Abstract
Purpose
Patients with symptomatic COPD are recommended to use inhaled bronchodilators containing long-acting muscarinic receptor antagonists (LAMAs). However, bronchodilators may cause gastrointestinal adverse effects due to anticholinergic reactions, especially in advanced-age patients with COPD. Dai-kenchu-to (TU-100, Da Jian Zhong Tang in Chinese) is the most frequently prescribed Japanese herbal Kampo medicine and is often prescribed to control abdominal bloating and constipation. The purpose of this study was to evaluate the role of Dai-kenchu-to as a supportive therapy in advanced-age patients with COPD.
Patients and methods
We used the Japanese Diagnosis Procedure Combination inpatient database and identified patients aged ≥75 years who were hospitalized for COPD exacerbation. We then compared the risk of re-hospitalization for COPD exacerbation or death between patients with and without Dai-kenchu-to using 1-to-4 propensity score matching. A Cox proportional hazards model was used to compare the two groups. We performed subgroup analyses for patients with and without LAMA therapy.
Results
Patients treated with Dai-kenchu-to had a significantly lower risk of re-hospitalization or death after discharge; the HR was 0.82 (95% CI, 0.67–0.99) in 1-to-4 propensity score matching. Subgroup analysis of LAMA users showed a significant difference in re-hospitalization or death, while subgroup analysis of LAMA non-users showed no significant difference.
Conclusion
Our findings indicate that Dai-kenchu-to may have improved the tolerability of LAMA in advanced-age patients with COPD and, therefore, reduced the risk of re-hospitalization or death from COPD exacerbation. Dai-kenchu-to may be recommended as a useful supportive therapy for advanced-age patients with COPD.
Introduction
COPD is a common disease worldwide. Both COPD and aging lead to progressive deterioration in physical activity and lung function. Physical inactivity impacts the prognosis and can cause symptoms that may induce a vicious circle in advanced-age patients with COPD.Citation1 In fact, functional constipation is one such symptom associated with physical inactivity,Citation2 and patients with COPD who develop severe exacerbation requiring hospitalization are reportedly likely to be aged ≥75 years.Citation3
Patients with COPD are prone to acute worsening in their condition; this is known as COPD exacerbation. Because COPD is not curable, pharmacotherapy for COPD primarily aims to improve symptoms and prevent exacerbations. The use of long-acting bronchodilators such as long-acting muscarinic receptor antagonists (LAMAs) and long-acting β2-agonists (LABAs) either alone or in combination is the first-line treatment for symptomatic COPD; such therapy is also effective for the prevention of COPD exacerbation.Citation4,Citation5 However, bronchodilators, particularly LAMAs, may potentially cause anticholinergic adverse events such as constipation in patients with COPD.Citation6
Recent studies have focused on the contribution of the gastrointestinal tract in patients with COPD in both the stable stateCitation7 and during exacerbation.Citation8 Dai-kenchu-to (TU-100, Da Jian Zhong Tang in Chinese), the most frequently prescribed traditional Japanese herbal Kampo medicine, activates intestinal motility.Citation9,Citation10 Dai-kenchu-to consists of natural food ingredients: Zingiberis processum rhizome, Ginseng radix, Zanthoxyli piperiti pericarpium (Chinese Da Jian Zhong Tang contains Zanthoxyli pericarpium instead), and Saccharum grano rum.Citation11 Dai-kenchu-to has been widely prescribed in patients with gastrointestinal disorders such as postoperative paralytic ileusCitation9,Citation12–Citation15 and chronic constipationCitation16,Citation17 and is referred to in the chronic constipation guideline in Japan. Dai-kenchu-to is different from laxatives in that it is taken more regularly than laxatives, which are sometimes prescribed for single use or for a short term. Advanced-age patients with COPD constitutively experiencing abdominal bloating and/or chronic constipation are likely to be treated with Dai-kenchu-to. Bridging the concept of Oriental herbal medicine (ie, modulation of the respiratory system through the maintenance of digestive function) to modern medicine is desired.
The objective of this study was to evaluate the effect of Dai-kenchu-to on COPD exacerbation in patients of advanced age with the intention to provide evidence regarding supportive therapy for advanced-age patients with COPD.
Patients and methods
Data source
For this retrospective cohort study, we used the Japanese Diagnosis Procedure Combination (DPC) database. The DPC database with outpatient data includes discharge abstracts and administrative claims data from around 250 acute-care hospitals in Japan. The DPC database includes the following data: age, sex, body height and weight (body mass index), primary and comorbid diagnosis, Barthel index at admission and discharge, smoking history, A-DROP (Age-Dehydration-Respiratory failure-Orientation disturbance-blood Pressure) score, Hugh–Jones score, information regarding operative procedures and anesthesia, discharge status, medications and treatment in both inpatient and outpatient settings, and dates of admission and discharge. All analyses and data reporting in this study complied with the Strengthening the Reporting of Observational Studies in Epidemiology guideline. This study was approved by the Institutional Review Board of The University of Tokyo, which waived the requirement for informed patient consent because of the anonymous nature of the data.
Patient selection
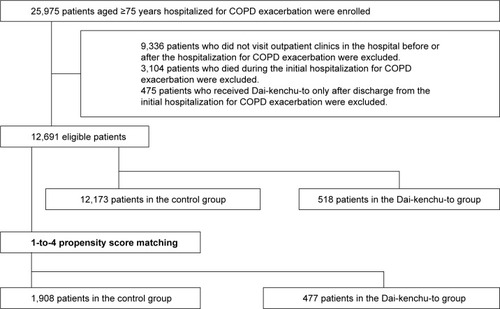
We extracted inpatient data of patients aged ≥75 years who were hospitalized for COPD exacerbation, and then extracted the outpatient data of these patients from July 2010 to March 2016. Patients with COPD were identified with the following ICD-10 codes: J41, J42, J43, J440, J441, and J449. Hospitalized patients with COPD exacerbation were identified with the following ICD-10 codes: J10, J11, J13, J14, J15, J16, J18, J20, J21, J22, J46, J170, J171, J178, J440, J441, J851, J12, A481, B012, B052, and B250. The Dai-kenchu-to group was defined as patients who received Dai-kenchu-to before or during hospitalization for COPD exacerbation. The control group comprised patients who did not receive Dai-kenchu-to throughout the observation period. Patients with no outpatient data before hospitalization, after hospitalization, or both before and after hospitalization and patients who died during the initial hospitalization for COPD exacerbation were excluded. Patients for whom Dai-kenchu-to was prescribed only after discharge from the initial hospitalization for COPD exacerbation were also excluded.
Outcomes
The outcome was re-hospitalization for COPD exacerbation or death.
Statistical analyses
We used the standardized difference to compare baseline characteristics, including comorbidities and treatments, between the Dai-kenchu-to group and the control group. Continuous variables are reported as mean ± SD. Dichotomous parameters and categorical variables are reported as frequency. Missing data for categorical variables are categorized as missing. The baseline characteristics were sex, fiscal year, season at admission, residential region, Hugh–Jones dyspnea score at admission, activities of daily living scores at admission and discharge (Barthel index), age, body mass index, and frequency of COPD exacerbations before hospitalization. COPD exacerbation before hospitalization was defined by episodes in which corticosteroids and/or antibiotics and/or anti-flu medicines were prescribed (). Comorbidities () and treatments assessed were lung cancer, other malignancy, interstitial pneumonia, bronchial asthma, bronchiectasis, bacterial lower respiratory tract infection, Mycobacterium infection, mycosis, cor pulmonale, congestive heart failure, ischemic heart disease, tachycardia, autoimmune disease, stroke, liver dysfunction, renal failure, gastroesophageal reflux disease, constipation and/or ileus, prostate hypertrophy, home ventilator support, home oxygen therapy, inhaled corticosteroids before hospitalization, long-acting musca-rinic receptor antagonists before hospitalization, LABA before hospitalization, other medications for COPD before hospitalization, ambulance transport, corticosteroid therapy during hospitalization, mechanical ventilation during hospi-talization, nasal tube feeding during hospitalization, surgery under general anesthesia during hospitalization, discharge to home, and length of stay. ICD-10 codes for comorbidities are shown in . An absolute standardized difference of >10% was considered to indicate significant imbalance.
We performed propensity score analysis to account for the differences in baseline characteristics between the two groups. The propensity scores were estimated using a multi-variable logistic regression model that included the aforementioned covariates as the dependent variables.Citation18 We performed 1-to-4 matching between the Dai-kenchu-to group and the control group. Kaplan–Meier curves were drawn, and a log-rank test was then used to compare the survival curves of the two groups. A Cox proportional hazards model was used to estimate the HR of re-hospitalization for COPD exacerbation or death. Patients who were neither re-hospitalized for COPD exacerbation nor died during the observation period were censored at the date of the last visit to each hospital.
All data were analyzed using SPSS version 23.0 (IBM Corporation, Armonk, NY, USA) and STATA software version 14.1 (StataCorp LP, College Station, TX, USA). Values of P<0.05 were considered to indicate statistical significance.
Subgroup analyses
We also performed subgroup analyses of patients with and without LAMA therapy. Patients with COPD who received either a LAMA alone or a LAMA in combination with a LABA (LABA/LAMA) before hospitalization may have been more likely to have worse symptoms and lower lung function with a higher risk of frailty and subsequent reduced gastrointestinal motility; therefore, we considered that they may benefit more from Dai-kenchu-to than patients without LAMA or LABA/LAMA therapy. Kaplan–Meier curves, a log-rank test, and a Cox proportional hazards model were used to compare the two groups.
Results
We identified 12,691 patients aged ≥75 years who were hospitalized for COPD exacerbation and had outpatient data before and after hospitalization for COPD exacerbation. Among them, Dai-kenchu-to was prescribed to 518 patients before or during hospitalization for COPD exacerbation ().
shows the baseline characteristics in the Dai-kenchu-to group and the control group. The number of patients in the Dai-kenchu-to group and control group after 1-to-4 propensity score matching was 477 and 1,908, respectively. Comorbidities during the initial hospitalization and treatments for COPD before and during hospitalization are shown in . Malignancies other than lung cancer and constipation and/or ileus were more common in the Dai-ken-chu-to group than the control group. Medications for COPD, including LAMAs, were more frequently administered in the Dai-kenchu-to group. After 1-to-4 propensity score matching, the distributions of most covariates were well balanced between the two groups.
Table 1 Baseline characteristics of patients with COPD aged ≥75 years with and without Dai-kenchu-to, before and after 1-to-4 propensity score matching
The median observation period after discharge from the first hospitalization for COPD exacerbation was 303 days (IQR, 104–594 days) in the Dai-kenchu-to group and 308 days (IQR, 100–678 days) in the control group. During the observation period, 127 (24.5%) and 3,379 (27.8%) patients were either re-hospitalized for COPD exacerbation or died in the Dai-kenchu-to group and control group, respectively.
Propensity scores were calculated using all the variables listed in and . After propensity score matching, the numbers of patients who were either re-hospitalized for COPD exacerbation or died during the observational period were 116 (24.3%) and 536 (28.2%) in the Dai-kenchu-to group and the control group, respectively.
Table 2 Comorbidities during hospitalization and treatments for COPD in patients aged ≥75 years with and without Dai-kenchu-to
and show the results of the survival analyses after 1-to-4 propensity score matching. The log-rank test showed a statistically significant difference (P=0.050) with better survival in the Dai-kenchu-to group (). In the analyses using the Cox proportional hazard model, the Dai-kenchu-to group showed a significantly reduced risk of re-hospitalization or death in the 1-to-4 propensity score analysis (HR, 0.82; 95% CI, 0.67–0.99; P=0.050), as shown in .
Figure 2 Kaplan–Meier survival curves of patients with COPD with or without Dai-kenchu-to after hospitalization for COPD exacerbation in the 1-to-4 propensity score-matched population. (A) Results for patients aged ≥75 years. (B) Results for patients with LAMA therapy. (C) Results for patients without LAMA therapy before hospitalization. The Kaplan–Meier curves were compared with a log-rank test. A P-value of <0.05 indicated statistical significance.
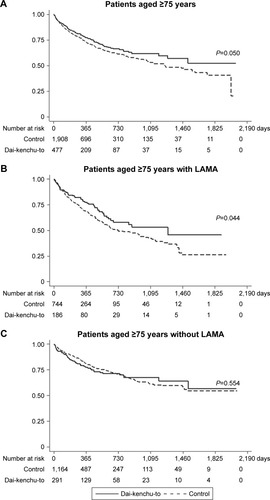
Table 3 HRs of re-hospitalization for COPD exacerbation or death after 1-to-4 propensity score matching in the Dai-kenchu-to group vs control group
In the subgroup analysis of patients with LAMA therapy, a significant difference in re-hospitalization or death was observed between the two groups (). Among patients without LAMA therapy, no significant difference in re-hospitalization or death was observed between the two groups ().
Discussion
This study showed a reduced risk of re-hospitalization for COPD exacerbation or death in the Dai-kenchu-to group vs control group among ≥75-year-old patients with COPD, using 1-to-4 propensity score matching. The subgroup analysis of patients without LAMA therapy showed no significant difference in the outcome between the Dai-kenchu-to users and non-users. Our findings indicate that Dai-kenchu-to may reduce the risk of severe COPD exacerbation requiring hospitalization, particularly in advanced-age patients with COPD, possibly by improving treatment adherence in LAMA users. Our study is the first to evaluate the relationship between Dai-kenchu-to (TU-100) and COPD. We also attempted to provide a bridge between the concept of traditional Oriental herbal medicine and modern medicine.
We were not able to clearly show the mechanisms that explain our findings in the present study. Despite numerous studies demonstrating the effect and mechanismCitation9–Citation17 of Dai-kenchu-to in the gut, no studies have yet evaluated the additional benefits of Dai-kenchu-to in the organs outside the digestive system. However, there are several possible explanations for why Dai-kenchu-to was preferable in advanced-age patients with COPD, especially in LAMA users. Improved gut motility is a major effect of Dai-kenchu-to, and this can be a plausible mechanism for reducing exacerbation in advanced-age patients with COPD. In general, patients of advanced age are susceptible to abdominal bloating and/or constipation.Citation19–Citation21 Patients aged >75 years using LAMAs are likely to have an advanced stage of COPD with a higher risk of frailty and reduced gastrointestinal motility than those not using LAMAs, which may explain their response to the treatment with Dai-kenchu-to. Moreover, such patients are vulnerable to adverse drug reactions,Citation22 which may also be true of LAMAs. Dai-kenchu-to may show improved patient adherence to long-acting bronchodilators, particularly advanced-age patients with COPD experiencing abdominal bloating and/or constipation, resulting in a lower risk of re-hospitalization.
Furthermore, because the thoracic lumen shares the diaphragm with the abdominal lumen, the diaphragmatic response to abdominal loading inevitably affects the lung function, particularly in advanced-age patients with COPD whose costal wall is unlikely to further expand horizontally to compensate for the limitation in vertical extension of the lung.Citation23 In fact, diaphragmatic motion during tidal breathing is reportedly greater in patients with than without COPD, suggesting a pivotal role of diaphragmatic motion in compensation for breathlessness.Citation24 Abdominal bloating and/or constipation may interfere with diaphragmatic motion and may aggravate symptoms in advanced-age patients with COPD, resulting in reduced physical activity and thereby increasing the risk of COPD exacerbation.Citation25 Further studies are required to assess whether the effect of Dai-kenchu-to simply relies on the improvement of gut motility.
Intestinal hyperpermeability following splanchnic hypoxia, possibly caused by blood flow redistribution, may lead to increased bacterial translocation in patients with COPD.Citation7,Citation8 Dai-kenchu-to in both animalsCitation26 and humansCitation27 increases intestinal blood flow. A protective effect of Dai-kenchu-to against bacterial translocation has been shown in rats.Citation28–Citation30 Dai-kenchu-to may have reduced the risk of COPD exacerbation in older patients in the present study by preserving intestinal blood flow and preventing bacterial translocation.
In addition, disruption of the microbiome in patients with lung disease, including COPD, has been reviewed,Citation31 and the modulatory effect of Dai-kenchu-to on the microbiome has been studied.Citation32,Citation33 In one study, Dai-kenchu-to preserved the diversity of the microbiome in stressed mice. In another study, it reshaped the proportion of the microbiome with increased short-chain fatty acids in rats. The increased short-chain fatty acids reportedly suppressed inflammation in the lungs and were protective in a mouse model of elastase-induced emphysema.Citation34 Moreover, short-chain fatty acids have been implicated in the immune responseCitation35 and non-eosinophilic hyperresponsiveness in the airway.Citation36 The modulatory effect of Dai-kenchu-to on the microbiome in the gut may serve as a basis for further exploration of the mechanism of Dai-kenchu-to in advanced-age patients with COPD.
The capability of Dai-kenchu-to to activate transient receptor potential ankyrin (TRPA) channels or transient receptor potential vanilloid (TRPV) channels is also of interest.Citation11 Activation of TRPA and TRPV channels on vagal sensory nerves in the airways initiates the cough reflex, which is considered to be a therapeutic target when excessive in disease states.Citation37 The protective effect of cough (ie, clearing harmful particles from the airway) is also acknowledged, particularly in stable states. However, whether Dai-kenchu-to can activate TRPA or TRPV channels in the airway has not been demonstrated. Therefore, further in vivo studies are required.
Several limitations of this study should be acknowledged. First, patients who switched hospitals during the study period and patients whose outpatient care hospital differed from the hospital for hospitalization were not captured. This may be a potential source of confounding. Second, the database does not contain data for each patient’s pulmonary function tests and symptoms. We, therefore, evaluated medications for COPD in the stable states (before hospitalization) as a surrogate for pulmonary function tests and symptoms. Third, factors such as the current smoking status and actual baseline physical activity, both of which may affect the outcomes, were not recorded in the DPC database. Fourth, in our propensity score-matching analysis, some variables such as residential regions, Hugh–Jones dyspnea, and activities of daily living at discharge were not well balanced. However, we performed a reanalysis with these three variables included as independent variables in the Cox regression model after 1-to-4 propensity score matching and obtained a similar result (HR, 0.81; 95% CI, 0.66–0.99; P=0.039).
Conclusion
Our study suggests that Dai-kenchu-to may reduce the risk of re-hospitalization for COPD exacerbation, most likely by improving the adherence to LAMAs in advanced-age patients with COPD. Prospective studies to confirm our findings and to elucidate the mechanisms of the protective effect of Dai-kenchu-to in advanced-age patients with COPD are needed.
Author contributions
TJ: study design, data analysis, data interpretation, and manuscript preparation. NM: data analysis, data interpretation, and manuscript preparation. HY: data analysis, data interpretation, and manuscript preparation. YS: data analysis and data interpretation. HM: data collection, data analysis, and data interpretation. HU: study design and data interpretation. AM: study design and data interpretation. YY: study design and data interpretation. KF: data collection and data interpretation. HY: study design and data interpretation. YH: study design, data interpretation, and manuscript preparation. All authors contributed to data analysis, draft or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by grants from the Ministry of Health, Labor and Welfare, Japan (H30-Policy-Designated-001, H30-Policy-Designated-004, and H29-ICT-Genral-004) and the Ministry of Education, Culture, Sports, Science and Technology, Japan (17H04141). The funding bodies had no role in the design of the study; collection, analysis, or interpretation of the data; or writing of the manuscript.
Supplementary materials
Table S1 List of drugs used to treat COPD and drugs used to define COPD exacerbation in outpatient settings
Table S2 ICD-10 codes used to identify comorbidities
Disclosure
TJ (corresponding author), NM, and HY have received financial contributions from Tsumura & Company (Tokyo, Japan) because they have academic affiliations with the Department of Health Services Research, Graduate School of Medicine, The University of Tokyo supported by Tsumura & Company. Tsumura & Company played no role in this study. The corresponding author also receives research funding from Tsumura & Company for research not related to this study. The authors report no other conflicts of interest in this work.
References
- JonesPWWatzHWoutersEFCazzolaMCOPD: the patient perspectiveInt J Chron Obstruct Pulmon Dis201611Spec Iss1320
- GeorgeSEBorello-FranceDFPerspective on physical therapist management of functional constipationPhys Ther201797447849327634920
- McGarveyLLeeAJRobertsJGruffydd-JonesKMcKnightEHaughneyJCharacterisation of the frequent exacerbator phenotype in COPD patients in a large UK primary care populationRespir Med2015109222823725613107
- GOLD reports 2018: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease Available from: https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdfAccessed December 19, 2018
- RabeKFWatzHChronic obstructive pulmonary diseaseLancet2017389100821931194028513453
- HanlonPNichollBIJaniBDExamining patterns of multimorbidity, polypharmacy and risk of adverse drug reactions in chronic obstructive pulmonary disease: a cross-sectional UK Biobank studyBMJ Open201881e018404
- RuttenEPALenaertsKBuurmanWAWoutersEFMDisturbed intestinal integrity in patients with COPD: effects of activities of daily livingChest2014145224525223928850
- SprootenRTMLenaertsKBraekenDCWIncreased small intestinal permeability during severe acute exacerbations of COPDRespiration201895533434229393240
- AkamaruYTakahashiTNishidaTEffects of daikenchuto, a Japanese herb, on intestinal motility after total gastrectomy: a prospective randomized trialJ Gastrointest Surg201519346747225564322
- KawasakiNNakadaKSuzukiYFurukawaYHanyuNKashiwagiHEffect of Dai-kenchu-to on gastrointestinal motility and gastric emptyingInt J Surg20097321822219371795
- KonoTShimadaMYamamotoMComplementary and synergistic therapeutic effects of compounds found in Kampo medicine: analysis of DaikenchutoFront Pharmacol201567315926300774
- YasunagaHMiyataHHoriguchiHKuwabaraKHashimotoHMatsudaSEffect of the Japanese herbal Kampo medicine Dai-kenchu-to on postoperative adhesive small bowel obstruction requiring long-tube decompression: a propensity score analysisEvid Based Complement Alternat Med2011201126428921584269
- IshizukaMShibuyaNNagataHPerioperative administration of traditional Japanese herbal medicine Daikenchuto relieves postoperative ileus in patients undergoing surgery for gastrointestinal cancer: a systematic review and meta-analysisAnticancer Res201737115967597429061775
- KatsunoHMaedaKOhyaMClinical pharmacology of Daiken-chuto assessed by transit analysis using radiopaque markers in patients with colon cancer undergoing open surgery: a multicenter double-blind randomized placebo-controlled study (JFMC39-0902 additional study)J Gastroenterol201651322222926162646
- KaidoTShimamuraTSugawaraYMulticentre, randomised, placebo-controlled trial of extract of Japanese herbal medicine Dai-kenchuto to prevent bowel dysfunction after adult liver transplantation (DKB 14 Study)BMJ Open201559e008356
- TsudaHKotaniTSumigamaSManoYHayakawaHKikkawaFEfficacy and safety of Daikenchuto (TJ-100) in pregnant women with constipationTaiwan J Obstet Gynecol2016551262926927243
- YukiMKomazawaYKobayashiYEffects of Daikenchuto on abdominal bloating accompanied by chronic constipation: a prospective, single-center randomized open trialCurr Ther Res Clin Exp201577586227069528
- RosenbaumPRRubinDBConstructing a control group using multivariate matched sampling methods that incorporate the propensity scoreAm Stat1985393338
- Fávaro-MoreiraNCKrausch-HofmannSMatthysCRisk factors for malnutrition in older adults: a systematic review of the literature based on longitudinal dataAdv Nutr20167350752227184278
- BailesKReeveKConstipation in older adultsNurse Pract201338382125
- GauJTAcharyaUHKhanMSKaoTCRisk factors associated with lower defecation frequency in hospitalized older adults: a case control studyBMC Geriatr20151514425887756
- WootenJMAdverse drug reactions: part ISouth Med J20101031025102820818299
- BarbaEQuirogaSAccarinoAMechanisms of abdominal distension in severe intestinal dysmotility: abdominothoracic response to gut retentionNeurogastroenterol Motil2013256e389e39423607758
- YamadaYUeyamaMAbeTDifference in diaphragmatic motion during tidal breathing in a standing position between COPD patients and normal subjects: time-resolved quantitative evaluation using dynamic chest radiography with flat panel detector system (“dynamic X-ray phrenicography”)Eur J Radiol201787768228065378
- KatajistoMKoskelaJLindqvistAKilpeläinenMLaitinenTPhysical activity in COPD patients decreases short-acting bronchodilator use and the number of exacerbationsRespir Med2015109101320132526298024
- KonoTOmiyaYHiraYDaikenchuto (TU-100) ameliorates colon microvascular dysfunction via endogenous adrenomedullin in Crohn’s disease rat modelJ Gastroenterol20114646111871196
- TakayamaSSekiTWatanabeMThe herbal medicine Dai-kenchuto increases blood flow in the superior mesenteric arteryTohoku J Exp Med2009219431933019966532
- TakasuCYismawWGKuritaNTU-100 exerts a protective effect against bacterial translocation by maintaining the tight junctionSurg Today201747101287129428421347
- YadaKIshibashiHMoriHThe Kampo medicine “Daikenchuto (TU-100)” prevents bacterial translocation and hepatic fibrosis in a rat model of biliary atresiaSurgery201615961600161126994485
- YoshikawaKKuritaNHigashijimaJKampo medicine “Dai-kenchu-to” prevents bacterial translocation in ratsDig Dis Sci20085371824183118446437
- ShuklaSDBuddenKFNealRHansbroPMMicrobiome effects on immunity, health and disease in the lungClin Transl Immunology201763e13328435675
- YoshikawaKShimadaMKuwaharaTEffect of Kampo medicine “Dai-kenchu-to” on microbiome in the intestine of the rats with fast stressJ Med Invest2013603–422122724190039
- HasebeTUenoNMuschMWDaikenchuto (TU-100) shapes gut microbiota architecture and increases the production of ginsen-oside metabolite compound KPharmacol Res Perspect201641e0021526977303
- TomodaKKuboKDairikiKWhey peptide-based enteral diet attenuated elastase-induced emphysema with increase in short chain fatty acids in miceBMC Pulm Med20151516426059026
- TrompetteAGollwitzerESYadavaKGut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesisNat Med201420215916624390308
- ChoYAbu-AliGTashiroHThe microbiome regulates pulmonary responses to ozone in miceAm J Respir Cell Mol Biol201859334635429529379
- BonviniSJBirrellMASmithJABelvisiMGTargeting TRP channels for chronic cough: from bench to bedsideNaunyn Schmiedebergs Arch Pharmacol2015388440142025572384