?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Introduction and aim
Abnormal brain structure and function in COPD has been reported on MRI. However, the deficit in local synchronization of spontaneous activity in patients with stable COPD remains unknown. The main aim of the present study was to explore spontaneous brain activity in patients with COPD compared with normal controls using the regional homogeneity (ReHo) method based on resting-state functional MRI.
Methods
Nineteen patients with stable COPD and 20 well-matched (including age, sex, and number of years of education) normal controls who were recruited for the present study underwent resting-state functional MRI examinations and a series of neuropsychological and clinical assessments. The ReHo method was used to assess the strength of local brain signal synchrony. The mean ReHo values in brain areas with abnormal ReHo were evaluated with a receiver operating characteristic curve. The relationships between the brain regions with altered ReHo values and the clinical and neuropsychological parameters in COPD patients were assessed using Pearson’s correlation.
Results
Patients with COPD showed significantly lower ReHo values in the left occipital lobe and the right lingual, bilateral precuneus, and right precentral gyrus. The result of receiver operating characteristic curve analysis showed that the altered average ReHo values have high efficacy for distinguishing function. The mean lower ReHo values in the precuneus gyrus showed a significant positive correlation with FEV1%, FEV1/FVC, and orientation function but a significant negative correlation with arterial partial pressure of carbon dioxide.
Conclusion
The COPD patients demonstrated abnormal synchrony of regional spontaneous activity, and the regions with abnormal activity were all correlated with visual processing pathways, which might provide us with a new perspective to further understand the underlying pathophysiology of cognitive impairment in patients with COPD.
Introduction
COPD is a common, treatable, and preventable disease that is characterized by chronic irreversible airflow obstruction.Citation1 Recently, a systemic review reported that COPD is currently the fourth leading cause of death worldwide and estimated to be the third by 2030.Citation2 COPD is a complex multisystem disease that is associated with many extrapulmonary features.Citation3,Citation4 In addition, the incidence of clinically relevant cognitive impairment has increased, and this feature has become one of the key extrapulmonary symptoms of COPD.Citation5–Citation8 Cognitive impairment not only increases the risk of acute exacerbation of COPD but also reduces function in several basic activities of daily life.Citation9,Citation10 The estimated prevalence of cognitive impairment among patients with COPD is approximately 36%, while only 12% of the general population presents evidence of cognitive impairment.Citation11 COPD is marked by hypoxia, which can lead to neuronal damage because the brain is highly sensitive to low oxygen and easily exposed to hypoxic damage.Citation12,Citation13 Brain pathology was considered as a potential systemic manifestation that may explain the increasingly reported cognitive deficits in COPD.Citation8 However, the mechanism of cognitive impairment in patients with COPD is unclear.
COPD-related brain structural and functional alterations have been explored by various advanced neuroimaging methods. For example, patients with COPD showed marked alterations in cerebral perfusion, and hypoxemic patients demonstrated poorer cognitive performance and cerebral perfusion than nonhypoxemic patients,Citation14 and severe COPD patients exhibited cerebral metabolite abnormities.Citation15–Citation17 Gray matter volume (GMV) atrophy in patients with COPD involves the limbic and paralimbic brain areas, such as the insula, precentral gyrus, and cingulate cortex. In addition, some of these regions with decreased GMV in COPD patients were positively correlated with arterial blood partial pressure of oxygen (PaO2), visual task performance,Citation18 hyperactivation in all resting-state networks (RSNs), with the exception of the visual network, and widespread white matter micro-structure impairment, which could contribute to cognitive defects.Citation19 Cortical thinning in the parietofrontal networks is significantly correlated with visuospatial construction dysfunction,Citation20 and the mechanism of increased functional activation may include early cortical reorganization in several regions.Citation21 A number of previous studies have confirmed that COPD can lead to structural and functional changes in the brain. However, how COPD affects the human brain remains largely unknown until now.
Resting-state functional MRI (rs-fMRI), which is a reliable, effective, and sensitive method, is widely used to investigate intrinsic functional activity in various neuropsychological and neuropsychiatric diseases, such as Alzheimer’s disease,Citation22,Citation23 post-traumatic stress disorder,Citation24 obstructive sleep apnea, and schizophrenia.Citation25,Citation26 In rs-fMRI, regional homogeneity (ReHo) can help to analyze the coherence and similarities of low-frequency (<0.08HZ) spontaneous fluctuations using voxel-wise analysis across the whole brain. ReHo assumes that voxels within a functional brain region are more temporally homogeneous when this area is under certain conditions, and the main advantage of ReHo, which model-driven methods have failed to reach, is the ability to detect unpredicted hemodynamic responses.Citation27 ReHo is a data-driven method, without any a priori knowledge of hemodynamic models or the start time of the stimulus, so this method can obtain more information and has better test–retest reliability than model-driven methods. To date, ReHo, which may help us to investigate the complexity of human brain function in patients with COPD, has not been used in COPD.
In the present study, we hypothesized that patients with COPD would show abnormal ReHo activity correlated with clinical status and cognitive impairment, and these alterations may be related to the severity of disease and cognitive defects. To test our hypothesis, we applied the ReHo method to explore whether the synchrony of regional spontaneous brain activity on rs-fMRI was altered in COPD patients when compared to normal controls (NCs). Then, we analyzed the relationships between regions with the altered ReHo values and demographic and neuropsychological data.
Materials and methods
Subjects
Nineteen patients with stable COPD who were recruited from the Respiratory Department of The First Affiliated Hospital of Nanchang University from December 2017 to May 2018 and 20 NCs were included in the present study. The two groups were well matched in age, gender, and number of years of education. All patients were confirmed to be stable (with no acute exacerbations during the past 8 weeks) by pulmonary function testing. Patients with COPD were diagnosed according to the Global Initiative for Chronic Obstructive Lung Disease guidelines from 2017.Citation1 The exclusion criteria for both the COPD patients and NCs were as follows: 1) comor-bidities such as cerebrovascular accidents, heart failure, liver failure, neurologic disorders, and obstructive sleep apnea, or other diseases that affect cognitive function; 2) illegal drug or alcohol abuse; 3) identification of a structural lesion during the brain MRI scan; and 4) contraindications for MRI, such as metallic implants, devices in the body, or claustrophobia.
The study protocol was approved by the Human Research Ethics Committee of the First Affiliated Hospital of Nanchang University and performed in accordance with the Declaration of Helsinki, and all subjects signed informed consent.
Arterial blood gas analysis
Arterial blood gas analysis was implemented using Stat Profile Critical Care Xpress (Nova Biomedical, Waltham, MA, USA) within 30 minutes. The following indicators were recorded: arterial PaO2, arterial partial pressure of carbon dioxide (PaCO2), blood oxygen saturation (SaO2), and power of hydrogen.
Pulmonary function tests
A dry spirometer device (Erich Jaeger GmbH, Hoechberg, Germany) was used 15 minutes after the patients inhaled 400 µg of salbutamol (Ventolin; GlaxoSmithKline, London, UK) to conduct the pulmonary function tests. The main indicators included the FEV1, FVC, and FEV1/FVC ratio after bronchodilator administration. An FEV1/FVC ratio <70% was defined as COPD. In addition, an FEV1 ≥80% predicted was defined as mild COPD, 50%≤ FEV1 <80% predicted was defined as moderate COPD, 30% ≤ FEV1 <50% predicted was classified as severe COPD, and FEV1 <30% predicted was classified as extremely severe COPD.
Neuropsychological evaluation
All subjects underwent cognitive assessment before MRI scans. Neuropsychological evaluation included the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA),Citation28,Citation29 both of which are 30-point questionnaires; the Chinese versions were used. The MoCA can evaluate many different cognitive fields including executive function, naming, calculation, language, attention, memory, orientation, and abstraction. A total MoCA score <26 indicated cognitive impairment, whereas a score ≥26 indicated normal cognition. In addition, if the length of schooling was <12 years, one point was added to the total score to compensate for educational bias. The MMSE is a standardized method used to classify cognitive function grades; participants were regarded as having no obvious cognitive impairment when they had MMSE scores >26, whereas a score ≤26 indicated cognitive impairment. These two scales were evaluated by two independent neuropsychologists. All participants completed these cognitive assessments.
MRI data acquisition
All subjects underwent MRI scan on the same imaging session on 3.0 Tesla MRI system with eight-channel head coil (Siemens, Erlangen, Germany) in our hospital. All participants lay in the scanner with their eyes closed and relaxed and avoided falling asleep or thinking. In order to reduce noise, all subjects were required to wear earplugs and use foam pads to decrease head movement. Firstly, the functional scans were located using a series of localizer scans. Secondly, conventional T1-weighted imaging (echo time [TE] =2.46 ms, repetition time [TR] =250 ms, field of view [FOV] =220×220 mm, gap =1.5 mm, slices =19, slice thickness =5 mm) and T2-weighted imaging (TE =113 ms, TR =4,000 ms, FOV =220×220 mm, gap =1.5 mm, slices =19, slice thickness =5 mm) were gained. Thirdly, 240 rs-fMRI images were obtained with the following parameters: TE =30 ms; TR =2,000 ms; FOV =230×230 mm; flip angle =90°; gap =1.2 mm; thickness =4.0 mm; acquisition matrix =64×64; and 30 axial slices from contiguous echo-planar imaging scans. Finally, a total of 176 high-resolution T1-weighted images were collected by using a three-dimensional spoiled gradient-recalled echo sequence in a sagittal orientation with the following parameters: TE =2.26 ms; TR =1,900 ms; gap =0 mm; thickness =1.0 mm; FOV =250×250 mm2; acquisition matrix =256×256; flip angle =9°.
fMRI data preprocessing
Initially, to eliminate macrostructural brain lesions that may have affected the function of the brain, all conventional T1- and T2-weighted imaging were reviewed by two senior radiologists in the Department of Radiology of the First Affiliated Hospital of Nanchang University. Two authors used MRIcro software (www.MRIcro.com) to evaluate all of the functional images carefully to exclude possible poor quality images. No subjects were excluded due to low image quality or brain lesions. All of the rs-fMRI data were preprocessed by Data Processing and Analysis for Brain Imaging (http://rfmri.org/DPABI) and Statistical Parametric Mapping (SPM8; http://www.fil.ion.ucl.ac.uk/spm), both of which are based on the MATLAB 2014a (Mathworks, Natick, MA, USA) platform. The following steps were performed: 1) The first ten volumes of each subject were eliminated to allow the participants to adapt to the scanning noise and allow the signal to reach equilibrium. 2) Three-dimensional head motion correction was performed for the remaining 230 volumes. According to the head motion criteria, which included a maximum cardinal direction displacement (x, y, z) of <2.0 mm and a maximum spin (x, y, z) of <2.0°, and according to the standard definition of head motion that frame-wise displacement (FD) was >2.5 standard deviations based on the method described by Van Dijk et al,Citation30 none of the participants were removed. 3) To achieve better registration, functional and anatomical images were manually reoriented to the anterior commissure. Then, using a linear transformation, structural images were coregistered to the functional images for each subject. 4) The transformed structural images were segmented into white matter, gray matter, and cerebrospinal fluid using the new segmentation in SPM8. 5) All functional images were normalized to Montreal Neurological Institute space by using the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra tool and resampled at a resolution of 3×3×3 mm voxels. 6) The cerebrospinal fluid signal, white matter signal, and Friston 24-parameter were regressed from the time series of all voxels via linear regression. 7) A temporal filter (0.01–0.08 Hz) was launched for the time series to reduce the effect of low-frequency drift and high-frequency noise.
ReHo calculation
Kendall’s coefficient of concordance (KCC) was calculated as a given voxel’s time series with those of its nearest neighbors (26 voxels). The calculation formula is as follows:Citation27
The ReHo value ranges from 0 to 1, which is the KCC of the given voxels; when a given cluster is more consistent with its neighboring cluster in a time series, the ReHo value is closer to 1. K refers to the number of time series within a measured cluster (in the present study, K=27; one given voxel located in the cubic center plus its 26 neighboring voxels) and is the smaller unit of the measured ReHo, which comprised more adjacent clusters; n is the number of ranks; Ri is defined as the sum rank of the ith time point, where is the mean of the Ri’s. We also used MATLAB to calculate the KCC program. The standard ReHo value is the average of the whole brain ReHo value; hence, an individual ReHo map was produced for each dataset. Normalization of the ReHo maps was performed by dividing the KCC for each voxel by the average KCC of the whole brain to reduce the influence of individual variations in KCC values. Then, the resulting fMRI data were spatially smoothed with a Gaussian kernel of 6×6×6 mm3 full width at half maximum.
Statistical analysis
The clinical and demographic data, such as number of years of education, age, pulmonary function indicators, arterial blood gas indexes, and MMSE and MoCA scores, were analyzed using independent sample t-tests with Statistical Package for Social Sciences version 19.0 (IBM SPSS 19.0).
The two-sample t-test was performed to compare the differences in the ReHo values between the COPD group and the NC group (two-tailed, voxel-level P <0.01; Gaussian random field theory correction, cluster-level P <0.05), and the age, number of years of education, intracranial volume, and mean FD were included as nuisance covariates within the default gray matter mask. Furthermore, receiver operating characteristic (ROC) analysis was used to verify use of the ReHo values, which were altered in some brain regions, as biomarkers to distinguish the COPD patients from NCs. Finally, we analyzed the correlation between the mean ReHo values in various brain regions and clinical performance in COPD patients using Pearson’s correlation analysis; P <0.05 was considered statistically significant.
Results
Demographic and clinical characteristics
The demographic and clinical characteristics of the two groups are shown in . Patients with COPD had markedly lower PaO2 (P <0.001), SaO2 (P =0.008), FVC (P <0.001), FEV1 (P <0.001), and FEV1/FVC (P <0.001) than NCs and had significantly higher PaCO2 (P <0.001). In addition, COPD patients presented significantly lower scores on the MoCA (P <0.001), MMSE (P <0.001), naming (P =0.026), visuospatial and executive (P =0.012), and memory testing (P <0.001) than NCs.
Table 1 Demographic and clinical characteristics of COPD patients and NCs
ReHo alterations in COPD patients compared with NCs
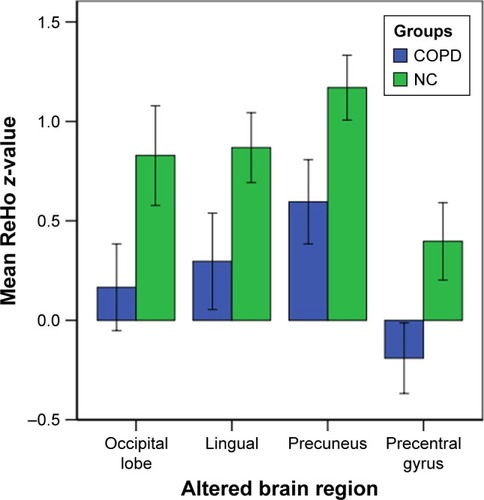
Patients with COPD showed significantly lower ReHo values in the left occipital lobe (BA 17) and the right lingual (BA 18), bilateral precuneus (BA 5/7), and right precentral gyri (BA 4) ( and and ). In addition, there were no statistically significant high ReHo values. The mean ReHo values of these altered areas are shown in .
Table 2 Two-sample t-test differences between COPD patients and NCs using ReHo method (GRF-corrected, voxel-level P <0.01, and cluster level of P <0.05)
Figure 1 The differences map in ReHo between COPD patients and NC groups (two-tailed GRF correction, voxel-level P <0.01, and cluster-level P <0.05) is shown in axial position. The blue areas indicate lower ReHo values.
Abbreviations: GRF, Gaussian random field theory; NC, normal control; ReHo, regional homogeneity.
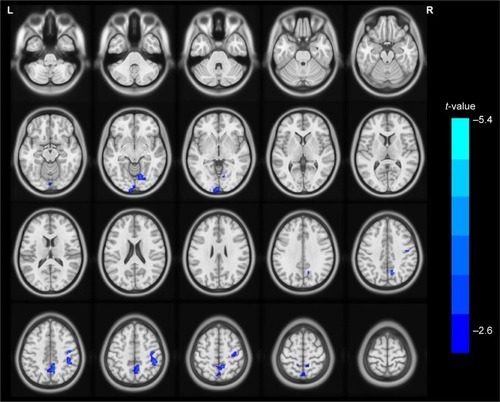
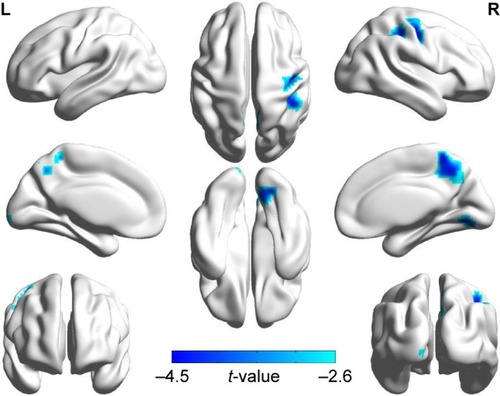
Figure 2 Significant abnormal activity map observed in the left occipital lobe, the right lingual, the bilateral precuneus, and the right precentral gyrus between patients with COPD and NCs (two-tailed, GRF correction, voxel-level P <0.01, and cluster-level P <0.05) in three-dimensional image.
Abbreviations: GRF, Gaussian random field theory; NCs, normal controls.
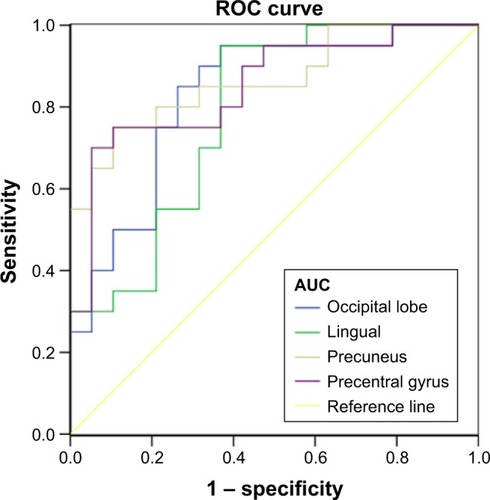
ROC curve analysis
The decreased mean ReHo values in the occipital lobe and lingual, precuneus, and precentral gyrus in COPD patients might distinguish COPD patients from NCs. In the present study, the area under the curve (AUC) values of the occipital lobe and lingual gyrus were 0.829 and 0.784, respectively, and the AUC values of the precuneus and precentral gyri were 0.866 and 0.850, respectively. Further analysis revealed that the sensitivity and specificity of the occipital lobe were 85.0% and 73.7%, respectively. Likewise, the sensitivity and specificity of the lingual, precuneus, and precentral gyrus were 95.0% and 63.2%, 75.0% and 89.5%, and 70.0% and 94.7% (), respectively, showing that the altered average ReHo values have high efficacy for distinguishing function.
Figure 4 ROC curve analysis of the mean ReHo values for abnormal activity brain regions.
Abbreviations: AUC, area under the curve; ReHo, regional homogeneity; ROC, receiver operating characteristic.
Correlation results
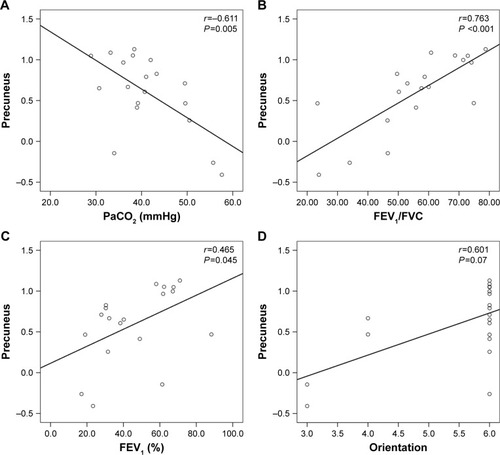
The observed lower mean ReHo values in the precuneus displayed significant positive correlation with FEV1% (r=0.465, P =0.045), FEV1/FVC (r=0.763, P <0.001), and orientation function (r=0.601, P =0.070), while the precuneus showed significant negative correlation with PaCO2 (r=−0.611, P =0.005; ).
Discussion
In our study, the ReHo method was used for the first time to investigate the synchrony of regional spontaneous brain activity in COPD patients and revealed abnormal activation in specific brain areas. According to the MMSE and MoCA scores, patients with COPD showed worse general cognitive performance than NCs, which is consistent with our hypothesis. The major findings of the present study were that COPD patients exhibited significantly lower ReHo values in the left occipital lobe and the right lingual, bilateral precuneus, and right precentral gyri compared with NCs. However, there was no statistically significantly higher ReHo values in patients with COPD. In addition, the precuneus gyrus had lower mean ReHo values that were significantly positively correlated with FEV1%, FEV1/FVC, and orientation function and were significantly negatively correlated with PaCO2 in COPD patients. Additional ROC curve analysis demonstrated the mean ReHo values in the bilateral precuneus gyrus and the right precentral gyrus could serve as biological indexes to distinguish stable COPD patients from NCs.
Interestingly, we found that these regions with lower ReHo values found in our present study were all correlated with visual processing pathways, including the occipital lobe, inferior temporal lobe (lingual gyrus), posterior parietal lobe (the precuneus gyrus), and premotor cortex (the precentral gyrus). A previous study reported that visuoconstructive deficits include two types of lesions, namely, parieto-occipital lesions and frontal lesions. The former can contribute to the loss of spatial organization of elements, while the latter leads to impairment in the regulation of sequential behavior and programming.Citation31 A later study presented two visuospatial processing streams, ie, posterior parietal to premotor and posterior parietal to prefrontal networks.Citation32 In COPD patients, the morbidities of impairments in intermediate visual memory and visuospatial memory are 19.2% and 26.9%, respectively.Citation33 A number of previous studies have shown visuospatial impairment in COPD patients. For example, COPD patients had markedly lower scores on the MMSE and demonstrated poorer performance on visual reproduction and visuospatial task than NCs.Citation34 White matter lesions were limited to the pathways of visual processing including inferior temporal, lingual and fusiform, posterior parietal lobe (precuneus, superior parietal lobule, and supramarginal gyrus), and optic radiation.Citation18 The visual network is the only RSN that showed deactivation in COPD patients compared with NCs; in other words, the deactive regions are restricted in the visual network, which is consistent with our findings.Citation19 In our study, patients with COPD also demonstrated a significant decrease in visuospatial function compared with NCs, which further shows that the visuospatial function of COPD patients is impaired.
The precuneus, a key part of the default mode network,Citation35 plays a core role in fundamental cognitive function and is involved in the processing of visual-spatial information, episodic memory retrieval, self-awareness, and working memory.Citation36–Citation39 A previous study showed a significant reduction in GMV in the precuneus.Citation40 Our previous research confirms that COPD patients had a significantly lower amplitude of low-frequency fluctuation (ALFF) in precuneus compared with NCs.Citation41 COPD patients showed obviously reduced cortical thickness in the parietal, motor, and prefrontal cortices, and the precuneus was included. This study indicates that cortical thinning is a core morphologic feature associated with COPD, which could be attributed to oxygen desaturation, drawing, and visual memory deficits.Citation20 Moreover, we found that the decreased ReHo area in the bilateral precuneus showed a significant positive relationship with orientation function, suggesting that impairment of the precuneus may lead to cognitive disorders. In addition, the precuneus ReHo value displayed a significant positive correlation with FEV1% and FEV1/FVC and a negative correlation with PaCO2, which suggests that hypercapnia is perhaps one of the causes of precuneus damage.
The precentral gyrus is the important area of the premotor cortex and is engaged in regulating motor function; the existing literature has reported that motor deficits are one of the cognitive deficits in patients with COPD.Citation8 Both the lingual gyrus and the occipital lobe are members of the occipitotemporal pathway, which is associated with object discriminationCitation41 and drawing,Citation42,Citation43 and they are considered to be involved in episodic memory consolidation.Citation44 The occipital lobe includes the main visual cortex V1–V5.Citation45 Research has demonstrated that late blindness in patients is correlated with altered ReHo in the occipital lobe.Citation46 The lingual gyri have been shown to be activated by angle discrimination tasks, visuospatial navigation, and tactile-guided drawing.Citation47–Citation49 The abnormal ALFF in the right lingual gyrus in COPD patients exhibited significant correlations with unsatisfactory performance in visual reproduction tasks.Citation50 These abovementioned regional lesions could result in multi-aspect cognitive impairments. The present study also confirmed the existence of cognitive impairment in multiple fields in patients with COPD, including naming, visuospatial, executive, and memory functions.
In line with the results of a previous study,Citation50 our study showed that patients with COPD had significantly lower PaO2, FVC, FEV1, FEV1/FVC, and significantly higher PaCO2 than NCs, suggesting that the reduced ReHo may result from hypoxia, hypercapnia, and altered lung function. Hypoxemia plays a crucial role in the development of cognitive impairment in patients with COPD because it impacts oxygen-dependent enzymes, which play an important role in the synthesis of neurotransmitters.Citation51 Studies have shown that chronic hypoxia decreases neuronal excitability.Citation52 A number of studies have confirmed the relationship between lung function and cognitive impairment in patients with COPD.Citation53,Citation54 Elevated PaCO2 levels are also related to delayed memory, deficits in attention and concentration, decreased reaction time, and slow information processing speed in COPD patients.Citation55 Although cigarette smoking appears to contribute to cognitive dysfunction in patients with COPD, a study revealed that healthy control smokers had normal regional GMVs when compared to healthy control nonsmokers and patients with COPD who smoked.Citation34 In addition, studies have found that associations between impaired cognitive function and COPD are not related to current smoking status.Citation54,Citation56
The present study also has several limitations that need to be considered. Systemic inflammation can exacerbate neuronal injury in patients with COPD,Citation57 and some inflammatory factors play key roles in neural activity in patients with COPD, such as C-reactive protein, interleukin (IL)-6, and IL-8. Regardless, a future study with an increased number of subjects should be conducted to more clearly investigate the effect of systemic inflammation on intrinsic brain activity. Drug therapy may have neurological effects on COPD patients; however, there is no clear evidence that these drugs have a negative impact on the brain.Citation58 The patients with acute exacerbation COPD are not suitable for undergoing rs-fMRI scan, as the image will have heavy respiratory artifact under this state, which can affect the measurement of metabolic activity in brain areas during rest state. To avoid this, we recruited only stable COPD patients. In addition, the present study was a cross-sectional study, and these results need to be observed in further longitudinal studies.
Conclusion
In conclusion, COPD patients demonstrated abnormal synchrony of regional spontaneous activity, which may result from hypoxia, hypercapnia, and altered lung function. All the regions with abnormal activity were correlated with visual processing pathways, and COPD patients showed a significant decrease in visuospatial function compared with NCs, both of which suggest that the altered synchrony of regional spontaneous activity may be related to impairments in visuospatial cognition. Furthermore, the lower ReHo in the precuneus was significantly positively correlated with FEV1% and FEV1/FVC but negatively correlated with PaCO2, which disclosed the relationship between the precuneus and clinical variables.
Acknowledgments
This study was supported by the Natural Science Foundation of China (grant numbers 81860307 and 81560285), the Natural Science Foundation Project of Jiangxi, China (grant numbers 20171BAB205070 and 20181ACB20023), Education Department Project of Jiangxi Province, China (grant numbers 700544006), Department of Health Project and Jiangxi Province, China (grant number 20181039), and the Graduate Innovation Foundation of Jiangxi, China (grant number YC2018-S099).
Disclosure
The authors report no conflicts of interest in this work.
References
- VogelmeierCFCrinerGJMartinezFJGlobal strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. Gold executive summaryAm J Respir Crit Care Med2017195555758228128970
- BairdCLovellJJohnsonMShiellKIbrahimJEThe impact of cognitive impairment on self-management in chronic obstructive pulmonary disease: a systematic reviewRespir Med201712913013928732820
- AgustíASystemic effects of chronic obstructive pulmonary disease: what we know and what we don’t know (but should)Proc Am Thorac Soc20074752252517878464
- OrganizerDCGoodeJASystemic Effects of Chronic Obstructive Pulmonary DiseaseNew YorkJohn Wiley & Sons, Ltd20087584
- Antonelli IncalziRMarraCGiordanoACognitive impairment in chronic obstructive pulmonary disease – a neuropsychological and SPECT studyJ Neurol2003250332533212638024
- KirkilGTugTOzelEBulutSTekatasAMuzMHThe evaluation of cognitive functions with p300 test for chronic obstructive pulmonary disease patients in attack and stable periodClin Neurol Neurosurg2007109755356017532116
- HungWWWisniveskyJPSiuALRossJSCognitive decline among patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2009180213413719423714
- DoddJWGetovSVJonesPWCognitive function in COPDEur Respir J201035491392220356988
- CaroneMBertolottiGAnchisiFZottiAMDonnerCFJonesPWAnalysis of factors that characterize health impairment in patients with chronic respiratory failure. Quality of life in chronic respiratory failure groupEur Respir J19991361293130010445604
- AllenSCJainMRagabSMalikNAcquisition and short-term retention of inhaler techniques require intact executive function in elderly subjectsAge Ageing200332329930212720616
- VilleneuveSPepinVRahayelSMild cognitive impairment in moderate to severe COPD: a preliminary studyChest201214261516152323364388
- KentBDMitchellPDMcnicholasWTHypoxemia in patients with COPD: cause, effects, and disease progressionInt J Chron Obstruct Pulmon Dis2011619920821660297
- HallJEGuytonACGuyton and Hall Textbook of Medical PhysiologyDenverSaunders/Elsevier2011773788
- OrtapamukHNaldokenSBrain perfusion abnormalities in chronic obstructive pulmonary disease: comparison with cognitive impairmentAnn Nucl Med20062029910616615418
- SicardKMDuongTQEffects of hypoxia, hyperoxia, and hypercapnia on baseline and stimulus-evoked BOLD, CBF, and CMRO2 in spontaneously breathing animalsNeuroimage200525385085815808985
- BorsonSScanlanJFriedmanSModeling the impact of COPD on the brainInt J Chron Obstruct Pulmon Dis20083342943418990971
- SinhaSKumarVJagannathanNRPandeyRMProton magnetic resonance spectroscopy of brain to study the cerebral metabolic abnormalities in COPD patients: a case control study in North IndiaIndian J Chest Dis Allied Sci2009511151919317358
- ZhangHWangXLinJGrey and white matter abnormalities in chronic obstructive pulmonary disease: a case–control studyBMJ Open201222e000844
- DoddJWChungAWvan den BroekMDBarrickTRCharltonRAJonesPWBrain structure and function in chronic obstructive pulmonary disease: a multimodal cranial magnetic resonance imaging studyAm J Respir Crit Care Med2012186324022652026
- ChenJLinI-TZhangHReduced cortical thickness, surface area in patients with chronic obstructive pulmonary disease: a surface-based morphometry and neuropsychological studyBrain Imaging Behav201610246447625986304
- RoosendaalSDSchoonheimMMHulstHEResting state networks change in clinically isolated syndromeBrain201013361612162120356855
- DaiZYanCLiKIdentifying and mapping connectivity patterns of brain network hubs in Alzheimer’s diseaseCerebral Cortex201525103723374225331602
- HanYWangJZhaoZFrequency-dependent changes in the amplitude of low-frequency fluctuations in amnestic mild cognitive impairment: a resting-state fMRI studyNeuroimage201155128729521118724
- LiSHuangXLiLPosttraumatic stress disorder: structural characterization with 3-T MR imagingRadiology2016280253754426928229
- PengDCDaiXJGongHHLiHJNieXZhangWAltered intrinsic regional brain activity in male patients with severe obstructive sleep apnea: a resting-state functional magnetic resonance imaging studyNeuropsychiatr Dis Treat2014101819182625278755
- TuPCHsiehJCLiCTBaiYMSuTPCortico-striatal disconnection within the cingulo-opercular network in schizophrenia revealed by intrinsic functional connectivity analysis: a resting fMRI studyNeuroimage201259123824721840407
- ZangYJiangTLuYHeYTianLRegional homogeneity approach to fMRI data analysisNeuroimage200422139440015110032
- FolsteinM“Mini-Mental State”. A practical method for grading the cognitive stage of patients for the clinicianJ Psychiatr Res199512189198
- NasreddineZSPhillipsNABédirianVThe Montreal Cognitive assessment, MoCA: a brief screening tool for mild cognitive impairmentJ Am Geriatr Soc200553469569915817019
- van DijkKRSabuncuMRBucknerRLThe influence of head motion on intrinsic functional connectivity MRINeuroimage201259143143821810475
- PillonBVisuo-constructive problems and methods of compensation. Results for 85 patients with brain lesionsNeuropsychologia1981193375383 French7266830
- KravitzDJSaleemKSBakerCIMishkinMA new neural framework for visuospatial processingNat Rev Neurosci201112421723021415848
- Antonelli-IncalziRCorsonelloATrojanoLCorrelation between cognitive impairment and dependence in hypoxemic COPDJ Clin Exp Neuropsychol200830214115018938666
- ZhangHWangXLinJReduced regional gray matter volume in patients with chronic obstructive pulmonary disease: a voxel-based morphometry studyAJNR Am J Neuroradiol201334233433922859277
- UtevskyAVSmithDVHuettelSAPrecuneus is a functional core of the default-mode networkJ Neurosci201434393294024431451
- CavannaAETrimbleMRThe precuneus: a review of its functional anatomy and behavioural correlatesBrain2006129356458316399806
- RigginsTGengFBlankenshipSLRedcayEHippocampal functional connectivity and episodic memory in early childhoodDev Cogn Neurosci201619C586926900967
- VogeleyKFinkGRNeural correlates of the first-person-perspectiveTrends Cogn Sci200371384212517357
- van SnellenbergJXSlifsteinMReadCDynamic shifts in brain network activation during supracapacity working memory task performanceHum Brain Mapp20153641245126425422039
- WangCDingYShenBAltered gray matter volume in stable chronic obstructive pulmonary disease with subclinical cognitive impairment: an exploratory studyNeurotoxicity Res2017314453463
- WangWLiHPengDAbnormal intrinsic brain activities in stable patients with COPD: a resting-state functional MRI studyNeuropsychiatr Dis Treat2018142763277230425494
- MishkinMUngerleiderLGMackoKAObject vision and spatial vision: two cortical pathwaysTrends Neurosci1983610414417
- OgawaKInuiTThe role of the posterior parietal cortex in drawing by copyingNeuropsychologia20094741013102219027762
- KukoljaJGöreciDYOnurÖARiedlVFinkGRResting-state fMRI evidence for early episodic memory consolidation: effects of ageNeurobiol Aging20164519721127459940
- MalikovicAVuceticBMilisavljevicMOccipital sulci of the human brain: variability and morphometryAnat Sci Int2012872617021993979
- HuangXYeCLZhongYLAltered regional homogeneity in patients with late monocular blindness: a resting-state functional MRI studyNeuroreport20172816108528858036
- PrvulovicDHublDSackATFunctional imaging of visuospatial processing in Alzheimer’s diseaseNeuroimage20021731403141412414280
- GrönGWunderlichAPSpitzerMTomczakRRiepeMWBrain activation during human navigation: gender-different neural networks as substrate performanceNat Neurosci20003440440810725932
- LikovaLTDrawing in the blind and the sighted as a probe of cortical reorganization [abstract]Proceedings IS&T/SPIE Electronic Imaging; Human Vision and Electronic Imaging XV75272010 Available from: https://www.spiedigitallibrary.org/conference-proceedings-of-spie/7527.tocAccessed February 14, 2019
- ZhangJChenJYuQAlteration of spontaneous brain activity in COPD patientsInt J Chron Obstruct Pulmon Dis20161111713171927555761
- HeatonRKGrantIMcsweenyAJAdamsKMPettyTLPsychologic effects of continuous and nocturnal oxygen therapy in hypoxemic chronic obstructive pulmonary diseaseArch Intern Med198314310194119476625781
- GoodallSTwomeyRAmannMAcute and chronic hypoxia: implications for cerebral function and exercise toleranceFatigue201422739225593787
- IncalziRAGemmaAMarraCMuzzolonRCapparellaOCarboninPChronic obstructive pulmonary disease. An original model of cognitive declineAm Rev Respir Dis199314824184248342906
- SachdevPSAnsteyKJParslowRAPulmonary function, cognitive impairment and brain atrophy in a middle-aged community sampleDement Geriatr Cogn Disord2006215–630030816484809
- KleinMGauggelSSachsGPohlWImpact of chronic obstructive pulmonary disease (COPD) on attention functionsRespir Med20101041526019748260
- RichardsMStrachanDHardyRKuhDWadsworthMLung function and cognitive ability in a longitudinal birth cohort studyPsychosom Med200567460260816046374
- BarnesPJChronic obstructive pulmonary disease: effects beyond the lungsPLoS Med201073e100022020305715
- OwensMYWallaceKLMamoonNWyatt-AshmeadJBennettWAAbsence of neurotoxicity with medicinal grade terbutaline in the rat modelReprod Toxicol201131444745321262341