Abstract
Background
The aim of this study was to determine whether long-term intermittent azithromycin therapy reduces the frequency of exacerbation in severe chronic obstructive pulmonary disease (COPD).
Methods
We retrospectively investigated the clinical benefits of long-term azithromycin (500 mg orally three times per week) over 12 months in patients with severe COPD and a minimum of four acute exacerbations (AECOPD) per year or chronic bronchial colonization by Pseudomonas aeruginosa, comparing the number of AECOPD, hospitalizations due to respiratory disease, days of hospital stay, and bacterial infections during azithromycin treatment and in the year prior to this therapy.
Results
Twenty patients who completed the 12-month treatment period were analyzed. No clinically significant adverse events were observed during azithromycin treatment. Compared with baseline data, azithromycin therapy significantly reduced the number of AECOPD (2.8 ± 2.5 versus 6.8 ± 2.8, P < 0.001), hospitalizations (1.4 ± 1.5 versus 3.6 ± 1.4, P < 0.001), and cumulative annual days of hospital stay (25 ± 32.2 versus 43.7 ± 21.4, P = 0.01). The improvement was particularly significant in patients with exacerbations caused by common potentially pathogenic microorganisms, who had 70% fewer AECOPD and hospitalizations. Patients colonized by P. aeruginosa had reductions of 43% in AECOPD and 47% in hospitalizations.
Conclusion
Long-term azithromycin is well tolerated and associated with significant reductions in AECOPD, hospitalizations, and length of hospital stay in patients with severe COPD.
Introduction
Acute exacerbations of chronic obstructive pulmonary disease (COPD) are a frequent event during evolution of the disease,Citation1 and the mortality risk increases with its frequency, especially when patients require hospitalization.Citation2,Citation3 Previous studies using protected specimen brushing have shown that bacterial infection could be the etiology in approximately 50% of acute exacerbations of COPD (AECOPD).Citation4 However, it is sometimes difficult to distinguish colonization from infection when organisms are found in sputum cultures from COPD patients.
Recently, interest in the use of prophylactic antibiotics to prevent AECOPD has increased,Citation5 and macrolides in this setting have the advantage of having both antibacterial and anti-inflammatory properties. Long-term macrolide therapy is routinely used in two diseases, ie, diffuse panbronchiolitis and cystic fibrosis, both of which involve chronic airway inflammation. Erythromycin is the most commonly used macrolide in diffuse panbronchiolitis, and obtains notable improvements in symptoms and survival. Studies in cystic fibrosis have mostly used azithromycin and have found improvement in lung function and a reduced exacerbation frequency.Citation7,Citation8 These effects are probably due to modulation of the inflammatory response and its ability to impede formation of biofilm.Citation9 Azithromycin has also been shown to be useful in the treatment of patients with bronchiectasis and chronic bronchial infection by Pseudomonas aeruginosa.Citation10,Citation11
Given the importance of inflammationCitation12 and bacterial infection in the pathogenesis of COPD, it has been proposed that macrolides may offer unique advantages as disease-modifying agents. Only two studies to date have analyzed the effectiveness and safety of long-term erythromycin in COPD over a 12-month period, reporting a significant reduction in moderate to severe AECOPD.Citation13,Citation14 It remains to be established whether the therapeutic effect of erythromycin reflects antimicrobial activity, an immunomodulatory effect, or both. Preliminary data have recently been reported for the MACRO study, a randomized controlled trial evaluating the utility of long-term azithromycin therapy to reduce AECOPD, with promising results.Citation15 Compared with erythromycin, the prototypical 15 member-ring macrolide, azithromycin, appears to have a better safety profile in long-term use, as well as improved bacteriological activity.Citation16
In this study, we investigated: the usefulness of long-term intermittent azithromycin therapy in reducing exacerbation frequency in severe COPD patients at a high risk of AECOPD despite conventional maximum treatment; its impact on the bacteriology of bronchial secretions, examining baseline and follow-up colonization by P. aeruginosa or other potentially pathogenic microorganisms; and its impact on the development of resistance to macrolides.
Materials and methods
Subjects
We identified a cohort of 203 patients with severe COPD, with a postbronchodilator forced expiratory volume in one second (FEV1) <50% of predicted and routinely controlled at the respiratory day care unit of the Sabadell Hospital in Barcelona, Spain, between March 2007 and August 2009. Patients with chronic bronchitis who had repeated AECOPD (at least four exacerbations in the previous year) or chronic bronchial colonization by P. aeruginosa treated with longterm azithromycin therapy were recruited for this study. Patients with asthma, significant bronchiectasis, malignancy, unstable heart disease, or liver disease were excluded. Ethical permission for the study was obtained from the Sabadell Hospital ethics committee.
Study design
Retrospective analysis of data for the year before initiation of long-term azithromycin therapy (for 12 months) in patients with severe COPD was undertaken in order to assess the clinical benefits of this treatment to reduce AECOPD frequency. Azithromycin (one 500 mg tablet) was administered three times per week (Monday, Wednesday, Friday), in accordance with standard practice in patients with bronchiectasis associated with cystic fibrosis and chronic bronchial colonization by P. aeruginosa.Citation17
Patients were classified into three groups according to the potentially pathogenic microorganisms isolated from their sputum samples during AECOPD in the year prior to azithromycin therapy, ie: Group 1, patients with at least two positive cultures for common potentially pathogenic microorganisms (Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis); Group 2, patients with chronic bronchial colonization by P. aeruginosa (at least three consecutive sputum cultures for potentially pathogenic microorganisms during a 6-month period of stability);Citation18 and Group 3, patients whose cultures alternated between being positive for common potentially pathogenic microorganisms and P. aeruginosa during exacerbations but without chronic bronchial colonization by this bacteria.
We compared the following parameters overall and by group before and after the 12-month azithromycin treatment period: number of registered AECOPD; number of hospitalizations due to respiratory disease; and length of hospital stay. We also traced the evolution of positive cultures for potentially pathogenic microorganisms during AECOPD in each group and the development of resistance to azithromycin before and after long-term therapy.
All patients underwent the same scheduled clinical assessments at the respiratory day care unitCitation19 by the same team of pulmonologists before and after starting long-term azithromycin therapy. At baseline, pulmonary function tests and use of home oxygen therapy were recorded. A high-resolution computed tomography of the chest was performed in all patients in a stable state to assess and quantify possible bronchiectasis. Monitoring visits were conducted every 3 months during the 2 years of study.
Number of registered AECOPD, hospitalizations due to respiratory disease, length of hospital stays, and microbiological isolates in the previous 12 months were recorded just before the initiation of azithromycin therapy and 1 year later. A sputum sample was routinely collected during each AECOPD. All patients received regular treatment with long-acting beta-agonists, long-acting anticholinergics, and inhaled corticosteroids during the 2 years of study.
When used in patients with chronic P. aeruginosa colonization, inhaled colimycin therapy was maintained during the full length of the study and recorded. During follow-up, an electrocardiogram, liver and renal function test, and complete blood count measurements were performed before and after initiation of azithromycin treatment. The appearance of possible adverse events was assessed at each monitoring visit.
Assessment of bronchiectasis
Bronchiectasis in the high-resolution computed tomography scan was scored in each lobe by consensus, using the grading system proposed by Smith et alCitation20 as follows: 0 if no bronchiectasis was present, 1 if <25% of bronchi were bronchiectatic, 2 if 2%–49% bronchi were bronchiectatic, 3 if 50%–74% bronchi were bronchiectatic, and 4 if ≥75% of the bronchi were bronchiectatic. The lingula was graded as a separate lobe, resulting in a maximum score of 24 per patient. Patients with a score ≤1 were considered normal.
Definition and treatment of exacerbations
AECOPD was defined as a sustained worsening of the patient’s condition from the stable state and beyond normal day-to-day variations, of acute onset, and requiring a change in regular treatment (oral corticosteroids and/or antibiotics and/or hospital admission) in a patient with underlying COPD.Citation21
A chest radiography was performed for each AECOPD to exclude acute pneumonia. AECOPD were attended from Monday to Friday (8 am to 5 pm) at the respiratory day care unit, always by the same team of pulmonologists.Citation19 Outside these hours, AECOPD were attended at the emergency department and registered by reviewing the emergency reports.
Antibiotics were prescribed during AECOPD according to Anthonisen’s criteria and were adjusted by antibiogram, maintaining azithromycin at the same dose.Citation22 Indications of hospital admission were made according to clinical practice guidelines.Citation23
Bacteriological assessments
Sputum samples were collected from all patients for each AECOPD and were processed locally for Gram stain and bacteriological culture. Identification and antibiogram were performed on all bacterial isolates following standardized microbiological protocols.Citation24 Positive cultures for P. aeruginosa during AECOPD were followed by performing additional sputum cultures at every follow-up visit to identify chronic colonization, diagnosed when three or more consecutive sputum cultures for this potentially pathogenic microorganism were found over a period of 6 months in clinically stable patients, in samples separated by at least 1 month.Citation18
Statistical analysis
All the information was entered into a database and analyzed using SPSS version 17 (SPSS Inc, Chicago, IL). Quantitative variables are expressed as mean ± standard deviation, and categorical variables as absolute and relative frequencies. The frequency and length of exacerbations and the microbiology of the sputum cultures before and after azithromycin treatment were compared. Statistical analysis was performed using the Student’s t-test for paired data and Chi-square tests as required. All statistical tests were performed with a confidence level of 95%.
Results
Twenty-four eligible patients with severe COPD and frequent AECOPD from the cohort of 203 patients controlled at the respiratory day care unit agreed to participate in this study. Baseline variables for the long-term azithromycin treatment group are shown in . Ten of 24 patients (41.6%) had no detectable bronchiectasis on high-resolution computed tomography. The median ± standard deviation of total bronchiectasis scoreCitation20 was 3.3 ± 3.5 (range 0–9).
Table 1 Baseline variables of the first year of follow-up for the long-term azithromycin therapy group
After initiation of azithromycin, four patients did not complete the scheduled 12-month antibiotic treatment period. One was withdrawn for mild dyspepsia, one because of a diagnosis of malignancy during follow-up, and the other two discontinued the treatment prematurely as a personal decision in the absence of reported side effects. No significant adverse events were observed among the 20 patients who completed the 12-month treatment period. One patient died in hospital as a result of an AECOPD during the last month of follow-up. These 20 patients comprised the sample for the comparative study.
In the 12 months prior to the start of azithromycin, the 20 study participants had a total of 136 AECOPD, of which 72 (52.9%) were severe and required hospitalization. Long-term azithromycin therapy achieved statistically significant reductions in AECOPD from 136 to 57 (a 58.9% decrease) and hospitalizations from 72 to 28 (a 61.2% decrease) and a reduction of 18.7 days in yearly mean hospital stay due to respiratory disease (see ).
Table 2 Number of AECOPD, hospitalizations due to respiratory disease, and days of hospitalization with and without azithromycin, overall and according to the group of potentially pathogenic microorganisms isolated from sputum samples before initiation of azithromycin
On the basis of potentially pathogenic microorganisms isolated in sputum samples during the year prior to starting azithromycin, seven patients with common potentially pathogenic microorganisms were included in Group 1, nine patients with chronic bronchial colonization by P. aeruginosa in Group 2 (of whom five [55.5%] were receiving inhaled colimycin therapy), and four patients with exacerbations due to common microorganisms and P. aeruginosa in Group 3. Long-term azithromycin therapy reduced the number of AECOPD, hospitalizations, and days of hospital stay in all groups. This reduction was particularly significant in Group 1 (common potentially pathogenic microorganisms) with a 70% reduction in AECOPD and hospitalizations, and a mean reduction of 25 days in mean hospital stay. In the group colonized by P. aeruginosa, a statistically significant reduction in AECOPD of 43.5% was observed, the number of hospitalizations fell by 47.1%, and their hospital stays by 32.5%, although these differences did not reach statistical significance. The group alternating between common potentially pathogenic microorganisms and P. aeruginosa during exacerbations showed improvements in all parameters studied, but no statistical comparisons were performed because of their small sample size (see and ). AECOPD with sputum cultures isolating only common potentially pathogenic microorganisms fell from 31 of an overall 136 (22.7%) to five of 57 (8.7%) during long-term azithromycin therapy (P < 0.05).
Figure 1 Number of AECOPD per patient before and after long-term azithromycin therapy. The continuous line represents Group 1 patients with potentially pathogenic microorganisms and the discontinuous line represents Group 2 patients with chronic bronchial colonization by Pseudomonas aeruginosa.
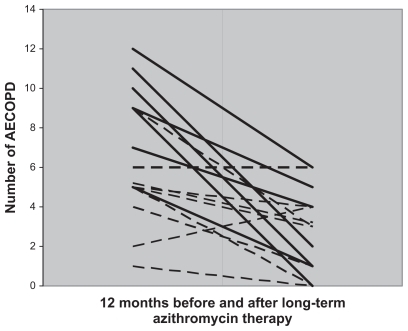
Long-term azithromycin therapy rendered sputum cultures negative during AECOPD in nine of 20 patients during follow-up, including four from the common potentially pathogenic microorganism group, four from the P. aeruginosa colonization group (without any significant association with the presence of mucoid forms or inhaled colimycin therapy, data not shown), and one from the common potentially pathogenic P. aeruginosa group. shows the microbiological evolution of patients during azithromycin treatment in relation to sputum cultures obtained during the first year.
Table 3 Microbiological evolution during long-term azithromycin therapy according to baseline groups and sputum culture isolates during AECOPD
shows the sensitivity of common potentially pathogenic microorganisms to macrolides before and after long-term azithromycin therapy. Interestingly, all strains of H. influenzae isolated were resistant to erythromycin and 30% to clarithromycin, while none were resistant to azithromycin prior to treatment. The macrolide-resistant S. pneumoniae strains were also resistant to clindamycin, representing a highly resistant phenotype.
Table 4 Common microorganisms isolated in sputum culture during exacerbations before and after starting azithromycin and its antibiogram to macrolides
On assessment of the bactericidal effect of long-term azithromycin therapy on common potentially pathogenic microorganisms and the development of bacterial resistance, we found no isolates of M. catarrhalis with azithromycin resistance during follow-up, a single isolate of H. influenzae with resistance to the antibiotic, and four isolates of S. pneumoniae, all resistant to azithromycin. The prevalence of resistance did not show statistically significant differences from the figures found in the first year of the study.
Discussion
This is the first study to examine long-term intermittent azithromycin therapy over a 12-month period in patients with severe COPD and repeated AECOPD, or chronically colonized by P. aeruginosa, assessing its usefulness in reducing exacerbation frequency and its bactericidal effect, and comparing the results of sputum cultures at exacerbation with the prevalence of bacteria-related exacerbation before the treatment. Our results show that long-term intermittent dosing of azithromycin as addon therapy to conventional maximum triple therapy (longacting anticholinergics, long-term beta-agonists, and inhaled corticosteroids) reduces the number of AECOPD and hospitalizations by more than a half, and also the days of hospital stay. The treatment was well tolerated over the 12-month period, and was stopped by only one patient due to dyspepsia.
Few studies focusing on the prophylactic use of antibiotics in COPD to prevent AECOPD have been published thus far, most of them examining long-term use of fluoroquinolones or macrolides. Sethi et al demonstrated that a 12-month regime with pulsed moxifloxacin 400 mg once a day for 5 days every 8 weeks achieved a 25% reduction in AECOPD, and a larger reduction (45%) in patients who reported mucopurulent sputum at baseline. The authors concluded that pulsed moxifloxacin might be indicated in COPD patients with a high frequency of AECOPD, but excluded patients colonized by P. aeruginosa, one of the specific subgroups discussed in our study, because of the risk of development of resistant strains.Citation25 Long-term macrolide therapy in COPD was also advocated in two published studies which analyzed erythromycin over a period of 12 months, with a significant reduction in the number and severity of AECOPD.Citation13,Citation14 In the first, Suzuki et al reported the results of a prospective randomized trial of erythromycin therapy 200–400 mg/day versus nonactive treatment for 12 months in 109 COPD patients (average FEV1 1.4 L).Citation13 They found a statistically significant increase in the relative risk of AECOPD in the control group versus the erythromycin group of 4.71 (95% CI, 1.53–14.5; P = 0.007) and more severe AECOPD in the control group than in the erythromycin group (P = 0.0007). In the second, a single-center, randomized, controlled trial, Seemungal et al administered erythromycin 250 mg twice daily or placebo for 1 year to 109 patients with moderate to severe COPD (mean FEV1 50% of predicted).Citation14 Erythromycin reduced AECOPD in relative terms by 35% and increased the median time to first exacerbation from 89 to 271 days, both differences being statistically significant.
Preliminary data from the prospective, placebo-controlled MACRO study in 1142 COPD patients randomized to receive azithromycin 250 mg or placebo daily for 1 year have been promising.Citation15 Inclusion criteria were use of supplemental oxygen or treatment with systemic steroids for an AECOPD within the previous year. The frequency of AECOPD for those receiving azithromycin was lower at 1.4 versus 1.8 patients/year (P = 0.004).
The microbiology data collected from the MACRO study have not yet been formally published.
Neither study assessed the impact of treatment in patients colonized by P. aeruginosa. Seemungal et al included patients with moderate to severe COPD in their series, of whom only 35% had three or more AECOPD during the year prior to inclusion. In the MACRO study, the frequency of AECOPD was also low. Our study enrolled an homogeneous population sample of severe or very severe COPD with very frequent AECOPD and patients with chronic colonization by P. aeruginosa.
Azithromycin offers clinical advantages over erythromycin. Its metabolism does not interfere with the metabolic pathway of cytochrome P450, thus avoiding possible metabolic interference with other drugs often used in COPD which share the same pathway, such as steroids and theophylline. It has better gastrointestinal tolerance and less hepatotoxicity, and because it is not associated with long QT syndrome, is better tolerated and has a better safety profile in long-term use.Citation26 Even though macrolides provide adequate coverage for the most frequent potentially pathogenic microorganisms identified in AECOPD, their activity against H. influenzae differs. Azithromycin, the prototypical 15-member ring macrolide, has greater bacteriological and clinical activity than 14-membered ring macrolides, such as erythromycin and clarithromycin.Citation27 Interestingly, in our study, all strains of H. influenzae isolated prior to the initiation of long-term azithromycin were resistant to erythromycin and 30% were resistant to clarithromycin, while none was resistant to azithromycin.
The subanalysis shows that Group 1, despite being the one with the highest number of AECOPD and hospitalizations prior to azithromycin therapy, demonstrated the greatest improvement with long-term azithromycin treatment, with highly significant reductions of around 70% in AECOPD and hospitalizations. The mechanism of improvement may be related to the antibacterial activity of azithromycin, particularly with regard to H. influenzae and M. catarrhalis. AECOPD with isolates positive for these potentially pathogenic microorganisms fell from 25 pre-azithromycin to just one (for H. influenzae) during long-term azithromycin therapy. However, we did not observe this level of bacterial eradication for S. pneumoniae, which has a higher prevalence of resistance to azithromycin, independently of the use of this antibiotic in the study.
We cannot exclude the possibility that the improvement observed may be due in part to the anti-inflammatory and immunomodulatory properties of azithromycin.Citation9 Azithromycin may decrease sputum volume and its viscoelasticity, and increase mucociliary transport.Citation28 Azithromycin also accumulates in neutrophils, interfering with chemotaxis to the inflammatory focus and promoting neutrophil apoptosis and clearance by macrophages.Citation29 In their trial with long-term erythromycin, Seemungal et al analyzed inflammatory mediators in sputum (interleukin-6, interleukin-8, myeloperoxidase) and plasma (interleukin-6, interleukin-8, C-reactive protein) as secondary outcomes, but found no statistically significant treatment-related differences.Citation14 The lack of effect of erythromycin on inflammatory markers suggests that the antimicrobial effects of macrolides in the treatment of COPD patients may be more important.
Patients with advanced COPD, especially those with repeated courses of antibiotic therapy or oral corticosteroids and requiring hospital admissions, have an increased risk of exacerbations caused by P. aeruginosa.Citation30,Citation31 In these patients, antibiotic treatment decisions should consider both the severity of AECOPD and the risk for isolation of P. aeruginosa.Citation23
The fact that 45% of our patients had chronic bronchial colonization by P. aeruginosa (Group 2) reflects the severity of their COPD. In these patients, long-term azithromycin therapy also improved all the outcomes analyzed, achieving a statistically significant reduction of 43% in AECOPD. Hospitalizations and days of hospital stay also fell to 47% and 32%, respectively, in these patients, although these differences did not reach statistical significance.
Macrolides, especially azithromycin, are useful in the treatment of chronic bronchial colonization by P. aeruginosa in patients with bronchiectasis, mainly in cases associated with cystic fibrosis.Citation7,Citation32 Azithromycin interferes with production of virulence factors by P. aeruginosa, reduces biofilm formation by inhibiting alginate production, and decreases bacterial adherence.Citation33 For these reasons, we think that longterm azithromycin is especially indicated in these patients. However, the reductions in hospitalizations and days of mean hospital stay are less evident, possibly because they often require prolonged hospitalizations for parenteral antibiotic treatment, because P. aeruginosa is often resistant to oral antibiotics in this clinical situation.
Potential limitations of our study are the small number of patients included and the absence of a control group. However, our findings are very promising, especially for selected patients with severe COPD at high risk of exacerbations despite conventional maximum treatment. This study may help in the design of future, randomized, controlled trials including more patients and assessing the impact of treatment in patients with different degrees of severity, focusing on aspects such as efficacy, safety, development of microbiological resistance, or economic burden.
In conclusion, we have shown that long-term intermittent azithromycin therapy, administered three times a week at a dosage of 500 mg, is well tolerated and associated with significant reductions in AECOPD, number of hospitalizations, and days of hospital stay in patients with severe COPD and repeated AECOPD. This improvement is especially significant in patients with AECOPD associated with common potentially pathogenic microorganisms, possibly due to the antibacterial activity of the drug, and in patients with chronic bronchial colonization by P. aeruginosa due to its ability to prevent AECOPD for common potentially pathogenic microorganisms also in these patients. These results suggest that long-term intermittent azithromycin therapy may be useful in the treatment of patients with severe COPD and frequent exacerbations.
Disclosure
The authors report no conflicts of interest in this work. Funded in part by Fondo de Investigaciones Sanitarias and Ciber de Enfermedades Respiratorias – CibeRes.
References
- HurstJRVestboJAnzuetoAEvaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) InvestigatorsSusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med20103631128113820843247
- Soler-CatalunaJJMartínezMARománPSalcedoENavarroMOchandoRSevere acute exacerbations and mortality in patients with chronic obstructive pulmonary diseaseThorax20056092593116055622
- MorenoABelmonteYGallegoMPomaresXRealJCauses of death and risk factors for mortality in patients with severe chronic obstructive pulmonary diseaseArch Bronconeumol200945181186 [Spanish.]19328612
- MonsóERuizJRosellABacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brushAm J Respir Crit Care Med1995152131613207551388
- KunisakiKMNiewoehnerDEAntibiotic prophylaxis for chronic obstructive pulmonary disease: Resurrecting an old ideaAm J Respir Crit Care Med20081781098109919023036
- KudohSAzumaAYamamotoMIzumiTAndoMImprovement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycinAm J Respir Crit Care Med1998157182918329620913
- SouthernKWBarkerPMSolisAMacrolide antibiotics for cystic fibrosisCochrane Database Syst Rev2004CD00220315106170
- ClementATameletALerouxERavillySFourouxBJaisJPLongterm effects of azithromycin in patients with cystic fibrosis: A double blind, placebo controlled trialThorax20066189590216809416
- ShinkaiMHenkeMORubinBKMacrolide antibiotics as immunomodulatory medications: Proposed mechanisms of actionPharmacol Ther200811739340518289694
- WolterJSeeneySBellSBowlerSMaselPMcCormackJEffect of long term treatment with azithromycin on disease parameters in cystic fibrosis: A randomised trialThorax20025721221611867823
- SaimanLMarshallBCMayer-HamblettNMacrolide Study GroupAzithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: A randomized controlled trialJAMA20032901749175614519709
- GanWQManSFSenthilselvanASinDDAssociation between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysisThorax20045957458015223864
- SuzukiTYanaiMYamayaMErythromycin and common cold in COPDChest200112073073311555501
- SeemungalTAWilkinsonTMHurstJRPereraWRSapsfordRJWedzichaJALong-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbationsAm J Respir Crit Care Med20081781139114718723437
- AlbertRKBaileyWCCasaburiRChronic azithromycin decreases the frequency of chronic obstructive pulmonary disease exacerbationsAbstract A6416 presented at the American Thoracic Society CongressMay 13–18, 2011Denver, CO
- Cobos-TriguerosNAtekaOPitartCVilaJMacrolides and ketolidesEnferm Infecc Microbiol Clin200927412418 [Spanish.]19625112
- FlumePAO’SullivanBPRobinsonKACystic Fibrosis FoundationPulmonary Therapies Committee Cystic fibrosis pulmonary guidelines: Chronic medications for maintenance of lung healthAm J Respir Crit Care Med200717695796917761616
- VendrellMde GraciaJOlveiraCDiagnosis and treatment of bronchiectasis. Spanish Society of Pneumology and Thoracic SurgeryArch Bronconeumol200844629640 [Spanish.]19007570
- PomaresXMontonCRespiratory day hospital: What have we learned?Med Clin (Barc)201113645445519819498
- SmithIEJurriaansEDiederichSAliNShneersonJFlowerCDRChronic sputum production: Correlation between clinical features and findings on high resolution computed tomographic scanning of the chestThorax1996519149188984702
- Rodriguez-RoisinRToward a consensus def inition for COPD exacerbationsChest200011739840110669681
- AnthonisenNRManfredaJWarrenCPWHershfieldESHardingGKMNelsonNAAntibiotic therapy in exacerbations of chronic obstructive pulmonary diseaseAnn Intern Med19871061962043492164
- PauwelsRABuistASCalverleyPMJenkinsCRHurdSSGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summaryAm J Respir Crit Care Med20011631256127611316667
- Clinical and Laboratory Standards InstitutePerformance Standards for Antimicrobial Susceptibility Testing; Twentieth Informational SupplementWayne, PAClinical and Laboratory Standards Institute2010
- SethiSJonesPWTheronMSPULSE Study GroupPulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: A randomized controlled trialRespir Res2010111020109213
- HuangBHWuCHHsiaCPYin ChenCAzithromycin-induced torsade de pointesPacing Clin Electrophysiol2007301579158218070319
- MartinezFJCurtisJLAlbertRRole of macrolide therapy in chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis2008333135018990961
- TamaokiJTakeyamaKTagayaEKonnoKEffect of clarithromycin on sputum production and its rheological properties in chronic respiratory tract infectionsAntimicrob Agents Chemother199539168816907486901
- ParnhamMJCulićOErakovićVModulation of neutrophil and inflammation markers in chronic obstructive pulmonary disease by shortterm azithromycin treatmentEur J Pharmacol200551713214315964564
- Garcia-VidalCAlmagroPRomaníVPseudomonas aeruginosa in patients hospitalised for COPD exacerbation: A prospective studyEur Respir J2009341072107819386694
- MiravitllesMEspinosaCFernández-LasoEMartosJAMaldonadoJAGallegoMRelationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Study Group of Bacterial Infection in COPDChest1999116404610424501
- SouthernKWBarkerPMAzithromycin for cystic fibrosisEur Respir J20042483483815516680
- TatedaKIshiiYKimuraSHorikawaMMiyairiSYamaguchiKSuppression of Pseudomonas aeruginosa quorum-sensing systems by macrolides: A promising strategy or an oriental mysteryJ Infect Chemother20071335736718095083