Table 2 Characteristics of the subjects classified by emphysema
The annual decline rate in the post-bronchodilator FEV1 was larger in ACO patients (−45 mL/year) than in COPD patients (−40 mL/year) and asthma patients with CAO (−25 mL/year). Among patients with COPD, emphysema (+) patients had a higher percentage of de novo malignant disease (P=0.006) but lower rates of complication with vascular disease (P=0.016) than emphysema (−) patients. In patients with ACO, similar tendencies were observed, but not to a significant degree.
Mortalities of the patients with ACO, COPD and asthma with CAO
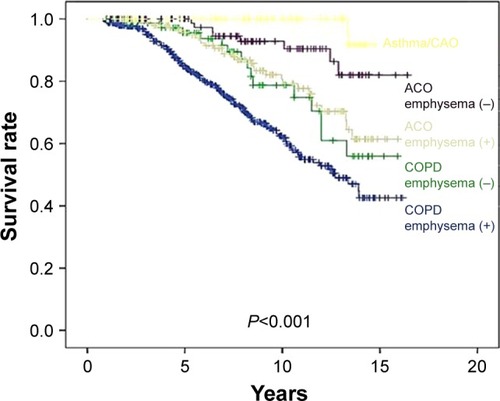
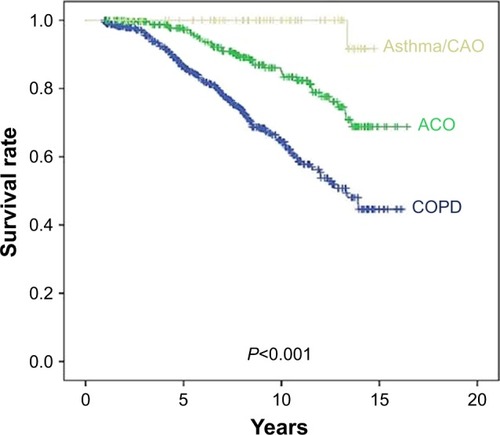
shows the overall survival rates of the groups. Patients with asthma with CAO (<10-pack-year smoking history) had a better survival rate than those with ACO (P=0.020) or COPD (P<0.001) ().
Table 3 Risk of all-cause mortality in asthma/CAO, ACO, and COPD
Figure 1 Kaplan–Meier survival curves for COPD, ACO, and asthma/CAO.
shows the overall survival rates of the subgroups classified by the presence of CT-diagnosed emphysema. Among both ACO and COPD patients, the subgroups without emphysema had a better survival rate than did the subgroups with emphysema (P=0.027 and P=0.023, respectively) ().
Frequencies of exacerbation among subgroups
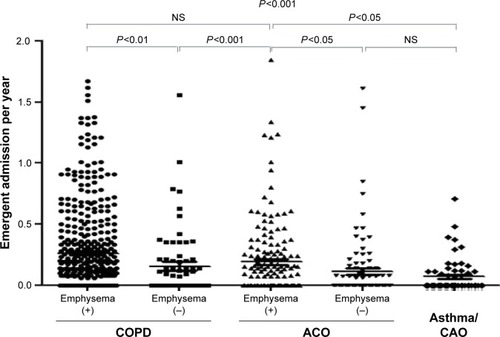
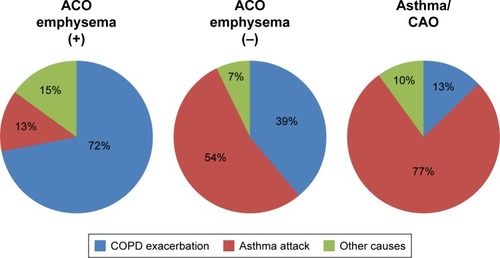
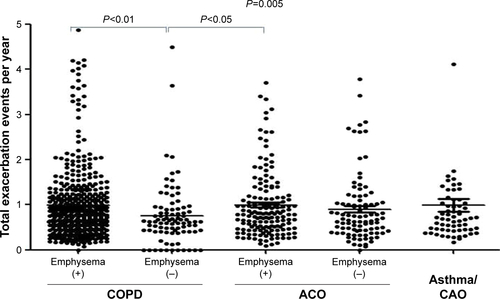
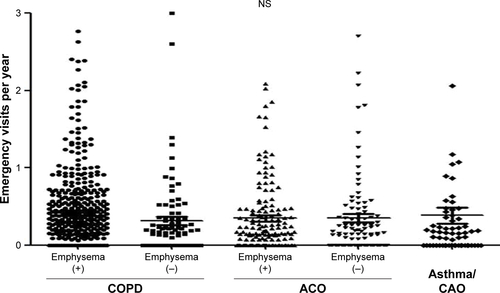
The frequencies of total exacerbation events were similar between the ACO and COPD patients (Figure S1). However, the frequency was lower in the COPD/emphysema (−) group than in the COPD/emphysema (+) group or ACO/emphysema (+) group. There were no significant differences in the frequencies of emergency visits among the subgroups (Figure S2). Subgroups with emphysema had higher frequencies of total emergent admission than subgroups without emphysema (P<0.001, ). The frequencies of emergent admission due to COPD exacerbation were higher in COPD patients than in ACO patients (P<0.001) and higher in patients with emphysema than in patients without emphysema (). ACO/emphysema (+) patients experienced more frequent admission due to COPD exacerbation but less frequent admission due to asthma attack than ACO/emphysema (−) patients (P<0.001 and P=0.014, respectively) ( and ).
Table 4 Causes of emergent admission
Discussion
The only difference in the entry criteria of this study between ACO patients and asthma patients with CAO was a 10-pack-year smoking history. Regardless, there were marked differences in the mortality and the nature of exacerbation that required emergent admission between these two patient groups. In addition, the rates of mortality and the frequency of severe exacerbation differed based on the presence of CT-diagnosed emphysema in ACO patients as well as in COPD patients. These results suggest that a 10-pack-year smoking history is a useful marker for differentiating ACO and asthma with CAO, and CT-diagnosed emphysema is a useful marker for classifying different phenotypes of ACO.
In 2000, we hypothesized that the differential diagnosis and longitudinal observation are important in the management of patients with CAO and developed an algorithm for the diagnosis of COPD, asthma and other airway diseases with CAO.Citation14 Fortunately, our approach and concept were very similar to the recent guideline of ACOCitation4 identifying asthma features and repeated follow-up of post-bronchodilator FEV1. However, until the publication of a recent consensus report in 2016,Citation6 some patients with asthma/CAO and less of a smoking history risked being classified as having ACO because the GINA/GOLD guidelineCitation4 does not provide a clear cut-off value for the smoking history. Subjects with asthma/CAO can be clearly classified into ACO and asthma with airway remodeling groups according to the proposed consensus report.Citation6
In previous reports, the coexistence of uncontrolled asthma and COPD was associated with more frequent exacerbation and worse prognosis than those observed in COPD.Citation5,Citation7,Citation8 However, the condition of ACO patients can be improved by ICS treatment,Citation16 and after a long period of time, they become less symptomatic.Citation4 In addition, growing evidence indicates that COPD patients with asthma features show a better prognosis and better response to ICS in terms of COPD exacerbation.Citation17–Citation22 In this study, all of the patients with ACO were treated with ICS and one or two long-acting bronchodilators (data not shown). In addition to the diagnostic algorithm for the patients with CAO,Citation14 we started “COPD school” and “asthma school” for all asthma and COPD patients twice a year in 2000. Each school provides individual assistance regarding inhalation procedures, a rehabilitation class and other educational programs. Part of the programs for inhalation procedures was recently described in a report.Citation23 Therefore, the subjects observed in this study had received relatively good medical care from respiratory specialists.
One of the strengths of this study is that we recruited all consecutive patients with respiratory symptoms who underwent spirometry. Such an approach may be particularly important for the screening of less symptomatic patients who might be overlooked in a multicenter study. From 2000 to 2011, we screened 3,289 patients with FEV1/FVC <0.7, and 1,960 of them had not-fully-reversible airflow limitation. However, 175 patients initially diagnosed as having asthma with not-fully-reversible airflow limitation were excluded through 2013, as their post-bronchodilator FEV1/FVC became >0.7 after ICS and other treatments were introduced.
In a report of GINA/GOLD, CT-diagnosed emphysema is referred to as a marker for distinguishing asthma and COPD in specialized investigations;Citation4 however, a recent study showed that a considerable portion of COPD patients were non-emphysema type.Citation24 The results of the present study suggest that emphysema is a good marker for classifying ACO as well as COPD. We showed that ACO/emphysema (−) patients are more similar to asthma patients with CAO, and ACO/emphysema (+) patients are more similar to COPD patients in terms of the mortality and nature of exacerbation. As in previous studies,Citation12,Citation25–Citation28 the present study showed that emphysema is a risk factor for de novo malignant tumor in patients with COPD.
To our knowledge, whether or not ACO patients experience more frequent COPD exacerbation or asthma attacks has been unclear. Although the differentiation of COPD exacerbation and asthma attack may be difficult, we tried to classify them using our study criteria in cases of admission. In the present study, the records of physical findings, response to treatments, laboratory data and findings of HRCT were available in most cases of emergent admission. ACO/emphysema (+) patients experienced more frequent admission due to COPD exacerbation, while ACO/emphysema (−) patients experienced more frequent admission due to asthma attack. A prospective study will be needed to determine whether or not exacerbations in ACO patients influence their mortality and decline in FEV1.
Several limitations associated with the present study warrant mention. First, we prospectively recruited patients with CAO, but this was a retrospective observational study, so some clinical data were not available. Some exacerbations may not have been documented in the records of our hospital. However, our hospital is the only respiratory center in the area, so we believe that most of the episodes that required emergent admission were properly recorded. The symptoms of wheezing, cough and chronic expectoration and smoking history were recorded by the paramedical staff at the annual pulmonary function tests in our clinic. We considered these data taken by the paramedical staff useful for strengthening the records of wheezing and smoking history taken by the doctors. Second, the female ratio in patients with COPD and ACO was very low in this study. However, this ratio accords with those in previous studies in JapanCitation17 and reflects the low smoking rate in Japanese women.Citation29 Third, treatments for COPD and ACO dramatically progressed from 2000 to 2016, but we tallied the number of exacerbations for the total period. Finally, the patients’ background characteristics likely vary considerably among countries due to differences in pollution exposure (eg, biomass) and comorbidities of COPD. In Japan, the air pollution level is too low to count as an etiology of COPD,Citation30 and all patients with COPD had a significant smoking history in this study. Therefore, we considered asthma patients with CAO but a low smoking history not to be ACO under the proposed criteria. The reported vascular diseases as a comorbidity of COPD are markedly lower in Japan than in other countries,Citation31–Citation33 and the data of this study were consistent with those of a recent study in Japan.Citation31
Conclusion
A smoking history of 10 pack-years is a useful cut-off value for differentiating ACO and asthma with airway remodeling. In addition, this study showed that airway-type ACO has more asthma features, while emphysema-type ACO has more COPD features. These markers are useful for predicting overall survival and frequency of exacerbation. Symptoms and airway obstruction of ACO could be improved by the treatments with ICS and long-acting bronchodilators; therefore, careful differential diagnosis and repeated evaluation are necessary for the recognition of ACO that might be treated as asthma or COPD.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The authors wish to thank the medical staff of Saitama Cardiovascular and Respiratory Center who cared for the patients.
Supplementary materials
Disclosure
The authors report no conflicts of interest in this work.
References
- SorianoJBDavisKJColemanBVisickGManninoDPrideNBThe proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United KingdomChest2003124247448112907531
- MarshSETraversJWeatherallMProportional classifications of COPD phenotypesThorax200863976176718728201
- WeatherallMTraversJScirtcliffePMDistinct clinical phenotypes of airways disease defined by cluster analysisEur Respir J200934481281819357143
- Global Initiative for AsthmaGlobal strategy for asthma management and prevention Updated 2017. Available from: http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention/Accessed May 1, 2017
- GibsonPGSimpsonJLThe overlap syndrome of asthma and COPD: what are its features and how important is it?Thorax200964872873519638566
- SinDDMiravitllesMManninoDMWhat is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussionEur Respir J201648366467327338195
- HardinMSilvermanEKBarrRGCOPDGene InvestigatorsThe clinical features of the overlap between COPD and asthmaRespir Res20111212721951550
- MeyerPAManninoDMReddSCOlsonDRCharacteristics of adults dying with COPDChest200212262003200812475839
- HospersJJPostmaDSJijckenBWeiseSTSchoutenJPHistamine airway hyper-responsiveness and mortality from chronic pulmonary disease: a cohort studyLancet200035692381313131711073020
- SilvaGESherrillDLGuerraSBarbeeRAAsthma as a risk factor for COPD in a longitudinal studyChest20041261596515249443
- TashkinDPAltoseMDConnettJEKannerRELeeWWWiseRAMethacholine reactivity predicts changes in lung function over time in smokers with early chronic obstructive disease. The Lung Health Study Research GroupAm J Respir Crit Care Med19961536 Pt 1180218118665038
- KurashimaKFukudaCNakamotoKCT-diagnosed emphysema and prognosis of chronic airflow obstruction: a retrospective studyBMJ Open2013311e003541
- CosioBGSorianoJBLópez-CamposJLCHAIN StudyDefining the asthma-COPD overlap syndrome in a COPD cohortChest20161491455226291753
- KurashimaKTakayanagiNSatoNHigh resolution CT and bronchial reversibility test for diagnosing COPDRespirology200510331632215955144
- The Japanese Respiratory SocietyGuidelines for the Diagnosis and Treatment of COPD3rd edTokyoMedical Review Co., Ltd2013
- SuzukiTTadaYKawataNClinical, physiological, and radiological features of asthma-chronic obstructive pulmonary disease overlap syndromeInt J Chron Obstruct Pulmon Dis20151094795426028967
- SuzukiMMakitaHKonnoSHokkaido COPD Cohort Study InvestigatorsAsthma-like features and clinical course of COPD: an analysis from the Hokkaido COPD Cohort StudyAm J Respir Crit Care Med2016194111358136527224255
- BrightlingCEMonterioWWardRSputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomized controlled trialLancet200035692401480148511081531
- BrightlingCEMcKennaSHargadonBSputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary diseaseThorax200560319319815741434
- LeighRPizzichiniMMMorrisMMMaltaisFHargreaveFEPizzichiniEStable COPD: predicting benefit from high-dose inhaled corticosteroid treatmentEur Respir J200627596497116446316
- FattahiFten HackenNHLöfdahlCGAtopy is a risk factor for respiratory symptoms in COPD patients: results from the EUROSCOP studyRespir Res2013141023356508
- PascoeSLocantoreNDransfieldMTBarnesNCPavordIDBlood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomized controlled trialsLancet Respir Med20153643544225878028
- TakakuYKurashimaKOhtaCHow many instructions are required to correct inhalation errors in patients with asthma and chronic obstructive pulmonary disease?Respir Med201712311011528137486
- BhattSPSierenJCDransfieldMTCOPDGene InvestigatorsComparison of spirometric thresholds in diagnosing smoking related airflow obstructionThorax201469540941423525095
- Sanchez-SalcedoPZuluetaJJLung cancer in chronic obstructive pulmonary disease patients, it is not just the cigarette smokeCurr Opin Pulm Med201622434434927077725
- OelsnerECCarrJJEnrightPLPer cent emphysema is associated with respiratory and lung cancer mortality in the general population: a cohort studyThorax201671762463227048196
- HenschkeCIYipRBoffettaPI-ELCAP InvestigatorsCT screening for lung cancer: importance of emphysema for never smokers and smokersLung Cancer2015881424725698134
- ZaynagetdinovRSherrillTPGleavesLAChronic NF-kB activation links COPD and lung cancer through generation of an immunosuppressive microenvironment in the lungsOncotarget2016755470548226756215
- MacKenzieREckhardtJPrastyaniAWJapan tobacco international: to ‘be the most successful and respected tobacco company in the world’Glob Public Health201712328129928139966
- SasakiHSekizawaKYanaiMAraiHYamayaMOhruiTEffects of air pollution and smoking on chronic obstructive pulmonary disease and bronchial asthmaTohoku J Exp Med1998186315116710348212
- ChubachiSSatoMKameyamaNKeio COPD Comorbidity Research (K-CCR) GroupIdentification of five clusters of comorbidities in a longitudinal Japanese chronic obstructive pulmonary disease cohortRespir Med201611727227927492541
- BhattSPWellsJMKinneyGLCOPDGene Investigatorsβ-Blockers are associated with a reduction in COPD exacerbationsThorax201671181426283710
- LaforestLRocheNDevouassouxGFrequency of comorbidities in chronic obstructive pulmonary disease, and impact on all-cause mortality: a population-base cohort studyRespir Med2016117333927492511