Abstract
Purpose
No consensus has been reached regarding appropriate nutritional intervention and rehabilitation during early acute exacerbation of COPD (AECOPD). Given the individual differences in symptoms of AECOPD, patients should be classified by their pathology. For example, it is known that there are differences in the inflammatory response between AECOPD with and without bacterial infection. However, there have been few reports on AECOPD from a nutritional perspective. The aim of this study was to investigate amino acid levels in patients with AECOPD.
Patients and methods
Blood was collected from patients who were hospitalized with AECOPD and from patients with COPD that was in a stable state. We divided the patients with AECOPD into those without bacterial infection (group A) and those with bacterial infection (group B). The patients with COPD that was stable served as controls (group C). The plasma levels of 9 essential amino acids, 13 nonessential amino acids, and total amino acids were compared between the three groups.
Results
In the early stages of AECOPD, differences in plasma levels of only three amino acids (glycine, phenylalanine, and arginine) were observed between groups C and A. Differences in total amino acids and 13 amino acids were observed between groups C and B. Group B had lower levels of total amino acids and of seven amino acids (asparagine, citrulline, glutamine, histidine, methionine, serine, and threonine) compared with the other study groups.
Conclusion
The findings of this study show that amino acid levels in plasma differ in patients with AECOPD depending on whether or not bacterial infection is present. Our results suggest that specific amino acids (ie, asparagine, citrulline, glutamine, histidine, serine, and threonine) have potential utility as diagnostic markers to distinguish between bacterial and nonbacterial AECOPD.
Plain language summary
It has been reported that the increases in inflammatory cytokine levels that occur in acute exacerbation of COPD (AECOPD) with bacterial infection differ from those in AECOPD without bacterial infection. We speculated that the metabolism of amino acids was also different between AECOPD with and without bacterial infection and felt that a distinction between the two was necessary for appropriate rehabilitation and/or nutritional interventions. In this study, we measured plasma amino acid levels in patients with stable COPD and acute-phase plasma amino acid levels in patients hospitalized for AECOPD. We found that levels of certain amino acids decreased in AECOPD with bacterial infection. Our results suggest that specific amino acids (ie, asparagine, citrulline, glutamine, histidine, methionine, serine, and threonine) have potential utility as diagnostic markers to distinguish between bacterial and nonbacterial AECOPD.
Introduction
Acute exacerbation of COPD (AECOPD) is known to accelerate the decline in quality of life and lung function that occurs in patients with COPD, resulting in deterioration of the prognosis.Citation1 BacterialCitation2 and viralCitation3 infections are important etiologic factors in AECOPD.Citation4 However, there have been reports of differences in increases in IL-6 and other inflammatory cytokines in patients with AECOPD depending on whether or not bacterial infection is present.Citation5 Differences in amino acid levels have also been reported in patients with influenza, a viral infection, according to whether there is concomitant bacterial infection.Citation6 Systemic inflammation associated with infection results in loss of appetite, and in order to compensate, the protein needed for gluconeogenesis is recruited from the muscles and dermal tissues.Citation7 The changes in amino acid levels that occur in response to loss of appetite have been reported to differ between patients with and without bacterial infection.Citation7 Therefore, even in patients who meet the commonly accepted criteria for AECOPD,Citation8,Citation9 differences in amino acid metabolism between those with and without bacterial infection would be expected. However, according to the GOLD, in relation to management of AECOPD, causes are classified solely on the basis of the criteria for administration of antibiotics.Citation10 Respiratory rehabilitation and nutritional intervention have previously been implemented in patients with COPD that is in a stable state,Citation11–Citation14 but rarely in patients in the early stages of AECOPD,Citation15,Citation16 for whom there is currently no consensus regarding appropriate rehabilitation and nutritional intervention. A negative report on the efficacy of nutritional supplementation during the early stage of AECOPD did not distinguish between cases with and without bacterial infection,Citation17 and the studies on rehabilitation during the early stage of AECOPD similarly did not make this distinction.Citation15,Citation16
We considered these opposing results to be based on the lack of a clear method or markers to distinguish between bacterial and nonbacterial AECOPD, and the lack of reports in which differences in nutritional status or amino acid metabolism in patients with AECOPD were noted. In this study, we measured acute-phase plasma amino acid levels in patients hospitalized for AECOPD with the objective of providing a landmark for research about nutritional status or amino acid metabolism in patients with AECOPD.
Patients and methods
Subjects were diagnosed to have COPD if they had a history of smoking (≥10 pack-years) and evidence of an obstructive pulmonary disorder on a lung function test (percentage of forced expiratory volume in 1 second for forced vital capacity [FEV1%] <70%).Citation10,Citation18 AECOPD was diagnosed according to the ASPEN,Citation9 GOLD,Citation10 and Japanese Respiratory Society guidelines for the diagnosis and treatment of COPD.Citation18 This study was approved by the ethics committee of Sanyudo Hospital. Written informed consent was obtained from each patient, in accordance with the tenets of the Declaration of Helsinki. These processes were performed in accordance with the ethical guidelines for medical and health research involving human subjectsCitation19 set out by the Ministry of Health, Labor and Welfare in Japan. Patients with diabetes (HBA1C >6.1 or receiving treatment for diabetes), chronic renal failure (estimated glomerular filtration rate <60 mL/min/1.73 mCitation2), a history of gastric resection, or terminal illness were excluded from all the study groups.
The AECOPD study population consisted of patients who were hospitalized in our Pulmonary Division of Internal Medicine from March 2016 to April 2018 and met the diagnostic criteria for AECOPD. Patients who visited the hospital as outpatients from April to June 2018 and had not suffered an acute exacerbation in the previous 3 months were considered to have COPD that was stable and were enrolled in group C.
Patients in the AECOPD group with the following features were diagnosed as having bacterial infection (pneumonia, group B): expectoration of purulent sputum;Citation4,Citation20 an increased white blood cell count or C-reactive protein level on hematology tests;Citation5 opacity on thoracic radiography or computed tomography; febrile symptoms;Citation21,Citation22 and detectable etiologic bacteria. Patients who did not display any of these features, except for fever, were deemed to have AECOPD without bacterial infection and were enrolled in group A. Sputum was collected from every patient and was screened with Gram’s stain method. Bacteria were cultured with sheep blood agar, chocolate agar, mannitol salt agar with egg yolk, and modified DRIGALSKI agar.
In the AECOPD group, we collected blood between 2 pm and 3 pm within 24 hours of admission, ie, 2–3 hours after eating for subjects who were permitted to eat. The nutritional manager planned all menus served from our nutrition division. The lunch menu for COPD patients was regulated to contain 600 kcal, 25 g of protein, and 80–100 g of carbohydrates. The nutritional manager measured leftover food from each served meal, and calculated the eating rate with food consumed expressed as a percentage of total food served to the patient. The control group was allowed to consume water only from 9 pm on the previous day and underwent blood collection on an outpatient basis at 9 am. Immediately after collection, each blood sample was placed in a test tube containing EDTA-Na2 and then transferred on ice to the testing laboratory, where it was immediately centrifuged and the plasma obtained was stored frozen at −40°C. Thirty-eight amino acids were subsequently analyzed by SRL Inc. (Tokyo, Japan) using liquid chromatography-mass spectrometry. We obtained the plasma levels of 9 essential amino acids and 13 nonessential amino acids from the reports supplied by SRL Inc. Because patients with AECOPD (groups A and B) were not fasted, we conducted a survey of eating rates based on the medical records.
The three groups were compared using a nonparametric multiple comparison test (the Steel–Dwass method). We used chi-squared tests for the three-group sex ratio and the two-group comparison of eating rates. Relationships between body mass index (BMI), albumin, or percentage of predicted value for FEV1 (%FEV1) as independent predictors and each amino acid as dependent variable were examined using multiple linear regression analysis. Post hoc power analysis was used to verify the sample size for comparisons between groups.
Results
We obtained consent and collected blood from 33 hospitalized patients who met the selection criteria for AECOPD. Eleven of these 33 patients did not have bacterial infection (group A; including 2 patients who were diagnosed to have influenza A within 2 days of onset) and 13 had bacterial infection (group B). The remaining nine subjects were excluded. They were considered AECOPD with bacterial infection from clinical data, but no etiologic bacteria were detected. We were able to obtain consent and samples from 26 patients with COPD in a stable state who served as controls (group C). There was no significant between-group difference in the age or gender distribution between the study groups (). There was also no significant difference in the proportions of patients in the AECOPD groups who had eaten before collection of the blood samples ().
Table 1 Mean patient age in each study group
Table 2 Eating rate in the study groups
Patients without bacterial infection received noninvasive nasal ventilation, if needed, and medication with crystalloid solution before blood sampling. Patients with bacterial infection received medication with crystalloid solution and antibiotics before blood sampling. Both groups of patients were served a similar meal. No patients received medication with systemic corticosteroids.
The etiologic agents in group B were Streptococcus pneumoniae, Haemophilus influenzae, Klebsiella pneumoniae, Staphylococcus aureus, and others (in four, three, two, two, and two patients, respectively).
The distributions for patient age, FEV1%, %FEV1, BMI, total protein, and serum albumin are shown in . There was no statistically significant difference between the groups except for %FEV1, BMI, and serum albumin between groups C and B (P<0.05). Therefore, for groups A and B combined, we examined relationships between BMI, albumin, or FEV1 and each amino acid using multiple linear regressions (). Concentrations of phenylalanine were related to BMI and %FEV1, glutamic acid with albumin, and tryptophan with albumin and %FEV1. The levels of isoleucine, leucine, methionine, ornithine, and tyrosine were related to %FEV1.
Figure 1 Body mass index and total serum protein, serum albumin, and C-reactive protein levels in each study group.
Abbreviations: AECOPD, acute exacerbation of COPD; FEV1%, forced expiratory volume in 1 second; %FEV1, percentage of predicted value for FEV1; NS, not statistically significant.
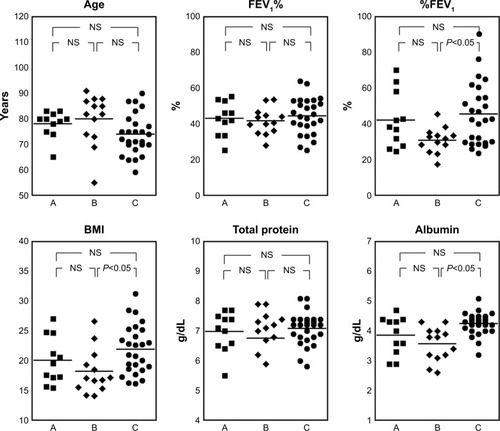
Table 3 Multiple linear regression analysis for each amino acid to BMI, albumin, and %FEV1
The distributions of levels of total amino acids, the 9 essential amino acids, and the 13 nonessential amino acids are shown in . There were statistically significant differences in levels between groups C and A for only three amino acids (glycine, phenylalanine, and arginine). In contrast, there were statistically significant differences in levels between groups C and B for total amino acids and 13 amino acids. Levels of total amino acids and seven amino acids (asparagine, citrulline, glutamine, histidine, methionine, serine, and threonine) were lower in group B than in group A. The ratio of these amino acids in group B to group C () was <70% of the control for four amino acids (citrulline, histidine, serine, and threonine) and 83% or less for the other three amino acids. Post hoc power analysis for these seven amino acids and for total amino acids showed that the numbers of patients were sufficient for statistical comparisons between groups A and B (). Glycine and arginine levels were lower in the AECOPD groups (A and B) than in group C and were also lower in group B than in group A. Levels of three amino acids (alanine, tryptophan, and lysine) were lower in group B than in group C. The aspartic acid level was higher in group B than in group C. The phenylalanine level was higher in both AECOPD groups (A and B) than in group C, but there was no difference between groups A and B. Furthermore, there was no difference in three branched-chain amino acids (BCAAs) (valine, leucine, and isoleucine) or in cystine, glutamic acid, proline, ornithine, or tyrosine between the groups. The capacity for nitric oxide (NO) synthesis compared using the global arginine bioavailability ratio (GABR;Citation23 defined as arginine/[ornithine + citrulline], was lower in the AECOPD groups [A and B] than in group C []).
Figure 2 Intergroup comparisons for each amino acid.
Notes: The horizontal solid lines show the mean values in each group. The statistical analysis was performed using the Steel–Dwass method. A, AECOPD without bacterial infection (group A); B, AECOPD with bacterial infection (group B); C, COPD in a stable state, control (group C).
Abbreviations: AECOPD, acute exacerbation of COPD; NS, not statistically significant.

Figure 3 Global arginine bioavailability ratio in each group.
Abbreviations: AECOPD, acute exacerbation of COPD; GABR, global arginine bioavailability ratio; NS, not statistically significant.
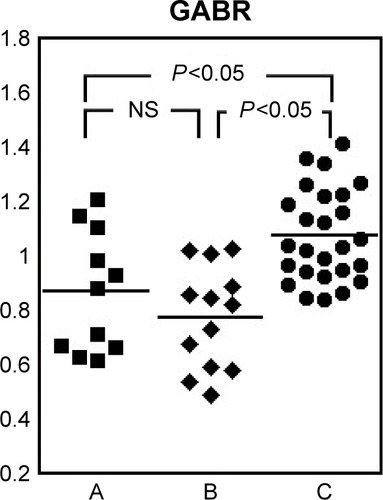
Table 4 Ratio of amino acids in AECOPD (groups A and B) to stable COPD (group C)
Table 5 Results of post hoc analysis
Discussion
Diagnosis and selection of subjects
Infections, including bacterial and viral infections, are estimated to cause 50%–70% of AECOPD,Citation24 and AECOPD without a clear cause is reported to occur at a rate of about 30%.Citation24 Bacterial infection and viral infection cannot be easily distinguished as AECOPD at the time of admission to hospital.Citation4 Therefore, in this study, we only classified patients in whom etiologic bacteria could be detected as having AECOPD with bacterial infection (group B) rather than those in whom bacterial infection was suspected on the basis of clinical symptoms and test results. In contrast, we classified patients who did not satisfy any of the selection criteria except for fever as having AECOPD without bacterial infection (group A). The nine excluded cases were suspected of having bacterial infection from their clinical symptoms and clinical test results, but we could not detect any bacteria that might conceivably be the etiologic agent. The etiologic agent for community-acquired pneumonia is reportedly detected on bacterial culture in 20%–49% of cases in Japan.Citation25 S. pneumoniae is reported to be the most common etiologic agent, followed by H. influenzae.Citation25 The etiologic agents detected in this study followed a similar trend.
Measurements obtained
Blood was collected after ~12 hours of fasting in group C. However, in the AECOPD groups, it was performed 2–3 hours after eating as a nonfasting collection. This is because the AECOPD groups could not fast to prioritize their treatment. Plasma amino acids may increase in response to dietary intake, but amino acid levels were lower in our patients with AECOPD (groups A and B) than in the controls (group C), except for phenylalanine and aspartic acid. There was no difference in the eating rate in the medical records between groups A and B, and the decreased amino acid levels were lower in group B than in group A. On the basis of these observations, we consider that the difference between group A and group B was not an effect of dietary intake. In young Japanese adults, it has been reported that amino acid levels return to predose values 2 hours after oral intake of BCAAs.Citation26 There have been two reports on plasma amino acid concentrations in Japanese patients with COPD in a stable state,Citation27,Citation28 and the results in group C in our study (excluding glutamic acid, histidine, and threonine) are similar to those in one of the earlier reports.Citation28 BCAA levels are reported to be lower in individuals with COPD in a stable state.Citation27 We found that isoleucine and leucine concentrations were influenced by %FEV1 (), and considered these results to be related to the reported relationship between the ratio of BCAA/(aromatic amino acids) and FEV1.Citation27 A detailed previous comparison between nonpneumonic and pneumonic AECOPDCitation5 showed that %FEV1 was lower in nonpneumonic than in pneumonic AECOPD, but our results did not support this relationship.
The dynamics and clinical significance of changes in amino acid levels in patients with AECOPD are largely unknown. However, a decrease in the total amino acid level has been reported to start before the onset of infection-related loss of appetite and to have no causal relationship with a reduced dietary intake of protein.Citation7 In this study, the total amino acid levels were lower only in group B, which we could not attribute to dietary intake. Of the seven amino acids that were observed to decrease in group B, four decreased to <70% of the value in group C and three to <83% (); in comparison, the levels in group A were within the range of 92.6%–106.6% of those in group C, except for citrulline (). Only methionine was correlated with %FEV1. Other amino acids were not related to BMI, albumin, or %FEV1. These results suggest that levels of six of the amino acids (ie, asparagine, citrulline, glutamine, histidine, serine, and threonine) decrease greatly in the acute phase of bacterial infection.
Immunonutrition has recently been introduced as a concept in nutritional intervention and emphasizes the importance of arginine and glutamine.Citation29 In this study, arginine levels were lower in the patients with AECOPD (groups A and B), as was the glutamine level in the patients with bacterial infection (group B). One recent report showed an association between the severity of COPD and changes in arginine metabolism.Citation30 Another report showed that endogenous production of arginine was upregulated but was unrelated to whole-body NO production in patients with moderate-to-severe COPD.Citation31 It has been suggested that the capacity to synthesize NO should be assessed by the GABR rather than the systemic arginine level.Citation23 The GABR was lower in the patients with AECOPD (groups A and B) than in the controls (group C), and there was no difference in this ratio between groups A and B. These findings suggest that there is a decrease in both plasma arginine levels and the capacity to synthesize NO in patients with AECOPD, regardless of whether or not bacterial infection is present. A review of the relationship between glutamine and various respiratory diseases highlights the important role of the lungs in glutamine homeostasis, which is therefore altered in various pulmonary diseases.Citation32
There have been reports concerning the dynamics of glycine and methionine specifically in relation to bacterial infection.Citation33,Citation34 Glycine is known to be involved in anti-inflammatory activity and the immune response,Citation33 and methionine is an essential amino acid for numerous organisms, including bacteria.Citation34 Therefore, it is possible that these changes in amino acid levels are a physiologically normal reaction to bacterial infection. Glycine levels have been reported to increase when influenza is complicated by bacterial infectionCitation6 and in severe infectious disorders such as sepsis.Citation35 However, in this study, glycine levels decreased in patients with AECOPD (groups A and B).
At the time of infection, there is an increase in release of tryptophan from skeletal muscles in response to increased catabolism and decreased anabolism, and this tryptophan is immediately taken up by the liver and used for protein synthesis in plasma.Citation7 Recent research has shown that tryptophan levels decrease in patients with AECOPD regardless of whether the infection is bacterial or nonbacterial.Citation36 According to recent reports, activation of indoleamine 2,3-dioxygenase and catabolism of tryptophan occur at times of infection and trigger anti-inflammatory activity in the lungs.Citation37 In the present study, there was no significant difference in the plasma concentration of tryptophan between groups A and B; however, the level was lower in group B than in group C.
In contrast, phenylalanine is known to increase in response to inflammation, including that caused by bacterial infection and viral infection,Citation38 and the details of this have been elucidated.Citation7 In this study, the plasma phenylalanine level was increased in patients with AECOPD (groups A and B).
Amino acids are directly involved in cell metabolism and also operate as neurotransmitters.Citation39 Serine, levels of which decreased markedly only in group B, has an inhibitory effect in the central nervous system, and glycine, which decreased in groups A and B, also has an inhibitory effect.Citation39 Aspartic acid is one of the amino acids with an excitatory effect, and an increase in aspartic acidCitation39 was observed in group B. This means that amino acid levels in group B changed in a direction that increased excitability in the central nervous system.
General comments
The above-mentioned findings indicate that differences in amino acid levels at the time of hospitalization for AECOPD are likely to be associated with bacterial infection. We think that these decreased amino acids (ie, asparagine, citrulline, glutamine, histidine, methionine, serine, and threonine) have potential utility as diagnostic markers to distinguish between bacterial and nonbacterial AECOPD. However, further research is needed to investigate the time course and clinical significance of changes in the individual amino acids associated with AECOPD.
COPD involves inflammation not only in the lungs but also throughout the entire body, including the skeletal muscles.Citation40,Citation41 Therefore, it is considered that inflammation and changes in amino acid metabolism related to AECOPD affect the skeletal muscles, so rehabilitation probably requires individualized approaches. In addition, amino acids are essential nutrients for infectious microorganisms,Citation34 and levels of some amino acids such as tryptophan decrease in response to infection and/or inflammation.Citation37 One study showed that arginine supplementation had adverse effects in sepsis related to pneumonia.Citation42 Trends in amino acid values in patients with AECOPD and their clinical significance will require further study.
Limitations
The COPD phenotype in AECOPDCitation43,Citation44 should have been considered but was not included in our analysis. Furthermore, there was no investigation of the duration of the decrease in plasma amino acid levels that occurred in response to bacterial infection. This question is being addressed in a current investigation by our group.
Conclusion
Our results suggest that specific amino acids (ie, asparagine, citrulline, glutamine, histidine, serine, and threonine) have potential utility as diagnostic markers to distinguish between bacterial and nonbacterial AECOPD. In addition, consideration must be given to distinguishing whether or not bacterial infection is present when embarking on rehabilitation and/or nutritional intervention for patients with AECOPD.
Author contributions
HI had full access to the study data, takes full responsibility for the integrity of the data and the accuracy of the analysis, and contributed to the study design. SI contributed to the nutritional management of patients. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
We would like to thank Editage (https://www.editage.jp) for English-language editing and SRL, Inc. (http://www.srl-group.co.jp/english/index.html) for analyzing the amino acids.
Disclosure
The authors report no conflicts of interest in this work.
References
- DonaldsonGCSeemungalTABhowmikAWedzichaJARelationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax2002571084785212324669
- MiravitllesMEspinosaCFernández-LasoERelationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Study Group of bacterial infection in COPDChest19991161404610424501
- GreenbergSBAllenMWilsonJAtmarRLRespiratory viral infections in adults with and without chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2000162116717310903237
- SapeyEStockleyRACOPD exacerbations 2: AetiologyThorax200661325025816517585
- ArturoHErnestoCRosarioMPneumonic and nonpneumonic exacerbations of COPDChest201314441134114223828375
- MohammadMBHansJVAalimMWCanadian Critical care translational biology group (CCCTBG). Plasma metabolomics for the diagnosis of H1N1 influenza pneumoniaCrit Care2017219728424077
- WannemacherRWKey role of various individual amino acids in host response to infectionAm J Clin Nutr197730812691280407785
- AnthonisenNRManfredaJWarrenCPAntibiotic therapy in exacerbations of chronic obstructive pulmonary diseaseAnn Intern Med198710621962043492164
- Rodriguez-RoisinRToward a consensus definition for COPD exacerbationsChest20001175 Suppl 2398S4015S10843984
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management and prevention of COPD2017 Available from: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/Accessed January 8, 2017
- Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based guidelinesACCP/AACVPR pulmonary rehabilitation guidelines panel. American College of chest physicians. American Association of cardiovascular and pulmonary rehabilitationChest19971125136313969367481
- SugawaraKTakahashiHKasaiCEffects of nutritional supplementation combined with low-intensity exercise in malnourished patients with COPDRespir Med2010104121883188920627502
- FerreiraIMBrooksDWhiteJGoldsteinRNutritional supplementation for stable chronic obstructive pulmonary diseaseCochrane Database Syst Rev201212CD000998
- CollinsPFStrattonRJEliaMNutritional support in chronic obstructive pulmonary disease: a systematic review and meta-analysisAm J Clin Nutr20129561385139522513295
- GreeningNJWilliamsJEAHussainSFAn early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: randomised controlled trialBMJ2014349g431525004917
- MatsuiHJoTFushimiKYasunagaHOutcomes after early and delayed rehabilitation for exacerbation of chronic obstructive pulmonary disease: a nationwide retrospective cohort study in JapanRespir Res20171816828431501
- HelgaSUJamesGMKatherineGDImpact of nutritional support on functional status during an acute exacerbation of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1997a56794799
- Japanese Respiratory SocietyThe JRS Guidelines for the Management of COPD 2018Tokyo, JapanJapanese Respiratory Society2018 Japanese
- Ethical guidelines for medical and health research involving human subjectsMinistry of health, labour and welfare of Japan2015 Available from: http://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000080278.pdfAccessed January 8, 2017
- StockleyRAO’BrienCPyeAHillSLRelationship of sputum color to nature and outpatient management of acute exacerbations of COPDChest200011761638164510858396
- NiedermanMSMandellLAAnzuetoAGuidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and preventionAm J Respir Crit Care Med200116371730175411401897
- MüllerBHarbarthSStolzDDiagnostic and prognostic accuracy of clinical and laboratory parameters in community-acquired pneumoniaBMC Infect Dis200771017335562
- TangWHWangZChoLBrennanDMHazenSLDiminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular riskJ Am Coll Cardiol200953222061206719477356
- SapeyEStockleyRACOPD exacerbations. 2: aetiologyThorax200661325025816517585
- Japanese Respiratory SocietyThe JRS guideline for management of pneumonia in adults 2017Tokyo, JapanJapanese Respiratory Society2017 Japanese
- ZhangYKobayashiHMawatariKEffects of branched-chain amino acid supplementation on plasma concentrations of free amino acids, insulin, and energy substrates in young menJ Nutr Sci Vitaminol201157111411721512300
- YonedaTYoshikawaMFuAPlasma levels of amino acids and hypermetabolism in patients with chronic obstructive pulmonary diseaseNutrition2001172959911240335
- KutsuzawaTShioyaSKuritaDHaidaMPlasma branched-chain amino acid levels and muscle energy metabolism in patients with chronic obstructive pulmonary diseaseClin Nutr200928220320819250720
- McCowenKCBistrianBRImmunonutrition: problematic or problem solving?Am J Clin Nutr200377476477012663270
- JonkerRDeutzNEErblandMLAndersonPJEngelenMPAlterations in whole-body arginine metabolism in chronic obstructive pulmonary diseaseAm J Clin Nutr201610361458146427146652
- WannemacherRWKlainerASDintermanREBeiselWRThe significance and mechanism of an increased serum phenylalanine-tyrosine ratio during infectionAm J Clin Nutr19762999971006822705
- OliveiraGde AbreuMPelosiPRoccoPExogenous glutamine in respiratory diseases: myth or reality?Nutrients2016827626861387
- ZhongZWheelerMDLiXL-glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agentCurr Opin Clin Nutr Metab Care20036222924012589194
- BasavannaSChimalapatiSMaqboolAThe effects of methionine acquisition and synthesis on Streptococcus pneumoniae growth and virulencePLoS One201381e4963823349662
- LongxiangSHuaLAimeiXDynamic changes in amino acid concentration profiles in patients with sepsisPLoS One201510e012193325849571
- GulcevMReillyCGriffinTJTryptophan catabolism in acute exacerbations of chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis2016112435244627729784
- SuzukiYSudaTYokomuraKSerum activity of indoleamine 2,3-dioxygenase predicts prognosis of community-acquired pneumoniaJ Infect201119436442
- ZinelluAFoisAGSotgiaSArginines plasma concentration and oxidative stress in mild to moderate COPDPLoS One2016118e016023727479314
- BruntonLChabnerBAKnollmanBGoodman and Gilman’s The Pharmacological Basis of Therapeutics12th edNew York, NYMcGraw-Hill Education/Medical2011
- AgustíAGNogueraASauledaJSystemic effects of chronic obstructive pulmonary diseaseEur Respir J200321234736012608452
- SorianoJBVisickGTMuellerovaHPayvandiNHansellALPatterns of comorbidities in newly diagnosed COPD and asthma in primary careChest200512842099210716236861
- DentDLHeylandDKLevyHImmunonutrition may increase mortality in critically ill patients with pneumonia: results of a randomized trialCrit Care Med200230SupplA17
- HurstJRExacerbation phenotyping in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2011184662562621920921
- ZhouAZhouZZhaoYChenPThe recent advances of phenotypes in acute exacerbations of COPDInt J Chron Obstruct Pulmon Dis2017121009101828392685
