Abstract
Purpose
Depression is reported in association with chronic obstructive pulmonary disease (COPD). However, to date, no multidimensional indices have taken depression into consideration to predict COPD patients’ prognosis. This study aimed to determine whether a new multidimensional index named CODEXS, based on comorbidities, airflow obstruction, dyspnea, previous exacerbation and depression assessed by Self-Rating Depression Scale (SDS), could predict 1-year exacerbations.
Methods
This was a prospective study, patients with stable COPD were used to develop CODEXS at the first visit, and followed up in the 3rd, 6th, and 12th months. After the last visit, patients were divided into frequent and infrequent exacerbators. Another cohort of COPD patients was used for validation. The SDS scoring system in the multidimensional indices ranged from 0 to 4 based on the modified SDS value, representing no depression (25–39 [0], 40–49 [1]), mild depression (50–59), moderate depression (60–69), and severe depression (≥70). Comorbidity, dyspnea, airflow obstruction, and severe exacerbations were calculated according to CODEX thresholds.
Results
Two sets of 105 and 107 patients were recruited in the development and validation cohorts, respectively. Depression was demonstrated as an independent risk factor for frequent exacerbators (odds ratio (OR)= 1.14, 95% confidence interval (CI) = 1.06–1.23, P < 0.001). The prevalence of depression in frequent exacerbators (35.09%) was higher than that in infrequent exacerbators. CODEXS was significantly associated with exacerbation (OR =2.91; 95% CI, 1.89–4.48, p<0.001). Receiver operating characteristic (ROC) curve comparison showed that CODEXS was superior to BODEX(BMI, airflow obstruction, dyspnea, previous exacerbation), BODE (BMI, airflow obstruction, dyspnea, exercise), and updated ADO (age, dyspnea, and airflow obstruction) indices, confirmed by the validation cohort with sensitivity at 85.94% and specificity at 76.74%.
Conclusion
Depression is an independent risk factor for COPD exacerbation. CODEXS is a useful predictor for predicting frequent exacerbators within 1 year and is superior to other previously published indices.
Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease which is characterized by persistent respiratory symptoms and persistent airflow limitation, and is currently the fourth leading cause of mortality worldwide. Acute exacerbation of COPD (AECOPD) is an event defined as acute worsening of respiratory symptoms that result in additional therapy.Citation1 It can accelerate deterioration of the disease,Citation2,Citation3 lead to a heavy economic burden,Citation4 and increase mortality.Citation5 Recently, there is an increasing recognition that COPD is a complex and heterogeneous disease.Citation6 In the review we published in 2017,Citation7 we concluded that there are different phenotypes in the exacerbation stage, one of which is called the “frequent exacerbator” phenotype. The frequency of AECOPD is variable among patients; a proportion of COPD patients seldom exacerbate, but others do so frequently.Citation8 As such, identifying those patients who are at high risk and intervening in a timely manner is a clinical priority. Many variables are proved to be related to outcome in patients with COPD; the most recognized variables are postbronchodilator FEV1, dyspnea, and historical exacerbations.Citation5,Citation9 GOLD (Global Initiative for Chronic Obstructive Lung Disease) recommendations have taken all these variables into consideration for grading COPD severity.Citation10 The use of multidimensional indices, such as CODEX (comorbidity, airflow obstruction, dyspnea, previous severe exacerbations),Citation11 BODE (BMI, airflow obstruction, dyspnea, and exercise capacity),Citation12 BODEX (BMI, airflow obstruction, dyspnea, and previous severe exacerbations), and updated ADO (age, dyspnea, and airflow obstruction),Citation13 have improved the prognostic capacity beyond individual variables.
Depression is a recognized complication of many chronic diseases, including COPD.Citation14,Citation15 Some studies have found that depression is common in COPD, with the prevalence increasing with the severity of disease, from 19.6% in the mild-to-moderate group to 25% in the severe group.Citation14 Patients with severe COPD are at increased risk of developing depression to increase the frequency of exacerbations.Citation16 The Self-Rating Depression Scale (SDS) is a 20-item self-report questionnaire that can be used to screen for and assess depression.Citation17 It consists of four common characteristics of depression: the pervasive effect, physiological equivalents, other disturbances, and psychomotor activities. There are 10 positive and 10 negative questions. Each question is scored on a scale of 1–4 (a little of the time, some of the time, a good part of the time, most of the time), and the total score ranges from 20 to 80. The raw score is then converted to an index score by dividing the raw score by the maximum score (80) and either expressing this as a decimal or multiplying by 100 to express it as a whole number with an index score range of 25 to 100. An index score of 25 to 49 indicates no depression, 50–59 indicates mild to moderate depression, 60–69 indicates moderate to severe depression, and a score of 70 or over indicates severe depression.Citation18
In this study, we investigated the relationship between depression and the outcome of patients with COPD, and determined whether the inclusion of SDS could improve the predictive value of the previously established multidimensional indices ADO, BODEX, BODE, and CODEX.
Patients and Methods
This research was approved by the local Ethics Committee of the First Affiliated People’s Hospital of Shaoyang College and conducted in accordance with the Declaration of Helsinki and its amendments, and all subjects gave written informed consent to participate in the study. The study was registered in the Chinese Clinical Trial Registry (ChiCTR-ROC-16009087; http://www.chictr.org.cn/).
Study Subjects
Two cohorts of patients diagnosed with stable COPD were recruited at the outpatient department of the First Affiliated People’s Hospital of Shaoyang College during different time periods from September 2015 to August 2018. One was used to develop updated multidimensional indices which included the SDS score for predicting frequent exacerbators; the other was collected to validate the new multidimensional indices. The diagnosis of COPD was based on the latest GOLD document.Citation1 Patients with a history of diagnosis of asthma, bronchiectasis, lung fibrosis, upper airway obstruction, or tuberculosis were excluded. Patients in the exacerbation stage were excluded. Patients who could not understand the SDS questionnaire or refused to complete the questionnaire were also excluded.
Study Design
This was a prospective study. All the patients in this study accepted four visits. At the first visit, all participants signed a written informed consent and provided the following information: age, sex, smoking history, career, education, comorbidities, BMI, COPD assessment test (CAT), modified Medical Research Council Dyspnea Scale (mMRC), Clinical COPD Questionnaire (CCQ), SDS, 6 min walk distance, history of exacerbations in the previous year, and lung function. Before using the SDS report, the researchers explained the content of the questionnaire to the patients in detail, making sure the patients understand the meaning of each item. Then, the patients were asked to rate each of the 20 items as to how it applied to them at the time of testing. If the patients forgot the meaning of any item in completing the form, the researcher could explain again without any intervention and guidance. Comorbidity was quantified according to the Charlson index.Citation19 CAT, mMRC, and CCQ were collected as described previously.Citation20,Citation21 The 6-mins walk test was performed according to the guideline.Citation22 The BODE, BODEX, CODEX, and ADO scores were calculated based on previous studies.Citation11–Citation13,Citation23 The SDS score included in the multidimensional indices ranged from 0 to 4 based on the severity of depression, representing 25–39, 40–49, 50–59, 60–69, and ≥70, respectively. Then, the SDS value was added into BODE, BODEX, ADO, and CODEX to develop the updated multidimensional indices, named BODES, BODEXS, ADOS, and CODEXS, respectively. After the baseline visit, every participant was followed up in the 3rd, 6th, and 12th months, respectively, to record the occurrence of exacerbations and the treatment therapy during 1 year of follow-up.
Definition of Exacerbations
Exacerbations were diagnosed based on event-based definition: worsening or new onset of any respiratory symptoms (cough, sputum volume or purulence, wheezing, or dyspnea) for at least 3 days that led to any of the following situations: 1. requiring prescription change under the guidance of a physician in the outpatient department; 2. requiring hospitalization or an emergency room visit with the diagnosis of AECOPD. Exacerbations separated by ≥14 days were considered distinct events. The frequent exacerbators were those who had at least two exacerbations or one hospitalization per year.
Statistical Analysis
Adjusted multiple logistic regression models were performed to determine the association between variables and COPD exacerbations. The variables age, gender, BMI, smoking status, exacerbation history, GOLD stage, CCQ, CAT, mMRC, and SDS were taken into consideration. Comparison of mean SDS score and GOLD stage was performed with one-way ANOVA. The Student t test or Mann–Whitney U-test was used to compare means difference for continuous variables and the chi-squared test was used for categorical variables. All statistical analyses above were performed using SPSS 25. Receiver operating characteristic (ROC) analysis was used to determine the predictive capacity of different multidimensional indices. Comparisons between the area under the curves (AUC) were made using the Hanley–McNeil test through Medcalc software, and a P value of <0.05 was considered statistically significant.
Results
Patient Demographic Characteristics
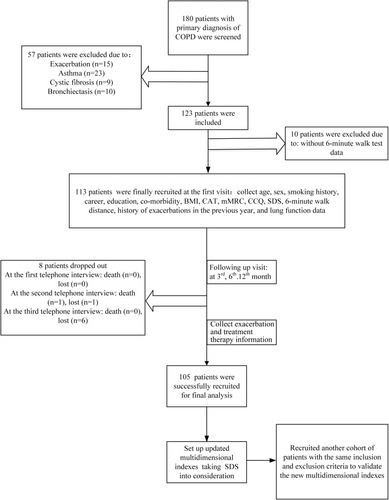
A flow chart of the study is shown in . Initially, a total of 180 subjects with a primary diagnosis of COPD were screened. Among them, 57 patients were excluded because of exacerbation (n = 15), asthma (n = 23), fibrosis (n = 9), or bronchiectasis (n = 10). Ten patients refused to perform the 6-mins walk test and dropped out. During the 1-year follow-up period, eight patients dropped out: one of them died because of extrapulmonary disease and seven of them could not be reached. Finally, 105 patients were recruited into the final analysis. Demographic and clinical data are presented in . The mean age of the patients was 67.97 ± 8.91 years, with 94.29% males and 5.71% females. The mean FEV1/FVC and predicted FEV1% were 49.90 ± 9.33% and 49.56 ± 15.42%. The average mMRC, CAT, CCQ, and SDS scores were 1.43 ± 0.85, 13.63 ± 7.10, 3.11 ± 3.88, and 40.59 ± 14.42, respectively. The average number of exacerbations was 1.10 ± 1.25 per year.
Table 1 Demographic and Clinical Characteristics of Patients
Figure 1 Study flow chart. A total of 180 subjects with a primary diagnosis of COPD were screened; 57 patients were excluded because of exacerbation (n = 15), asthma (n = 23), fibrosis (n = 9), or bronchiectasis (n = 10). Ten patients refused to perform the 6-mins walk test and dropped out. Seven of them could not be reached. During the 1-year follow-up period, eight patients dropped out; one of them died because of extrapulmonary disease and seven of them could not be reached. After developing the updated multidimensional indices, another cohort of patients was recruited to validate the indices.
Depression Is an Independent Risk Factor for COPD Exacerbation
After 1 year of follow-up, 57 (54.29%) patients were grouped as frequent exacerbators. A logistic regression model was used to evaluate the prognostic value of each variable. Univariate analysis showed that several factors, including FEV1%, CAT, mMRC, SDS, comorbidities, treatment, and history of exacerbations contributed to frequent exacerbations (). These factors except CAT were then further analyzed by multivariate analysis. The multivariate model showed that SDS (odds ratio (OR) = 1.14, 95% confidence interval (CI) = 1.06–1.23, P < 0.001) and history of exacerbations (OR = 2.33, 95% CI = 1.20–4.51, P < 0.001) were independent indicators of frequent exacerbations; regular treatment (OR = 0.03, 95% CI = 0.01–0.14, P < 0.001) could decrease exacerbation significantly ().
Table 2 Univariate and Stepwise Multivariate Analysis of Risk Factors for Frequent Exacerbators
Depression Severity Is Correlated with GOLD Stage and Symptom Severity
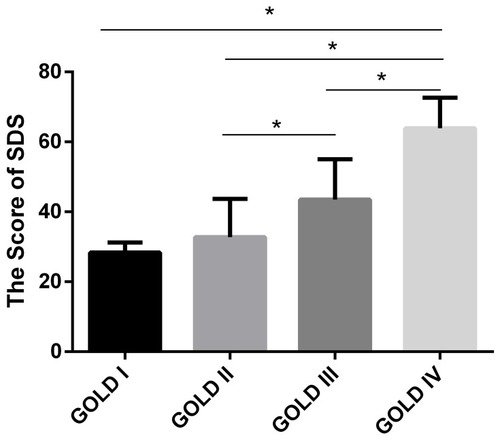
The prevalence of depression in the frequent exacerbators (35.09%) was higher than that in infrequent exacerbators (12.5%) (). The SDS score in patients at GOLD stage IV was significantly higher than that in other stages. The average SDS score of patients at GOLD stages I, to IV was 28.33 ± 2.89, 32.73 ± 10.96, 43.50 ± 11.51, and 63.91 ± 8.76, respectively (). The SDS score was positively correlated with the GOLD stage (rho = 0.641, P < 0.001), CAT score (rho = 0.528, P < 0.001), and mMRC (rho = 0.425, P < 0.001).
Figure 2 Prevalence of depression in COPD patients. The prevalence of depression in the frequent exacerbators (35.09%) was higher than that in infrequent exacerbators (12.5%). *Means P < 0.05.
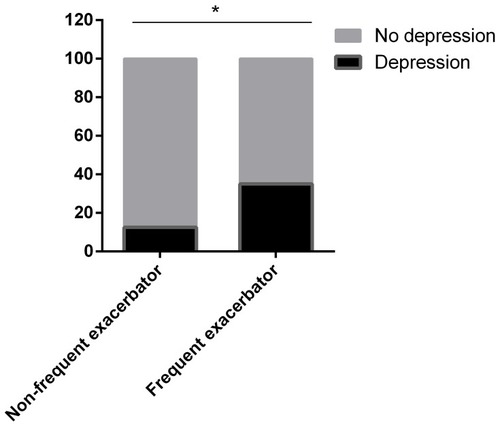
Figure 3 SDS score for different GOLD stages. The SDS score increased with disease severity. The average SDS score of patients at GOLD stages I to IV was 28.33 ± 2.89, 32.73 ± 10.96, 43.50 ± 11.51, and 63.91 ± 8.76, respectively. *Means P < 0.05.
Comparison of the Predictive Value of Different Multidimensional Indices
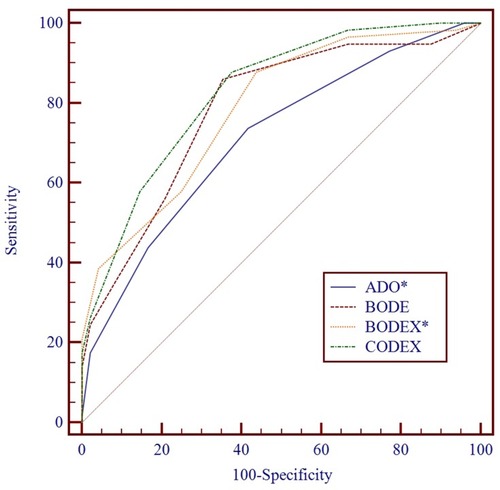
The ADO, CODEX, BODE, and BODEX indices could predict the frequent exacerbators effectively: the OR were 2.0, 2.79, 1.91, and 2.17, respectively. Comparison of the ROC curves showed that CODEX had a better predictive capacity for the frequent exacerbators compared with ADO (P = 0.008) and BODEX (P = 0.03); the AUC were 0.82, 0.71, and 0.79, respectively ( and ).
Table 3 Predictive Value of Different COPD Multivariate Indices for Frequent Exacerbators
Figure 4 ROC comparison between previously established multidimensional indices for predicting frequent exacerbators. CODEX had a better predictive capacity for frequent exacerbators compared with ADO (P = 0.008) and BODEX (P = 0.03); the AUC were 0.82, 0.71, and 0.79, respectively. *Means P < 0.05.
Combined Variables Improve the Predictive Value for Frequent Exacerbators
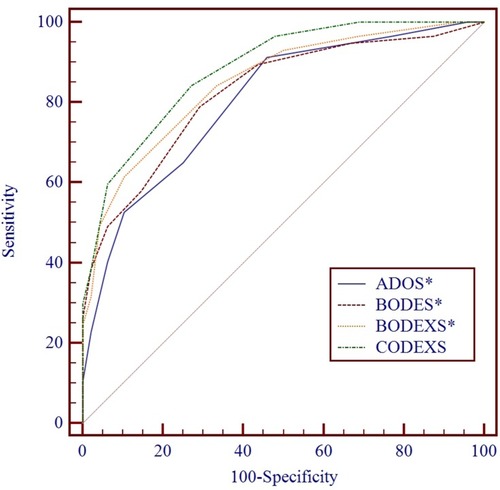
With respect to the combined variable (SDS value), all indices reached statistical significance for predicting frequent exacerbators (). ROC curve comparison showed that the inclusion of SDS value improved the predictive capacity of all the previously established indices (ADOS vs ADO, 0.80 [0.71], P = 0.0002 []; CODEXS vs CODEX 0.88 [0.82], P = 0.02 []; BODES vs BODE 0.83 [0.79], P = 0.03 []; BODEXS vs BODE 0.85 [0.79], P = 0.004 []). The cut-off point for all the multidimensional indices to differentiate frequent exacerbators was 3.5. ROC comparison showed that CODEXS had a better capacity to predict exacerbations than ADOS (P = 0.005), BODES (P = 0.01), and BODEXS (P = 0.007) ().
Table 4 Predictive Value of Updated COPD Multivariate Indices for Frequent Exacerbators
Figure 5 ROC comparison between updated multidimensional indices and previous indices. The updated multidimensional indices improved the predictive capacity of all the previous indices (A) (ADOS vs ADO, 0.80 [0.71], P = 0.0002; (B) CODEXS vs CODEX 0.88 [0.82], P = 0.02; (C) BODES vs BODE 0.83 [0.79], P = 0.03; (D) BODEXS vs BODEX 0.85 [0.79], P = 0.004). *Means P < 0.05.
![Figure 5 ROC comparison between updated multidimensional indices and previous indices. The updated multidimensional indices improved the predictive capacity of all the previous indices (A) (ADOS vs ADO, 0.80 [0.71], P = 0.0002; (B) CODEXS vs CODEX 0.88 [0.82], P = 0.02; (C) BODES vs BODE 0.83 [0.79], P = 0.03; (D) BODEXS vs BODEX 0.85 [0.79], P = 0.004). *Means P < 0.05.](/cms/asset/cca9891c-9ce5-4c93-8801-f73827c6b207/dcop_a_12198155_f0005_c.jpg)
Figure 6 ROC comparison between updated multidimensional indices for predicting frequent exacerbators. CODEXS had better a capacity to predict exacerbations than ADOS (P = 0.005), BODES (P = 0.01), and BODEXS (P = 0.007). *Means P < 0.05.
Validation of Multidimensional Indices
In order to validate these new indices, we recruited 107 patients with COPD from the First Affiliated People’s Hospital of Shaoyang College. The demographic characteristics are shown in . No significant statistic changes of the variables were found between the two cohorts of patients. After 1 year of follow-up, 64 patients had frequent exacerbations (59.81%). Taking 3.5 as the cut-off point for CODEXS, BODEXS, and ADOS, the sensitivity to identify frequent exacerbators was 85.94%, 82.81%, and 84.38%, respectively, and the specificity was 76.74%, 60.47%, and 53.49%, respectively ().
Table 5 Validation of Multicomponent Indices
Discussion
In this study, we found depression was an independent risk factor for frequent exacerbation in patients with COPD. The combination of SDS score with the existing indices BODE, BODEX, CODEX, and ADO improved the predictive value for frequent exacerbators significantly. We named these new indices BODES, BODEXS, CODEXS, and ADOS. We found CODEXS was superior to the other three multidimensional indices, and validated the finding in another cohort of patients with COPD. To our knowledge, no multicomponent indices took depression into consideration in previous studies. In our study, we developed and validated a new multidimensional index to predict frequent exacerbators among patients with COPD, which can be easily applied to clinical practice.
Depression is the leading cause of increased disability and impaired quality of life in older people worldwide. Many studies have reported a high rate of depression among patients suffering from COPD.Citation16,Citation24,Citation25 The prevalence of depression in COPD varies from 6% to 57% due to different sample size, age group, and depression screening tool.Citation26 There are many questionnaires used to study depression in COPD patients, including the Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), SDS, and the Structured Clinical Interview for DSM-I. CES-D was designed to cover most depression symptoms, with emphasis on affective components; GDS was designed for screening depression in older populations, HADS was designed for screening depression in the general medical population, and SDS can screen for depression, as well as classify its severity.Citation27 In our study, we wanted to include the SDS score in a multidimensional index, so we chose SDS to assess the severity of depression and assigned a different value, ranging from 0 to 4 based on the SDS score, to the previously reported indices BODE, BODEX, ADO, and CODEX. We found the percentage of COPD patients with depression was 25.71%, similar to a previous study.Citation28 The prevalence of depression (35.09%) was much higher in frequent exacerbators compared with infrequent exacerbators (12.50%), indicating that depression is related to exacerbations in COPD patients. More severe depression was found in patients at a higher GOLD stage. A possible reason for this result may be that COPD and depression are deeply interrelated. It is well known that depression plays a role in the initiation and maintenance of smoking, that smoking is a critical risk factor for the development of COPD, and that COPD, in turn, contributes to the worsening of depression.Citation29,Citation30
Through logistic regression analysis, we found that depression is an independent risk factor for predicting frequent exacerbators among COPD patients. To determine whether taking depression into consideration could improve the predictive value of a multidimensional index, we converted the SDS score into a 5-point scale, ranging from 0 to 4, representing 25–39, 40–49, 50–59, 60–69, and ≥70, respectively. The rationale for setting up this scale was based on the severity of depression. Index scores of 25 to 49 indicate no depression, 50–59 indicates mild depression, 60–69 indicates moderate depression, and scores ≥70 indicate severe depression;Citation18 0–1 represents no depression, and 2–4 represent mild, moderate, and severe depression, respectively.
The most widely recognized variables associated with exacerbations in patients with COPD are FEV1%, dyspnea, and exacerbations in the previous year.Citation31–Citation33 The first two are the basis of multicomponent indices; the number of exacerbations in the previous year has been proved to be an independent risk factor, and some indices have also included this factor. Other factors, such as age, comorbidities, BMI, and 6-mins walk test, have also been used to develop some multicomponent indices. Several multicomponent indices are widely recognized, including CODEX, BODEX, ADO, and BODE. In this study, we demonstrated that all these indices could predict 1-year exacerbations in COPD patients, which was similar to previous studies.Citation11,Citation34–Citation37 ROC comparison analysis showed that CODEX was superior to BODEX, ADO, and BODE for predicting 1-year exacerbation. CODEX was first proposed by Pedro Almagro; it was an evolution of the BODE and BODEX indices, retaining their cut-offs for dyspnea, obstruction, and previous exacerbations, but replacing BMI with comorbidity measured by the original Charlson index modified by age. Although low BMI has been proved to be associated with a poor survival, the Charlson index shows a better prognostic capacity.Citation11 This may be why CODEX showed a better capacity for predicting frequent exacerbators.
The importance of depression in COPD is well established. However, to date, none of the COPD indices have included depression as a prognostic variable. In our study, we found that SDS score was correlated to symptoms and airflow obstruction, and confirmed that adding the SDS value into previous multicomponent indices improved their predictive value for frequent exacerbators significantly. Similar to CODEX, CODEXS is the most useful, with the highest sensitivity and specificity. As such, clinicians should pay more attention to those who are judged to be frequent exacerbators, making proper clinical interventions.
Our study has several limitations. Firstly, the population for validating the indices came from the same hospital in different time periods with the same inclusion and exclusion criteria; it would be better to validate these indices with a multicenter study, and it is worth having a longer follow-up period to assess the predictive value of CODEXS for the long-term outcome. Secondly, our study had a clear predominance of men. But this finding is similar to all studies of COPD performed in our area and it is probably to the fact that there are a higher proportion of men to smoke than that in women.Citation20,Citation21,Citation38 Finally, although we found that depression is an independent risk factor for exacerbation, we did not treat the patients with antidepressant or psychological intervention; in a future study, it would be worth determining whether proper psychological intervention improves the prognosis of patients with COPD.
Conclusion
In conclusion, depression is an independent risk factor for COPD exacerbations. CODEXS is a simple, effective tool for predicting the risk of COPD exacerbation.
Abbreviations
ADO, age, dyspnea, airflow obstruction; ADOS, age, dyspnea, airflow obstruction, depression; AECOPD, acute exacerbations of chronic obstructive pulmonary disease; AUC, area under the curve; BMI, body mass index; BODE, body mass index, airflow obstruction, dyspnea, exercise; BODES, body mass index, airflow obstruction, dyspnea, exercise, depression; BODEX, body mass index, airflow obstruction, dyspnea, previous severe exacerbation; BODEXS, body mass index, airflow obstruction, dyspnea, previous severe exacerbation, depression; CAT, COPD assessment test; CCQ, Clinical COPD Questionnaire; CES-D, Center for Epidemiologic Studies Depression Scale; CI, confidence interval; CODEX, comorbidities, airflow obstruction, dyspnea, previous severe exacerbation; CODEXS, comorbidities, airflow obstruction, dyspnea, previous severe exacerbation, depression; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GDS, Geriatric Depression Scale; GOLD, Global Initiative of Chronic Obstructive Lung Disease; HADS, Hospital Anxiety and Depression Scale; mMRC, modified Medical Research Council Dyspnea Scale; OR, odds ratio; ROC, receiver operating characteristic; SD, standard deviation; SDS, Self-Rating Depression Scale.
Data Sharing Statement
The authors confirm that the data supporting the findings of this study are available within the article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request, the authors will not share any individual deidentified participant date or other relevant study documents.
Ethics Approval and Consent to Participate
The research protocol was approved by the local Ethics Committee of the First Affiliated People’s Hospital of Shaoyang College (number: C2016123) and conducted in accordance with the Declaration of Helsinki and its amendments. All subjects gave written informed consent to participate in the study.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This study was funded by grants from the Fundamental Research Funds for the Central Universities of Central South University (2017zzts228 to Doctor Aiyuan Zhou). We want to thank Dr. Alexandra Racanelli (Division of Pulmonary and Critical Care Medicine, Weill Cornell Medical College) for proofreading the manuscript. Dingding Deng and Aiyuan Zhou are co-first authors for this study.
References
- Global Initiative for Chronic Obstructive Lung Disease(GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2019 Available from: http://goldcopd.org/. Accessed 122, 2020.
- MakrisD, MoschandreasJ, DamianakiA, et al. Exacerbations and lung function decline in COPD: new insights in current and ex-smokers. Respir Med. 2007;101:1305–1312. doi:10.1016/j.rmed.2006.10.01217112715
- DonaldsonGC, SeemungalTA, BhowmikA, WedzichaJA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi:10.1136/thorax.57.10.84712324669
- ByngD, LutterJI, WackerME, et al. Determinants of healthcare utilization and costs in COPD patients: first longitudinal results from the German COPD cohort COSYCONET. Int J Chron Obstruct Pulmon Dis. 2019;14:1423–1439. doi:10.2147/COPD.S20189931308648
- Soler-CatalunaJJ, Martinez-GarciaMA, Roman SanchezP, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi:10.1136/thx.2005.04052716055622
- BafadhelM, McKennaS, TerryS, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184:662–671. doi:10.1164/rccm.201104-0597OC21680942
- ZhouA, ZhouZ, ZhaoY, ChenP. The recent advances of phenotypes in acute exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1009–1018. doi:10.2147/COPD28392685
- HurstJR, VestboJ, AnzuetoA, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi:10.1056/NEJMoa090988320843247
- NishimuraK, IzumiT, TsukinoM, OgaT. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121:1434–1440. doi:10.1378/chest.121.5.143412006425
- VestboJ, HurdSS, AgustiAG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi:10.1164/rccm.201204-0596PP22878278
- AlmagroP, SorianoJB, CabreraFJ, et al. Short- and medium-term prognosis in patients hospitalized for COPD exacerbation: the CODEX index. Chest. 2014;145:972–980. doi:10.1378/chest.13-132824077342
- CelliBR, CoteCG, MarinJM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi:10.1056/NEJMoa02132214999112
- PuhanMA, Garcia-AymerichJ, FreyM, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet. 2009;374:704–711. doi:10.1016/S0140-6736(09)61301-519716962
- van ManenJG, BindelsPJ, DekkerFW, et al. Risk of depression in patients with chronic obstructive pulmonary disease and its determinants. Thorax. 2002;57:412–416. doi:10.1136/thorax.57.5.41211978917
- Di MarcoF, VergaM, ReggenteM, et al. Anxiety and depression in COPD patients: the roles of gender and disease severity. Respir Med. 2006;100:1767–1774. doi:10.1016/j.rmed.2006.01.02616531031
- QuintJK, Baghai-RavaryR, DonaldsonGC, WedzichaJA. Relationship between depression and exacerbations in COPD. Eur Respir J. 2008;32:53–60. doi:10.1183/09031936.0012010718321938
- ZungWW. A Self-Rating Depression Scale. Arch Gen Psychiatry. 1965;12:63–70. doi:10.1001/archpsyc.1965.0172031006500814221692
- ZungWW. From art to science. The diagnosis and treatment of depression. Arch Gen Psychiatry. 1973;29:328–337. doi:10.1001/archpsyc.1973.042000300260044724142
- QuanH, LiB, CourisCM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682. doi:10.1093/aje/kwq43321330339
- ZhouA, ZhouZ, PengY, et al. The role of CAT in evaluating the response to treatment of patients with AECOPD. Int J Chron Obstruct Pulmon Dis. 2018;13:2849–2858. doi:10.2147/COPD.S17508530237709
- ZhouZ, ZhouA, ZhaoY, DuanJ, ChenP. A comparison of the assessment of health status between CCQ and CAT in a Chinese COPD clinical population: a cross-sectional analysis. Int J Chron Obstruct Pulmon Dis. 2018;13:1675–1682. doi:10.2147/COPD29872285
- Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi:10.1164/ajrccm.166.1.at110212091180
- Espantoso-RomeroM, Roman RodriguezM, Duarte-PerezA, et al. External validation of multidimensional prognostic indices (ADO, BODEx and DOSE) in a primary care international cohort (PROEPOC/COPD cohort). BMC Pulm Med. 2016;16:143. doi:10.1186/s12890-016-0305-227835945
- MikkelsenRL, MiddelboeT, PisingerC, StageKB. Anxiety and depression in patients with chronic obstructive pulmonary disease (COPD). A review. Nord J Psychiatry. 2004;58:65–70. doi:10.1080/0803948031000082414985157
- YohannesAM, AlexopoulosGS. Depression and anxiety in patients with COPD. Eur Respir Rev. 2014;23:345–349. doi:10.1183/09059180.0000781325176970
- ZhangMW, HoRC, CheungMW, FuE, MakA. Prevalence of depressive symptoms in patients with chronic obstructive pulmonary disease: a systematic review, meta-analysis and meta-regression. Gen Hosp Psychiatry. 2011;33:217–223. doi:10.1016/j.genhosppsych.2011.03.00921601717
- MatteDL, PizzichiniMM, HoepersAT, et al. Prevalence of depression in COPD: a systematic review and meta-analysis of controlled studies. Respir Med. 2016;117:154–161. doi:10.1016/j.rmed.2016.06.00627492526
- HananiaNA, MullerovaH, LocantoreNW, et al. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am J Respir Crit Care Med. 2011;183:604–611. doi:10.1164/rccm.201003-0472OC20889909
- NorwoodR. Prevalence and impact of depression in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2006;12:113–117. doi:10.1097/01.mcp.0000208450.50231.c616456380
- SchneiderC, JickSS, BothnerU, MeierCR. COPD and the risk of depression. Chest. 2010;137:341–347. doi:10.1378/chest.09-061419801582
- ZiderAD, WangX, BuhrRG, et al. Reduced COPD exacerbation risk correlates with improved FEV1: a meta-regression analysis. Chest. 2017;152:494–501. doi:10.1016/j.chest.2017.04.17428483609
- Garcia-GutierrezS, QuintanaJM, UnzurrunzagaA, et al. Predictors of change in dyspnea level in acute exacerbations of COPD. COPD. 2016;13:303–311. doi:10.3109/15412555.2015.107878426667827
- GuerraB, GaveikaiteV, BianchiC, PuhanMA. Prediction models for exacerbations in patients with COPD. Eur Respir Rev. 2017;26:160061.28096287
- AlmagroP, Martinez-CamblorP, MiravitllesM, et al. External validation and recalculation of the CODEX index in COPD patients. A 3CIAplus Cohort study. COPD. 2019;16:8–17. doi:10.1080/15412555.2018.148444030870059
- MarinJM, CarrizoSJ, CasanovaC, et al. Prediction of risk of COPD exacerbations by the BODE index. Respir Med. 2009;103:373–378. doi:10.1016/j.rmed.2008.10.00419013781
- ChenCZ, OuCY, YuCH, et al. Comparison of global initiative for chronic obstructive pulmonary disease 2013 classification and body mass index, airflow obstruction, dyspnea, and exacerbations index in predicting mortality and exacerbations in elderly adults with chronic obstructive pulmonary disease. J Am Geriatr Soc. 2015;63:244–250. doi:10.1111/jgs.1325825641518
- JonesRC, PriceD, ChavannesNH, et al. Multi-component assessment of chronic obstructive pulmonary disease: an evaluation of the ADO and DOSE indices and the global obstructive lung disease categories in international primary care data sets. NPJ Prim Care Respir Med. 2016;26:16010. doi:10.1038/npjpcrm.2016.1027053297
- ZhouA, LuoL, LiuN, et al. Prospective development of practical screening strategies for diagnosis of asthma-COPD overlap. Respirology. Epub 2019 Nov 27.
