Abstract
Background
Etiologies of acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are heterogeneous. We phenotyped severe AECOPD based on molecular pathogen detection of sputum samples collected at hospitalization of COPD patients and determined their outcomes.
Methods
We phenotyped 72 sputum samples of COPD patients who were hospitalized with a primary diagnosis of AECOPD using a molecular array that detected common bacterial and viral respiratory pathogens. Based on these results, the patients were classified into positive or negative pathogen groups. The pathogen-positive group was further divided into virus or bacteria subgroups. Admission day 1 blood samples were assayed for N-terminal prohormone brain natriuretic peptide, CRP, and complete blood counts.
Results
A total of 52 patients had a positive result on the array, while 20 patients had no pathogens detected. The most common bacterial pathogen detected was Haemophilus influenzae and the most common virus was rhinovirus. The pathogen-negative group had the worse outcomes with longer hospital stays (median 6.5 vs 5 days for bacteria-positive group, P=0.02) and a trend toward increased 1-year mortality (P=0.052). The bacteria-positive group had the best prognosis, whereas the virus-positive group had outcomes somewhere in between the bacteria-positive and pathogen-negative groups.
Conclusion
Molecular diagnostics on sputum can rapidly phenotype serious AECOPD into bacteria-, virus-, or pathogen-negative groups. The bacteria-positive group appears to have the best prognosis, while pathogen-negative group has the worst. These data suggest that AECOPD is a heterogeneous event and that accurate phenotyping of AECOPD may lead to novel management strategies that are personalized and more precise.
Introduction
Acute exacerbations of COPD (AECOPD) are caused by a variety of etiological factors.Citation1 In AECOPD, the major drivers are respiratory tract infections; however, in roughly 30% of cases no clear inciting factor is found.Citation2 An autopsy study of COPD patients who died within 24 hours of hospital admission due to AECOPD demonstrated that acute heart failure and pulmonary embolism were common primary causes of death.Citation3 These data highlighted the importance of phenotyping AECOPD to target and treat underlying causes of AECOPD. According to the latest GOLD document,Citation4 sputum cultures are generally not considered useful for guiding initial antibiotic choice or in phenotyping AECOPD. This is because sputum cultures have relatively poor sensitivity in identifying respiratory pathogens and determining therapeutic responsiveness to antimicrobials.Citation5 Therefore, AECOPD are empirically treated with antibiotics and/or systemic corticosteroids, irrespective of the microbial or nonmicrobial drivers.Citation4 The advent of molecular diagnostics in sputum has significantly increased the sensitivity of pathogen detection compared with traditional culture-based methods. However, its role in the prognosis or management of AECOPD has not been well defined.
In this study, our primary aim was to phenotype severe AECOPD by using a molecular pathogen-detection method. We hypothesized that a nucleic acid-based assay for detecting pathogens in sputum would enable classification of AECOPD into viral, bacterial, and uninfectious groups, which in turn would be associated with different health outcomes.
Methods
Study patients
This study consisted of patients who were able to provide adequate sputum samples in the COPD Rapid Transition Program. This program’s cohort has been described in detail previously.Citation6,Citation7 In brief, all patients included in this study were hospitalized with a confirmed primary diagnosis of AECOPD by board-certified general internists or pulmonologists who cared for these patients. All diagnoses were validated through a detailed chart review by at least one additional pulmonologist using the criteria recommended by the GOLD committee.Citation4 Samples were classified as being adequate sputum samples based on color, transparency, and viscosity by study personnel, who were blinded to characteristics of the study patients. All patients received standard antiexacerbation therapy, including prednisone and antibiotics. The study is registered with ClinicalTrials.gov (NCT2050022, registered January 28, 2014). The study was approved by the University of British Columbia Providence Health Care Research Ethics Board (certificate H11-00786) for patients enrolled at St Paul’s Hospital, Vancouver, Canada and the University of British Columbia Clinical Research Ethics Board (certificate H13-00790) for patients enrolled at Vancouver General Hospital, Vancouver, Canada.
Specimens and measurement technique
Following receipt of written informed consent from patients, blood samples were collected in PaxGene, EDTA, and serum tubes on days 1 and 3 of hospitalization, at discharge, and on days 30 and 90 postdischarge. Blood components were processed as per standardized protocol and stored at −80°C until analysis. N-terminal prohormone brain natriuretic peptide (NT-proBNP), CRP, and complete blood count and differentials were measured using standard techniques (Supplementary material).
Spontaneously expectorated sputum samples were collected on day 1 in OmniGene oral (OM505) tubes and stored in −80°C freezers until measurement. The tubes were thawed in a hot bath at 50°C for 1 hour and then placed in an air incubator at 24°C for 30 minutes. Next, Sputolysin was added in a 1:1 ratio to liquefy the samples, after which samples were subaliquoted into 500 μL volumes and stored at −80°C. Bacterial load was quantified (Supplementary material), and for detecting pathogenic microorganisms, the Randox Respiratory Multiplex Array II was used. This array detects 22 common bacterial and viral pathogens within 6 hours using nucleic acids extracted from sputum samples (Supplementary material). In brief, the assay combines multiplex PCR and biochip-array hybridization. Baseline lung-function measurements were performed at the time of convalescence (ie, at day 30 or day 90) for AECOPD patients. Spirometry was used to obtain lung-function parameters after bronchodilator administration during clinical stability according to recommendations from American Thoracic Society–European Respiratory Society guidelines.Citation8
Statistical analysis
Continuous variables that were normally distributed are reported as mean ± SD, abnormally distributed variables as medians and IQR, and categorical variables as percentages. Continuous variables that were not normally distributed were log10-transformed prior to application of a parametric test where appropriate. Student’s t-test and Mann–Whitney–Wilcoxon tests were used to determine differences between the pathogen-negative and pathogen-positive groups, and ANOVA and Kruskal–Wallis tests were used to determine differences between pathogen-negative, virus, and bacteria groups. Fisher’s exact test was used to test for differences in categorical variables between the groups. Comparisons of 1-year mortality rate across groups were analyzed by Kaplan–Meier survival curves using a log-rank test, and a Cox proportional-hazard model adjusted for age and sex was used to calculate HRs. Statistical tests were two-sided, and significance was assigned to results with P<0.05.
Ethics approval and consent to participate
The study is registered with ClinicalTrials.gov (NCT2050022, registered January 28, 2014). The study was approved by the University of British Columbia Providence Health Care Research Ethics Board (certificate H11-00786) for patients enrolled at St Paul’s Hospital, Vancouver, Canada and the University of British Columbia Clinical Research Ethics Board (certificate H13-00790) for patients enrolled at Vancouver General Hospital, Vancouver, Canada. Written informed consent was obtained from all enrolled patients. The study was conducted in accordance with the Declaration of Helsinki.
Results
Patient characteristics
Demographic and clinical data for the 72 patients studied are displayed in . Patients had a mean age of 65.8±11.5 years, 63.9% were male, 80.6% were Caucasian, and 61.1% were current smokers. All patients had airflow limitation, with mean FEV1 of 46.6%±16.7% predicted, and 38.9% had a history of cardiac comorbidities (heart failure, coronary artery disease, myocardial infarction, and arrhythmia).
Table 1 Demographic and clinical characteristics of study patients
Pathogens detected
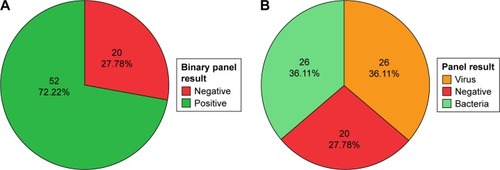
Pathogens that were detected in our 72-patient cohort are presented in . The most common pathogen was Haemophilus influenzae, accounting for 33.7% of all pathogens detected. Rhinovirus was the most common virus detected, accounting for 13.3% of all pathogens detected. Details on the pathogens detected for each patient are provided in the Supplementary material.
Table 2 Pathogens detected on the panel
Phenotyping COPD exacerbations
Twenty of 72 patients (27.8%) had a negative result on the array. Nucleic acid concentrations were similar between samples that did and did not have a positive result on the array (P=0.71, Supplementary material). Patients with a negative result on the array were considered to have had an uninfectious exacerbation, whereas those who had a positive result on the array were considered to have had an infectious exacerbation. We further subdivided the patients with an infectious exacerbation into either a bacteria- or a virus-associated exacerbation based on the results. The virus group consisted of those who had a virus identified on the array, with or without detection of bacteria ().
Figure 1 AECOPD-phenotype pie charts.
Abbreviation: AECOPD, acute exacerbations of chronic obstructive pulmonary disease.
Demographics and clinical data for AECOPD exacerbations
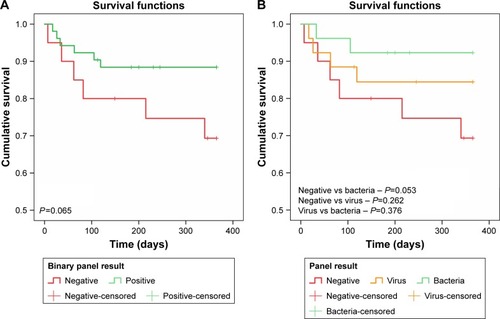
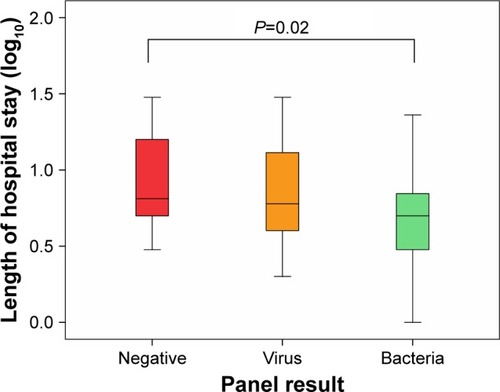
Demographic and clinical data for the groups are listed in the Supplementary material. There were no statistically significant differences between the pathogen-negative and pathogen-positive groups in terms of age, sex, cardiac comorbidities, inhaled corticosteroid use, or baseline FEV1% predicted. Of note, the pathogen-positive group was less likely to be on home (domiciliary) oxygen therapy (35% in the negative group and 14% in the positive group), but this comparison did not reach statistical significance (P=0.094). All hospitalizations were right-censored at 30 days, as hospital stays beyond this time frame were likely driven by factors other than AECOPD. There were no significant differences in length of hospital stay between the pathogen-negative and the pathogen-positive groups (P=0.096). However, a subgroup analysis revealed that there were significant differences in length of hospital stay across the bacteria, virus, and pathogen-negative groups (P=0.046 for overall ANOVA). These differences were largely driven by the comparison between the bacteria-associated AECOPD group and the pathogen-negative group (P=0.02 on post hoc analysis with Fisher’s least-significant-difference test). Consistent with this analysis, there was a significant trend in length of hospitalization across the three groups, with the pathogen-negative group having the longest stay and the bacteria group having the shortest (Ptrend=0.017, ).
Figure 2 Box plots depicting length of hospital stay in the three groups.
NT-proBNP, CRP, complete blood count, and bacterial load
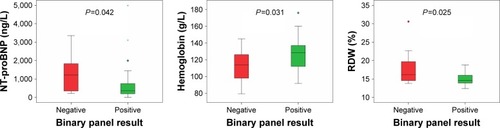
Concentrations of NT-proBNP, CRP, and complete blood counts for each of the groups are provided in the Supplementary material. We examined NT-proBNP, CRP, and complete blood counts at the date of hospital admission for all patients. The pathogen-negative group had significantly higher NT-proBNP concentrations (P=0.042), lower concentrations of hemoglobin (P=0.031), and higher red-blood-cell distribution width (P=0.025) compared with the pathogen-positive group (). Subgroup analyses demonstrated a statistically significant difference between the pathogen-negative and virus groups for red-blood-cell distribution width (P=0.046 on overall Kruskal–Wallis test, Bonferroni-adjusted P=0.04 on post hoc pairwise comparison between the pathogen-negative and virus groups). There was no statistically significant difference among the three groups for NT-proBNP concentrations (P=0.081), but there was a significant trend in NT-proBNP concentrations across the three groups (P=0.029 on Jonckheere–Terpstra test for trend), with the pathogen-negative group having the highest concentrations and the bacteria group having the lowest (Supplementary material). Bacterial load measured by droplet digital PCR is shown in the Supplementary material. There was no statistically significant difference between the pathogen-negative and -positive groups (P=0.503) or the virus and other groups (P=0.625). Of note, we had day 30 follow-up NT-proBNP levels in 23 patients, which showed that subjects in the negative group had a median decrease in NT-proBNP levels of 1,102 (61−1,768) ng/L, while the positive group had a median decrease of 127.5 (30−458) ng/L. However, the results did not reach statistical significance, most likely due to the small sample.
Figure 3 Box plots depicting the significantly different variables between groups.
Abbreviations: NT-proBNP, N-terminal prohormone brain natriuretic peptide; RDW, red-blood-cell distribution width. *Represents an extreme outlier, >3× the IQR from a quartile. °Outliers with values between 1.5–3 box lengths from the upper or lower edge of the boxplot.
One-year mortality
Of the 72 patients included in our study, 12 died within 1 year of follow-up (). On Kaplan–Meier survival analysis, there were no statistically significant differences between the pathogen-negative and -positive groups (P=0.065, ). There were no statistically significant differences in survival between the virus and pathogen-negative groups (P=0.262), or between the virus and bacteria groups (P=0.376). However, there was moderate evidence of a difference in survival between the pathogen-negative and bacteria groups, which did not reach the predefined level of statistical significance (P=0.053, ). Across the three groups, there was a trend toward increased mortality in the pathogen-negative group compared with the bacteria group (P=0.052). On the Cox proportional-hazard model adjusting for age and sex (), the pathogen-negative group was approximately 4.7 times more likely to die than the bacteria group during the 1-year follow-up period (HR 4.69, 95% CI 0.92–23.8).
Table 3 Survival characteristics of AECOPD phenotypes
Table 4 Cox proportional-hazard model comparing the pathogen-negative, virus, and bacteria groups
Discussion
To our knowledge this is the first study to examine the utility of a molecular method for pathogen detection in AECOPD for the purpose of phenotyping exacerbations. Here, we showed that one in four patients hospitalized for AECOPD did not have detectable (common) respiratory pathogens in their sputum, one in three demonstrated a potential viral pathogen, and the rest had potential bacterial pathogens detected in their sputum. Most importantly, we showed that patients with a negative sputum-pathogen-array test had worse outcomes, including longer stays in hospital and a trend toward increased 1-year mortality. Interestingly, the group that demonstrated only bacteria in their sputum had the best outcomes, including the shortest hospital stay.
In this study, we demonstrated the feasibility of using molecular pathogen-detection methods that have been proven to be more sensitive in detecting viral pathogens than culture and serology methods in patients with serious AECOPD.Citation9 Multiple studies have shown that molecular methods have higher sensitivity in detecting pathogens compared to traditional culture-based methods.Citation10–Citation12 Moreover, molecular methods enable simultaneous detection of multiple microorganisms, including both viral and bacterial pathogens, from a single clinical specimen. This approach, known as multiplexing, is increasingly being utilized for the diagnosis of a variety of different infectious diseases, and currently, there are multiple US Food and Drug Administration-approved panels designed to aid in the diagnosis of respiratory, gastrointestinal, and central nervous system infections.Citation13 A strength of our study is that we chose a clinically approved panel that detects common respiratory pathogens. This enabled us to use a single clinical specimen, in contrast to other studies, which have used different tests and different sampling sites (ie, nasopharyngeal swabs for viruses, blood and urine samples for atypical bacteria, and sputum for typical bacteria).Citation14–Citation17 The pathogens detected in our study are consistent with the published literature on AECOPD in terms of type and prevalence.Citation18
The current paradigm of AECOPD pathogenesis suggests that roughly 80% of cases are infectious in origin,Citation15,Citation19 with a third being caused by virusesCitation20 and the remainder being attributed to multiple uninfectious etiologies. Uninfectious exacerbations in COPD are frequently attributed to heart failure,Citation21 atrial fibrillation,Citation22 gastroesophageal reflux disease,Citation23 and acute pulmonary embolism.Citation24 Here, we showed that patients with a negative result on the array had higher NT-proBNP concentrations, which suggests that these patients may have experienced acute cardiac dysfunction.Citation25,Citation26 This raises the possibility that at least in a subset of patients with serious AECOPD, cardiac dysfunction may play a significant role in their AECOPD.
We did not observe any significant differences in CRP concentrations across groups. Several explanations are possible for this observation. First, we had a relatively small sample, which may have limited our ability to detect statistically significant differences in CRP concentrations. Second, the array does not cover all the bacterial organisms that are associated with AECOPD. Most notably, Staphylococcus aureus and Pseudomonas aeruginosa are not on this array. Therefore, patients with these infectious organisms may have been overlooked and included in the pathogen-negative group. We did not find any significant differences in CRP concentrations between viral and bacterial groups either, which have been previously noted by other groups.Citation27,Citation28
In AECOPD, there are specific treatments for bacterial infections that have very high cure rates, while in the case of viral infections, influenza is the only respiratory virus that has an available treatment.Citation29 We showed here that patients who had only bacteria detected in their sputum had the shortest hospitalization, while interestingly patients who had a negative result on the array had the longest hospitalization. Previous studies have shown that patients with a high burden of comorbidities, such as anemia, have longer hospitalizations for AECOPD, independently of age, sex, or FEV1.Citation30 Similarly, those who have elevated NT-proBNP concentrations on admission also experience longer hospitalizations for their AECOPD.Citation6
Interestingly, we observed a trend toward a higher 1-year mortality rate in patients who were part of the pathogen-negative group compared to the bacteria group. The pathogen-negative group was fourfold more likely to die within 1 year of follow-up than the bacteria group. The most common causes of death in COPD patients according to death-certificate data are cardiac diseases,Citation31 and elevated NT proBNP concentrations are strongly associated with mortality in AECOPD,Citation6,Citation32 which might explain the increased mortality rate observed in patients who had a negative result on the array.
There are several limitations to our study and studies utilizing molecular methods of pathogen detection in general. First, we used a qualitative diagnostic method, and colonization could have led to false-positive results.Citation33 Colonization rates may also be increased in those with concomitant bronchiectasis, though in our study all patients had chest imaging and those with significant bronchiectasis were excluded from the study (data not shown). Second, we used sputum samples to phenotype exacerbations. However, a sputum sample does not necessarily represent the whole lung. Moreover, there are regional differences in detection rates of bacterial pathogens within the same lung.Citation34,Citation35 Third, our study was a retrospective study in which stored sputum samples in −80°C were tested for respiratory pathogens, and the effects of prolonged storage at low temperatures on microbial pathogen detection have not been systematically studied.Citation36 However, in a study that examined the ability of the Xpert Mycobacterium tuberculosis–resistance to rifampicin assay to detect M. tuberculosis in sputum samples that had been stored in −80°C freezers for up to 4 years, the assay showed sensitivity of 95.7%, which was within the range reported in fresh samples.Citation37 Another study that examined microbial communities in stored bronchoalveolar lavage samples for cystic fibrosis patients in −80°C for >5 years showed results that were consistent with historical culturing results.Citation38 Fourth, a limitation of the array is that it does not cover all pathogenic organisms implicated in AECOPD, and of these, P. aeruginosa and S. aureus are the most notable. For Staphylococcus, as with other organisms that possess thick cell walls, specialized DNA-extraction methods are required.Citation39–Citation42 If these methods are used, it has been shown that increased detection of Staphylococcus comes at the expense of microbial organisms with a fragile cell wall and viruses.Citation43,Citation44 Currently, there is no commercial nucleic acid-extraction kit that can simultaneously extract Gram-positive, Gram-negative, and viruses from the same sample. Kajiura et alCitation44 proposed a method for achieving this goal through techniques that prevent the loss of smaller viral particles while still extracting Gram-positive bacteria. This has been successfully used on 300 archived clinical samples of respiratory origin. This method appears promising, and if externally and prospectively validated, it could become the gold standard for nucleic acid extraction for molecular pathogen detection. Fifth, we did not have data on whether the subjects had received antibiotics or systemic steroids prior to presenting to our emergency department. Sixth, the small sample of the study may be a potential reason for not uncovering statistically significant outcomes. Lastly, we did not have echocardiography data to confirm with the elevated NT-proBNP concentrations the evidence of cardiac dysfunction in the pathogen-negative group.
Conclusion
Comprehensive molecular methods of pathogen detection are soon to be considered a cornerstone for diagnosing respiratory infectious diseases. Molecular diagnostic methods are significantly faster and more sensitive than culture methods, and not affected by prior antibiotic use to the same extent as culture methods. One important aspect is that these methods are not only capable of simultaneously detecting a wide gamut of different pathogens (bacteria, viruses, and fungi) but also are capable of simultaneously and accurately detecting antibiotic-resistance genes.Citation45 We show here that a commercially available respiratory multiplex array could aid in phenotyping AECOPD into infectious or uninfectious exacerbations. These results are encouraging to explore further and develop better molecular pathogen-detection panels that possess broader pathogen coverage and address the issue of colonization. Prospectively examining respiratory multiplex arrays in AECOPD that requires hospital admission and assessing the point-of-care advantages that these panels might possess would be the logical next step to validate their value in the utilization of hospital resources and clinical outcomes.
Availability of data and materials
Data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
NMA had full access to all the data in the study, takes responsibility for the integrity of the data and the accuracy of the data analysis, and contributed to drafting the manuscript. DDS contributed substantially to the study design, data analysis and interpretation, and had authority over manuscript preparation and the decision to submit the manuscript for publication. CJH interpreted the radiological studies. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
We would like to thank all patients for their participation and Sara Assadian, Roxanne Rousseau, and Breanne Crouch for their roles in patient recruitment for the study and collecting and entering clinical data, and Sheena Tam, Grace Lee, Yeni Oh, and David Ngan for processing samples, as well as Meghan McLennan and Jackson Wu for performing clinical laboratory analyses. An abstract of this paper was presented at the 2018 American Thoracic Society International Conference as a poster discussion with interim findings. The poster’s abstract was published in the 2018 abstract issue of the American Journal of Respiratory and Critical Care Medicine (https://www.atsjournals.org/doi/abs/10.1164/ ajrccm-conference.2018.197.1_MeetingAbstracts.A2749). The study was funded by Genome Canada, Genome British Columbia, Genome Quebec, Canadian Institutes of Health Research, Providence Health Care, St Paul’s Hospital Foundation, and the PROOF Center.
Disclosure
DDS reports grants and personal fees from AstraZeneca and Boehringer Ingelheim, grants from Merck Frosst, and personal fees from Novartis, Regeneron, and Sanofi Aventis outside the submitted work. JAL reports grants from GE Healthcare outside the submitted work. The authors report no other conflicts of interest in this work.
References
- PavordIDJonesPWBurgelPRRabeKFExacerbations of COPDInt J Chron Obstruct Pulmon Dis201611Spec Iss213026937187
- ConnorsAFDawsonNVThomasCOutcomes following acute exacerbation of severe chronic obstructive lung disease. The support Investigators (study to understand prognoses and preferences for outcomes and risks of treatments)Am J Respir Crit Care Med19961544 Pt 19599678887592
- ZvezdinBMilutinovSKojicicMA postmortem analysis of major causes of early death in patients hospitalized with COPD exacerbationChest2009136237638019318666
- VogelmeierCFCrinerGJMartinezFJGlobal strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. Gold executive summaryAm J Respir Crit Care Med2017195555758228128970
- SethiSMolecular diagnosis of respiratory tract infection in acute exacerbations of chronic obstructive pulmonary diseaseClin Infect Dis201152Suppl 4S290S29521460287
- ChenYRChenVHollanderZC-reactive protein and N-terminal prohormone brain natriuretic peptide as biomarkers in acute exacerbations of COPD leading to hospitalizationsPLoS One2017123e017406328328968
- AlotaibiNMChenVHollanderZPhenotyping COPD exacerbations using imaging and blood-based biomarkersInt J Chron Obstruct Pulmon Dis20181321722929386890
- MillerMRHankinsonJBrusascoVStandardisation of spirometryEur Respir J200526231933816055882
- BeckhamJDCadenaALinJRespiratory viral infections in patients with chronic, obstructive pulmonary diseaseJ Infect200550432233015845430
- SimpsonJLBainesKJHorvatJCCOPD is characterized by increased detection of Haemophilus influenzae, Streptococcus pneumoniae and a deficiency of Bacillus speciesRespirology201621469770426781464
- MurphyTFBrauerALSchiffmacherATSethiSPersistent colonization by Haemophilus influenzae in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2004170326627215117742
- GarchaDSThurstonSJPatelARChanges in prevalence and load of airway bacteria using quantitative PCR in stable and exacerbated COPDThorax201267121075108022863758
- HansonKECouturierMRMultiplexed molecular diagnostics for respiratory, gastrointestinal, and central nervous system infectionsClin Infect Dis201663101361136727444411
- KimHCChoiSHHuhJWDifferent pattern of viral infections and clinical outcomes in patient with acute exacerbation of chronic obstructive pulmonary disease and chronic obstructive pulmonary disease with pneumoniaJ Med Virol201688122092209927187664
- PapiABellettatoCMBraccioniFInfections and airway inflammation in chronic obstructive pulmonary disease severe exacerbationsAm J Respir Crit Care Med2006173101114112116484677
- BafadhelMMcKennaSTerrySAcute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkersAm J Respir Crit Care Med2011184666267121680942
- ShimizuKYoshiiYMorozumiMPathogens in COPD exacerbations identified by comprehensive real-time PCR plus older methodsInt J Chron Obstruct Pulmon Dis2015102009201626451098
- LeungJMTiewPYMac AogáinMThe role of acute and chronic respiratory colonization and infections in the pathogenesis of COPDRespirology201722463465028342288
- RangelovKSethiSRole of infectionsClin Chest Med20143518710024507839
- MohanAChandraSAgarwalDPrevalence of viral infection detected by PCR and RT-PCR in patients with acute exacerbation of COPD: a systematic reviewRespirology201015353654220415983
- RuttenFHCramerMJLammersJWGrobbeeDEHoesAWHeart failure and chronic obstructive pulmonary disease: an ignored combination?Eur J Heart Fail20068770671116531114
- BuchPFribergJScharlingHLangePPrescottEReduced lung function and risk of atrial fibrillation in the Copenhagen City Heart studyEur Respir J20032161012101612797497
- HurstJRVestboJAnzuetoASusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- AlevaFEVoetsLSimonsSOde MastQvan der VenAHeijdraYFPrevalence and localization of pulmonary embolism in unexplained acute exacerbations of COPD: a systematic review and meta-analysisChest2017151354455427522956
- BuchanABennettRCoadABarnesSRussellRManuelARThe role of cardiac biomarkers for predicting left ventricular dysfunction and cardiovascular mortality in acute exacerbations of COPDOpen Heart201521e00005225852947
- HawkinsNMKhoslaAViraniSAMcMurrayJJFitzGeraldJMB-type natriuretic peptides in chronic obstructive pulmonary disease: a systematic reviewBMC Pulm Med20171711128073350
- ChangCHTsaoKCHuHCProcalcitonin and C-reactive protein cannot differentiate bacterial or viral infection in COPD exacerbation requiring emergency department visitsInt J Chron Obstruct Pulmon Dis20151076777425926728
- KwakHJParkDWKimJEPrevalence and risk factors of respiratory viral infections in exacerbations of chronic obstructive pulmonary diseaseTohoku J Exp Med2016240213113927725531
- FioreAEFryAShayDAntiviral agents for the treatment and chemoprophylaxis of influenza – recommendations of the Advisory Committee on Immunization Practices (ACIP)MMWR Recomm Rep2011601124
- AlmagroPCabreraFJDiezJComorbidities and short-term prognosis in patients hospitalized for acute exacerbation of COPD: the EpoC en Servicios de medicina interna (ESMI) studyChest201214251126113323303399
- HansellALWalkJASorianoJBWhat do chronic obstructive pulmonary disease patients die from? A multiple cause coding analysisEur Respir J200322580981414621089
- ChangCLRobinsonSCMillsGDBiochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPDThorax201166976476821474497
- MarinAGarcia-AymerichJSauledaJEffect of bronchial colonisation on airway and systemic inflammation in stable COPDCOPD20129212113022458940
- GoddardAFStaudingerBJDowdSEDirect sampling of cystic fibrosis lungs indicates that DNA-based analyses of upper-airway specimens can misrepresent lung microbiotaProc Natl Acad Sci U S A201210934137691377422872870
- GutierrezJPGrimwoodKArmstrongDSInterlobar differences in bronchoalveolar lavage fluid from children with cystic fibrosisEur Respir J200117228128611334132
- DollowJMGreenJASignificant roadblocks exist in developing sputum sample libraries for clinical validation of novel in vitro diagnosticsDrug Des Devel Ther20148175182
- SinghSSinghAPrajapatiSXpert MTB/RIF assay can be used on archived gastric aspirate and induced sputum samples for sensitive diagnosis of paediatric tuberculosisBMC Microbiol20151519126420261
- WillnerDDalyJWhileyDGrimwoodKWainwrightCEHugenholtzPComparison of DNA extraction methods for microbial community profiling with an application to pediatric bronchoalveolar lavage samplesPLoS One201274e3460522514642
- ZemanickETWagnerBDRobertsonCEAssessment of airway microbiota and inflammation in cystic fibrosis using multiple sampling methodsAnn Am Thorac Soc201512222122925474078
- WuGDLewisJDHoffmannCSampling and pyrosequencing methods for characterizing bacterial communities in the human gut using 16S sequence tagsBMC Microbiol20101020620673359
- Ó CuívPAguirre de CárcerDJonesMThe effects from DNA extraction methods on the evaluation of microbial diversity associated with human colonic tissueMicrob Ecol201161235336221153634
- BagSSahaBMehtaOAn improved method for high quality Metagenomics DNA extraction from human and environmental samplesSci Rep201662677527240745
- von WintzingerodeFGöbelUBStackebrandtEDetermination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysisFEMS Microbiol Rev19972132132299451814
- KajiuraLNStewartSDDresiosJUyeharaCFTSimultaneous extraction of viral and bacterial nucleic acids for molecular diagnostic applicationsJ Biomol Tech201526411812426543438
- FrickmannHMasantaWOZautnerAEEmerging rapid resistance testing methods for clinical microbiology laboratories and their potential impact on patient managementBiomed Res Int201420146119