Abstract
Background
Assessing risk of future exacerbations is an important component in COPD management. History of exacerbation is a strong and independent predictor of future exacerbations, and the criterion of ≥2 nonhospitalized or ≥1 hospitalized exacerbation is often used to identify high-risk patients in whom therapy should be intensified. However, other factors or “treatable traits” also contribute to risk of exacerbation.
Objective
The objective of the study was to develop and externally validate a novel clinical prediction model for risk of hospitalized COPD exacerbations based on both exacerbation history and treatable traits.
Patients and methods
A total of 237 patients from the COPD Registry of Changi General Hospital, Singapore, aged 75±9 years and with mean post-bronchodilator FEV1 60%±20% predicted, formed the derivation cohort. Hospitalized exacerbation rate was modeled using zero-inflated negative binomial regression. Calibration was assessed by graphically comparing the agreement between predicted and observed annual hospitalized exacerbation rates. Predictive (discriminative) accuracy of the model for identifying high-risk patients (defined as experiencing ≥1 hospitalized exacerbations) was assessed with area under the curve (AUC) and receiver operating characteristics analyses, and compared to other existing risk indices. We externally validated the prediction model using a multicenter dataset comprising 419 COPD patients.
Results
The final model included hospitalized exacerbation rate in the previous year, history of acute invasive/noninvasive ventilation, coronary artery disease, bronchiectasis, and sputum nontuberculous mycobacteria isolation. There was excellent agreement between predicted and observed annual hospitalized exacerbation rates. AUC was 0.789 indicating good discriminative accuracy, and was significantly higher than the AUC of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) risk assessment criterion (history of ≥1 hospitalized exacerbation in the previous year) and the age, dyspnea, and obstruction index. When applied to the independent multicenter validation cohort, the model was well-calibrated and discrimination was good.
Conclusion
We have derived and externally validated a novel risk prediction model for COPD hospitalizations which outperforms several other risk indices. Our model incorporates several treatable traits which can be targeted for intervention to reduce risk of future hospitalized exacerbations.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
COPD exacerbations are associated with high healthcare costs,Citation1 reduced quality of life,Citation2,Citation3 accelerated lung function decline,Citation4 and excess mortality.Citation5 Hence, assessing future risk of exacerbation is an important component in current COPD management.Citation6 History of exacerbation in the previous year, either ≥2 nonhospitalized or ≥1 leading to hospital admission, is often used to identify high-risk patients in whom bronchodilator or inhaled steroid therapy should be intensified.Citation6 This approach is based on history of exacerbation being a strong and consistent predictor of future exacerbations,Citation7 and is simple to use in clinical practice.
There are several limitations of using history of exacerbation alone to assess risk. First, exacerbations are heterogeneousCitation8 and multiple risk factors besides history of exacerbation may predispose to subsequent exacerbation such as comorbidities, inflammatory phenotype, infection, smoking, low body mass index (BMI), and lung function.Citation9 Whether inclusion of other factors (also known as “treatable traits”)Citation10 can improve the predictive accuracy for future exac-erbations is unknown. Second, a significant subset of COPD patients may exacerbate without a history of exacerbation in the past year.Citation11 In such patients, exacerbation risk is not accurately predicted by history of exacerbation. Preemptive risk assessment – using variables which are independent of the occurrence of the first exacerbation – may trigger interventions to mitigate occurrence of the first exacerbation and its associated adverse sequelae which may be irreversible such as accelerated lung function decline and mortality.Citation4,Citation5 Third, using ≥1 hospitalized or ≥2 nonhospitalized exacerbations in the past year to stratify risk is problematic because the optimal threshold for dichotomizing risk is not known,Citation7,Citation11,Citation12 and probably varies depending on the patient and health system factors. More importantly, annual exacerbation rate occurs as a gradient with a range of possible values, and dichotomizing risk based on a predetermined cut-point ignores risk differences falling outside the cut-point that might still be clinically relevant. For example, a patient who has had five exacerbations in the past year clearly has worse prognosis and needs to be managed differently compared to an individual who has had two, but both would be classified as high-risk if using a threshold of ≥2 exacerbations.
Here, our aim was to derive a model for predicting risk of hospitalized COPD exacerbations which uses multiple clinically-relevant parameters and is capable of stratifying patients along a gradient of risk. We compared the predictive accuracy of the model to the GOLD risk assessment criterion (history of ≥1 exacerbation leading to hospitalization in the previous year) and two other existing multicomponent risk indices. Finally, we validated the model in a multicenter patient cohort with distinct clinical features compared to the derivation group.
Patients and methods
Participants
We used data from the Changi General Hospital, Singapore COPD Registry for this study. The registry recruited consecutive patients aged ≥40 years, who attended the specialist COPD clinic at Changi General Hospital between January 1, 2008 and January 31, 2018. Diagnosis of COPD was based on persistent respiratory symptoms (cough, dyspnea, and sputum production) and supported by post-bronchodilator FEV1/FVC of <0.7.Citation6 We also included patients formerly classified as “GOLD 0”, who demonstrated persistent respiratory symptoms with emphysema or airway thickening on chest computed tomography, but absent airflow obstruction on spirometry.Citation13 This group of patients who do not meet standard criteria for airway obstruction are often excluded from clinical studies, despite having occult airway disease, high rate of exacerbation-like events, activity limitation, and frequent use of respiratory medications when compared to COPD patients diagnosed according to spirometric criteria.Citation13 The derivation cohort consisted of patients with complete data on all candidate variables analyzed in the model derivation phase. The multicenter validation cohort was formed by pooling patients from three sources: 1) the remaining patients from the Changi General Hospital COPD Registry who had incomplete data for model derivation but sufficient data on variables in the final model, 2) patients from the Singapore General Hospital COPD clinic recruited between 1 March 2014 and 6 October 2018 with sufficient data on variables in the final model, and 3) the first 50 consecutive patients who presented to Tan Tock Seng Hospital, Singapore with a diagnosis of COPD in 2016. The datasets used in this analysis were deidentified and anonymized in accordance with personal data protection regulations. In addition, waiver of informed consent was granted by the Singhealth Centralized Institutional Review Board (Changi General Hospital and Singapore General Hospital) and National Healthcare Group Domain Specific Review Board (Tan Tock Seng Hospital). Informed consent was waived based on ethical consideration and on the following grounds: the research was purely observational and did not interfere with patients’ usual care, the research could not reasonably be carried out without the use of health information, use of data involved no more than minimal risk to the research subject, and the waiver would not otherwise adversely affect the rights and welfare of subjects. This study complies with the Declaration of Helsinki.
Variables
We extracted the following variables from electronic discharge summaries and outpatient consult records: demographics, anthropomorphic data, COPD Assessment Test (CAT) or Modified Medical Research Council dyspnea scale (mMRC), smoking and comorbidities (coronary artery disease, atrial fibrillation, heart failure, hypertension, stroke, peripheral vascular disease, pulmonary hypertension, depression, anxiety, gastroesophageal reflux, peptic ulcer disease, gastritis, cancer, diabetes, dyslipidemia, sinonasal disease, asthma, obstructive sleep apnea, osteoporosis, chronic kidney disease, pulmonary tuberculosis [TB], and bronchiectasis). Comorbidities were obtained based on electronic medical record of International Classification of Diseases codes. Diagnosis of bronchiectasis was further confirmed by high-resolution computed tomography of the thorax. Spirometry was performed according to American Thoracic Society/ European Respiratory Society guidelines,Citation14 with predicted values obtained from Morris et alCitation15 and adjusted by a factor of 0.94 as recommended for Asian patients.Citation16 Bronchodilator reversibility was assessed 10–15 minutes after administration of 400 µg of inhaled salbutamol via a spacer.Citation14 Results of any previous sputum gram-stain, aerobic culture, Ziehl–Neelsen stain for acid-fast bacilli and mycobacterial cultures were also recorded.
Hospitalized COPD exacerbation was defined according to consensus definitionCitation17 as primary or secondary discharge diagnosis of acute COPD exacerbation. All subjects had data on number of hospitalized exacerbations in each of the two consecutive years. The primary outcome was number of hospitalized exacerbations in the second year.
Model derivation, assessment of predictive accuracy, and external validation
We modeled the primary outcome using zero-inflated negative binomial (ZINB) regression, which is suitable for over-dispersed count outcome variables with excessive zeros. To derive the prediction model, we used a univariate-based method.Citation18 Univariate ZINB regression was first performed on each candidate variable to identify factors associated with increased risk of hospitalized exacerbation. Significant variables arising from the univariate analysis were then used in multivariate ZINB regression to identify variables independently associated with increased risk of hospitalized exacerbation. Finally, we discarded variables which lost significance in the multivariate model to form the final model.
To assess model calibration, we graphed the probability of observed vs predicted annual hospitalized exacerbations rate. Receiver operating characteristics (ROC) and area under the curve (AUC) analyses were used to evaluate discriminative predictive accuracy for the identifying high-risk patients, defined as experiencing ≥1 hospitalized exacerbation. We compared AUCs between our model with three other risk assessment methods: the GOLD risk assessment criterion (history of ≥1 exacerbation leading to hospitalization in the previous year); the age, dyspnea, and obstruction (ADO) index;Citation19 and the dyspnea, obstruction, smoking, and exacerbation (DOSE) index.Citation20
To evaluate model generalizability, we assessed calibration and discrimination in the validation cohort.
The entire dataset was used in order to maximize statistical power and the present analysis fulfilled rule-of-thumb criterion of ratio of ≥10 outcome events (ie, hospitalized exacerbations) to the number of predictors used in the final model.
Statistics
Student’s t-test, chi-squared test, or Mann–Whitney U test was used to compare parametric, categorical, or nonparametric data, respectively. Statistics calculations were performed on Stata 13 (StataCorp LP, College Station, TX, USA).
Results
A total of 237 patients from the Changi General Hospital COPD Registry had complete data for model derivation, and the remaining 169 were pooled with 200 patients from the Singapore General Hospital and 50 patients from Tan Tock Seng Hospital to form the independent validation cohort (N=419). Characteristics and differences between the derivation and validation cohorts are shown in . Compared to the validation cohort, patients in the derivation cohort were significantly older and had lower BMI. In addition, the derivation cohort had lower use of short-acting bronchodilators only and higher use of triple therapy. Patients in the derivation cohort were also more likely to have a history of acute invasive/noninvasive ventilation, pulmonary hypertension, cancer, asthma, and osteoporosis than patients in the validation cohort, but prevalence of gastroesophageal reflux was lower than in the validation cohort. The derivation cohort demonstrated higher rates of long-term macrolide therapy compared to the validation cohort. Average prospective hos-pitalized exacerbation rates (primary outcome) were 1.41 and 1.04 per patient-year in the derivation and validation cohorts, respectively, (Mann–Whitney U test, P=0.03). Univariate ZINB regression using data from the derivation cohort () found that the following nontreatment variables were associated with increased risk of hospitalized exacerbation: number of hospitalized exacerbations in the previous year, previous invasive/ noninvasive ventilation, lower post-bronchodilator FEV1, CAT ≥10 or mMRC ≥2, coronary artery disease, anxiety, bronchiectasis, history of TB, and sputum nontuberculous mycobacteria (NTM) isolation. The final multivariate model, shown in , found that number of hospitalizations in previous year, previous invasive/noninvasive ventilation, coronary artery disease, bronchiectasis, and sputum NTM isolation were independently associated with increased risk of hospitalized exacerbation.
Table 1 Patient characteristics
Table 2 Univariate ZINB regression for the primary outcome of number of hospitalized exacerbations/year
Table 3 Final multivariate ZINB regression model for the primary outcome of number of hospitalized exacerbations/year
Observed and predicted annual hospitalized exacerbation rates for the final multivariate model were in good agreement, as depicted in . ROC curves of our model vs GOLD risk assessment criterion for identifying high-risk patients are shown in . Predictive accuracy of our model was significantly greater than using the GOLD risk assessment criterion (AUC: 0.789 [95% CI: 0.732–0.846] vs AUC: 0.690 [95% CI: 0.631–0.748], P=0.001). A subset of the derivation cohort, n=222, had complete data for calculating ADO and DOSE risk scores (). Predictive accuracy of our model was higher than that for the ADO index (AUC: 0.789 [95% CI: 0.731–0.848] vs AUC: 0.656 [95% CI: 0.586–0.727], P=0.002). AUC of the DOSE index was 0.717 (95% CI: 0.652–0.782), which was lower than the AUC of our model at borderline significance, P=0.05.
Figure 1 Observed and predicted values for the primary outcome of annual hospitalized exacerbation rate for (A) the derivation cohort and (B) the multicenter independent validation cohort. Data are shown for hospitalized exacerbation rate of up to eight per year.
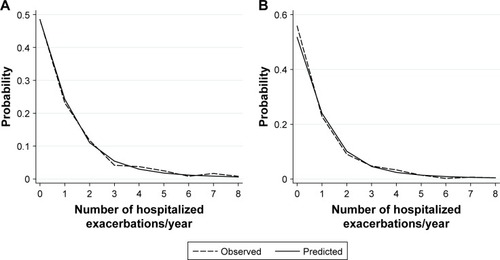
Figure 2 ROC curves of our model (solid line and closed circles) and GOLD risk assessment criterion (history of ≥1 exacerbation leading to hospitalization in the previous year, dashed line and open circles) for identifying high-risk patients in (A) the derivation cohort and (B) the independent multicenter validation cohort.
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; ROC, receiver operating characteristics.
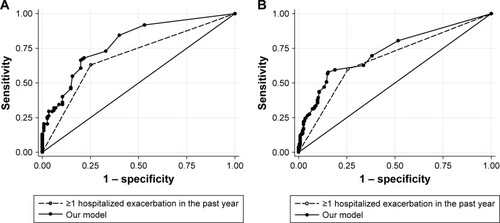
Figure 3 ROC curves of our model, DOSE index, ADO index, and GOLD risk assessment criterion (history of ≥1 exacerbation leading to hospitalization in the previous year) for identifying high-risk patients.
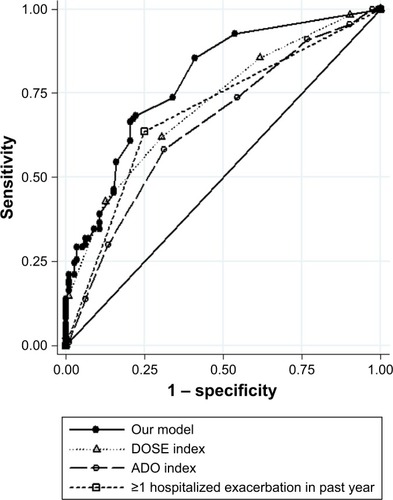
Applying our multivariate model to the validation cohort, we again found good agreement between observed and predicted annual hospitalized exacerbation rates (). In addition, predictive accuracy for high-risk patients remained good and was significantly superior to the GOLD risk assessment criterion in the validation cohort (, AUC: 0.728 [95% CI: 0.680–0.777] vs AUC: 0.671 [95% CI: 0.627–0.717], P=0.01).
Discussion
In this study, our aim was to develop and externally validate a novel clinical prediction model for risk of hospitalized COPD exacerbations based on both exacerbation history and treatable traits. We identified several factors independently associated with increased prospective risk of hospitalized COPD exacerbation: number of hospitalized exacerbations in the previous year, previous invasive/noninvasive ventilation, bronchiectasis, sputum NTM isolation, and coronary artery disease. A risk prediction model comprising these multiple variables demonstrated good predictive accuracy and significantly outperformed the GOLD risk assessment criterion (history of ≥1 exacerbation leading to hospitalization in the previous year) and the ADO index for identifying patients at high-risk of hospitalized exacerbation. Predictive accuracy of our model was also superior to the DOSE index, but statistical significance was borderline (P=0.05). In addition, our model was capable of predicting number of hospitalized exacerbations over a range of possible values, and stratified patients along a gradient of risk instead of two broad risk categories. We validated the risk prediction model in a multicenter cohort of COPD patients with distinct clinical characteristics and found that predictive accuracy remained good.
History of exacerbation is the strongest predictor of future exacerbations and, therefore, forms the basis for assessing future risk in the GOLD strategy. There are several multidimensional prognostic indices available for use in COPD patients, including for the prediction of exacerbations,Citation21 but to our knowledge, no study has demonstrated that inclusion of other variables besides history of exacerbation leads to better risk prediction. Motegi et alCitation22 prospectively evaluated the discriminative accuracies of the ADO, DOSE, and Body mass index, airflow Obstruction, Dyspnea, and Exercise (BODE) indices for predicting exacerbations ascertained from symptom diaries. AUC values obtained for ADO and DOSE were similar to those found in the present study despite methodological differences, but in that study, none of the indices was significantly better than history of exacerbation alone for predicting future exacerbations. ADO and DOSE were evaluated in another study by Jones et al using international multicenter datasets and found to be weak predictors of hospitalized and nonhos-pitalized exacerbations.Citation23 In contrast, our model demonstrated better discriminative accuracy for predicting hospitalized exacerbations than both ADO and the GOLD criterion, and a trend toward superior predictive accuracy compared to DOSE. Inclusion of comorbidities and infection, important dimensions known to affect exacerbation risk, is unique to our model and not used by other existing risk indices.
History of exacerbation,Citation7,Citation11,Citation24,Citation25 requirement for invasive/ noninvasive ventilation,Citation26 bronchiectasis,Citation27,Citation28 and sputum NTMCitation29,Citation30 have all been described as independent associations of severe exacerbations. In addition, some of the significant univariate associations such as anxiety,Citation31 dyspnea ratings,Citation11,Citation24,Citation32,Citation33 and lower FEV Citation7,Citation32 Citation1 have been reported in other studies to predict exacerbations, although in the present study they became nonsignificant in the final, fully-adjusted model. Only a few studies have investigated whether coronary artery disease is a risk factor for frequent exacerbations, and their results were conflicting. At least two studies did not find increased rate of exacerbations,Citation34,Citation35 although one study reported longer duration of exacerbation.Citation35 Notably, studies on risk factors associated with exacerbations have variable findings. This likely reflects differences in the patient population and risk factors studied, healthcare systems as well as definitions of exacerbation. An important source of variability is the inherent heterogeneity of exacerbation events, which may represent myriad clinicopathologic subtypes and mechanisms,Citation8 each with different risk factors.
In addition to ADO, DOSE, and the GOLD criterion, there are several other risk prediction models for COPD hospitalizations or readmissions, including SCOPEXCitation36 and PEARL.Citation37 Which prediction model to use depends not only on predictive accuracy but also on ease of use and practicability, such as whether the predictor variables are available or routinely collected in clinical practice, and this will vary depending on clinician or hospital practice patterns. Importantly, some of the variables in our full prediction model represent potential modifiable targets or “treatable traits”Citation10 for reducing hospitalized exacerbation risk. For example, domiciliary noninvasive ventilation has been demonstrated to prolong time to readmission in COPD patients with persistent hypercapnia after exacerbation associated with acute hypercapnic respiratory failure.Citation38 The presence of comorbid bronchiectasis may focus the attention of the clinician on treating both COPD and bronchiectasis, the latter mainly aimed at airway secretion clearance and treating acute or chronic airway infections.Citation39 There is insufficient evidence to guide the management of patients with COPD-bronchiec-tasis overlap. However, COPD-bronchiectasis overlap may influence the selection of therapies, including avoidance of inhaled corticosteroids, or use of long-term macrolides which have efficacy in reducing exacerbations in both COPDCitation40 and bronchiectasis.Citation41 Similarly, NTM lung disease represents a treatable pulmonary infection, although treatment of NTM in the context of COPD has not been studied. The armamen-tarium of interventions for coronary artery disease is wide and includes coronary revascularization, antiplatelet therapy, beta-blockers, statins, and angiotensin-converting enzyme inhibitors. Evidence from observational studies indicate that beta-blockers may have a beneficial effect on reducing COPD exacerbations in patients with coronary artery disease.Citation42–Citation44
The strengths of our study are: use of a real-world nonclinical trial COPD patient population with significant comorbidities and overlap with other respiratory diseases, and external validation in a multicenter cohort with several significantly different characteristics compared to the derivation cohort. In addition, bronchiectasis was diagnosed by computed tomography, which is the gold standard. There are several important limitations. First, although the prediction model was validated in other hospitals, the wider general-izability of the prediction model remains unassessed. The study was conducted in Singapore and applicability in other countries may be limited due to different underlying disease processes and prevalence of comorbidities. Thus, the risk prediction model needs to be prospectively evaluated and further validated in other countries and healthcare settings. Second, the model does not include biomarkers such as blood eosinophils and fibrinogen which are known to predict exacerbations and hospitalizations,Citation45 and should be a focus of future research. Third, clinical utility and impact of the model has not been evaluated. For example, estimating a patient’s risk using our prediction model would require a risk calculator, and some of the variables in the model may not be available in primary care, eg, computed tomography diagnosis of bronchiectasis or sputum cultures, thereby limiting usefulness of the risk prediction model in the primary care setting. Whether the risk prediction model will result in meaningful improvements in patient outcomes, care processes, or cost has not been evaluated. Fourth, we focused exclusively on severe exacerbations requiring hospitalization and did not have data on mild or moderate exacerbations not leading to hospitalization, which may impact on effect size of the incident rate ratios obtained here.Citation46 Nevertheless, ascertainment from primary hospital discharge diagnosis of COPD exacerbation is objective and widely accepted,Citation17 and could be reliably assessed using our electronic medical records-based methodology.
Conclusion
To conclude, we have derived and validated a novel risk prediction model based on history of exacerbation and other “treatable traits” which are known to contribute to exacerbation risk. Our model outperforms several other existing risk indices and is capable of stratifying patients along a gradient of risk. Future studies should aim to assess whether using this prediction model to target treatable traits has any clinical utility in preventing COPD exacerbations. Further impact analysis is also needed to investigate if implementation of our risk prediction model is better than usual care for the patient, care processes or cost outcomes.
Acknowledgments
The authors would like to thank Mr Gerald Sim and Changi General Hospital Clinical Trials Research Unit for their assistance in conducting this study. The abstract of this paper was presented at Asian Pacific Society of Respirology 2018 Congress as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Res-pirology: https://onlinelibrary.wiley.com/doi/full/10.1111/ resp.13420_131.
Disclosure
AA received grants and/or personal fees from GlaxoSmith-Kline, AstraZeneca, Novartis, and Boehringer Ingelheim. PYT is supported by Singapore Ministry of Health National Medical Research Council under its Research Training Fellowship (NMRC/Fellowship/0049/2017). The other authors report no conflicts of interest in this work.
References
- DalalAChristensenLLiuFRiedelAADirect costs of chronic obstructive pulmonary disease among managed care patientsInt J Chron Obstruct Pulmon Dis2010534134921037958
- SeemungalTADonaldsonGCPaulEABestallJCJeffriesDJWedzi-chaJAEffect of exacerbation on quality of life in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19981575 Pt 1141814229603117
- MiravitllesMFerrerMPontAEffect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up studyThorax200459538739515115864
- DransfieldMTKunisakiKMStrandMJAcute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20171953330
- SuissaSdell’anielloSErnstPLong-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortalityThorax2012671195796322684094
- Global Initiative for Chronic Obstructive Lung Disease (GOLD)GOLD 2018 global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2018 report2018
- HurstJRVestboJAnzuetoASusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- BafadhelMMckennaSTerrySAcute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkersAm J Respir Crit Care Med2011184666267121680942
- ViniolCVogelmeierCFExacerbations of COPDEur Respir Rev20182714717010329540496
- AgustiABelEThomasMTreatable traits: toward precision medicine of chronic airway diseasesEur Respir J201647241041926828055
- HanMKQuibreraPMCarrettaEEFrequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohortLancet Respir Med20175861962628668356
- Le RouzicORocheNCortotABDefining the “Frequent Exac-erbator” phenotype in COPD: a hypothesis-free approachChest201815351106111529054347
- WoodruffPGBarrRGBleeckerEClinical significance of symptoms in smokers with preserved pulmonary functionN Engl J Med2016374191811182127168432
- MillerMRHankinsonJBrusascoVStandardisation of spirometryEur Respir J200526231933816055882
- MorrisJFKoskiAJohnsonLCSpirometric standards for healthy nonsmoking adultsAm Rev Respir Dis1971103157675540840
- PellegrinoRViegiGBrusascoVInterpretative strategies for lung function testsEur Respir J200526594896816264058
- BurgeSWedzichaJACOPD exacerbations: definitions and classificationsEur Respir J Suppl20034146s53s12795331
- AdamsSTLevesonSHClinical prediction rulesBMJ2012344d831222250218
- PuhanMAGarcia-AymerichJFreyMExpansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated bode index and the ado indexThe Lancet20093749691704711
- JonesRCDonaldsonGCChavannesNHDerivation and validation of a composite index of severity in chronic obstructive pulmonary disease: the dose indexAm J Respir Crit Care Med2009180121189119519797160
- DijkWDBemtLHaak-RongenSMultidimensional prognostic indices for use in COPD patient care. A systematic reviewRespir Res201112115122082049
- MotegiTJonesRCIshiiTA comparison of three multidimensional indices of COPD severity as predictors of future exacerbationsInt J Chron Obstruct Pulmon Dis2013825927123754870
- JonesRCPriceDChavannesNHMulti-component assessment of chronic obstructive pulmonary disease: an evaluation of the ado and dose indices and the global obstructive lung disease categories in international Primary care data setsNPJ Prim Care Respir Med20162611601027053297
- Montserrat-CapdevilaJGodoyPMarsalJRBarbéFGalvánLRisk factors for exacerbation in chronic obstructive pulmonary disease: a prospective studyInt J Tuberc Lung Dis201620338939527046722
- MüllerovaHMaselliDJLocantoreNHospitalized exacerbations of COPDChest20151474999100725356881
- ChuCMChanVLLinAWWongIWLeungWSLaiCKReadmission rates and life threatening events in COPD survivors treated with non-invasive ventilation for acute hypercapnic respiratory failureThorax200459121020102515563699
- MinovJStoleskiSMijakoskiDVasilevskaKAtanasovskaAExacerbations in COPD patients with bronchiectasisMedical Sciences2017527
- NiYShiGYuYHaoJChenTSongHClinical characteristics of patients with chronic obstructive pulmonary disease with comorbid bronchiectasis: a systemic review and meta-analysisInt J Chron Obstruct Pulmon Dis2015101465147526251586
- DielRJacobJLampeniusNBurden of non-tuberculous myco-bacterial pulmonary disease in GermanyEur Respir J2017494160210928446559
- HuangCTTsaiYJWuHDImpact of non-tuberculous mycobac-teria on pulmonary function decline in chronic obstructive pulmonary diseaseInt J Tuberc Lung Dis201216453954522325332
- PoolerABeechRExamining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic reviewInt J Chron Obstruct Pulmon Dis2014931533024729698
- McgarveyLLeeAJRobertsJGruffydd-JonesKMcknightEHaughneyJCharacterisation of the frequent exacerbator phenotype in COPD patients in a large UK primary care populationRespir Med2015109222823725613107
- WanESDemeoDLHershCPClinical predictors of frequent exacerbations in subjects with severe chronic obstructive pulmonary disease (COPD)Respir Med2011105458859421145719
- JonesPWMullerovaHAgustiACardiovascular disease does not predict exacerbation rate or mortality in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2018197340040328665686
- PatelARCDonaldsonGCMackayAJWedzichaJAHurstJRThe impact of ischemic heart disease on symptoms, health status, and exacerbations in patients with COPDChest2012141485185721940771
- MakeBJErikssonGCalverleyPMA score to predict short-term risk of COPD exacerbations (SCOPEX)Int J Chron Obstruct Pulmon Dis20151020120925670896
- EchevarriaCSteerJHeslop-MarshallKThe pearl Score predicts 90-day readmission or death after hospitalisation for acute exacerbation of COPDThorax201772868669328235886
- MurphyPBRehalSArbaneGEffect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbationJAMA2017317212177218628528348
- PolverinoEGoeminnePCMcdonnellMJEuropean Respiratory Society guidelines for the management of adult bronchiectasisEur Respir J2017503170062928889110
- AlbertRKConnettJBaileyWCAzithromycin for prevention of exacerbations of COPDN Engl J Med2011365868969821864166
- KellyCChalmersJDCrossinghamIMacrolide antibiotics for bronchiectasisCochrane Database Syst Rev20183CD01240629543980
- BhattSPWellsJMKinneyGLβ-Blockers are associated with a reduction in COPD exacerbationsThorax201671181426283710
- duQSunYDingNLuLChenYBeta-blockers reduced the risk of mortality and exacerbation in patients with COPD: a meta-analysis of observational studiesPLoS One2014911e11304825427000
- RuttenFHZuithoffNPHakEGrobbeeDEHoesAWBeta-blockers may reduce mortality and risk of exacerbations in patients with chronic obstructive pulmonary diseaseArch Intern Med20101701088088720498416
- CouillardSLarivéePCourteauJVanasseAEosinophils in COPD exacerbations are associated with increased readmissionsChest2017151236637327746201
- EffingTWKerstjensHAMMonninkhofEMDefinitions of exacerbationsChest2009136391892319736196
