Abstract
Purpose
The co-existence of chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea (OSA) has been described as the overlap syndrome. The objective of the study is to investigate the performance of Berlin Questionnaire (BQ), modified Berlin Questionnaire (MBQ), and STOP-Bang score in screening overlap syndrome from COPD subjects and investigate how pulmonary function interferes with questionnaire scoring.
Subjects and Methods
Among 116 COPD subjects included in this study, 62 were overlap syndrome subjects and 54 were COPD subjects without OSA. Subjects included were asked to fill out the questionnaires to collect demographic characteristics of subjects and questionnaire scores of BQ, MBQ, and STOP-Bang; perform pulmonary function test to confirm their COPD diagnosis; and perform polysomnography.
Results
AUC (area under the curve) of BQ, MBQ, and STOP-Bang score in screening OSA among patients with COPD was 0.71 (0.64–0.79), 0.75 (0.67–0.83), and 0.72 (0.64–0.80). In COPD subjects without OSA, FEV1%pred was statistically associated with the misdiagnosis of BQ (P= 0.0091), MBQ (P= 0.0143), and STOP-Bang (P= 0.0453). In patients with overlap syndrome, FVC%pred affected the risk of misdiagnosis of the three questionnaires (BQ: P= 0.0413; MBQ: P= 0.0150; STOP-Bang: P= 0.0241). BMI and neck circumferences (NC) were negatively correlated with FEV1%pred (BMI: P= 0.0454; NC: P= 0.0230) and FVC%pred (BMI: P= 0.0042; NC: P= 0.0367) in overlap subjects. In contrast, BMI was positively correlated with FEV1/FVC (P= 0.0141) and FEV1%pred (P= 0.0391) in COPD subjects without OSA.
Conclusion
BQ, MBQ, and STOP-Bang score performed well in COPD subjects for screening OSA. The diagnosis of the three questionnaires was more accurate in subjects with lower FEV1%pred or FVC%pred value. Pulmonary function might exert influence on the diagnosis efficacy of the three questionnaires through BMI and neck circumference.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable, and treatable disease that is characterized by persistent respiratory symptoms and largely irreversible airflow obstruction,Citation1,Citation2 which affects over 10% of adult population worldwide.Citation3 Obstructive sleep apnea (OSA) is characterized by periods of partial or total intermittent collapse of the upper airway, resulting in nocturnal hypoxemia and arousals from sleep,Citation4 with the prevalence of 9% to 38% in general population,Citation5 and 10% to 66% among COPD subjects.Citation6 The co-existence of both disorders has been described as the overlap syndrome (OVS).Citation7
Studies have demonstrated, both daytime and nocturnal, PaO2 was significantly lower, while PaCO2 was significantly higher in OVS subjects than those of COPD subjects without OSA, and the pulmonary artery pressure (PAP) was higher in overlap patients both at rest and during steady-state exercise.Citation8 The OVS is associated with an increased risk of comorbid cardiovascular disease and diabetes, death, and hospitalization.Citation9,Citation10 Furthermore, if OSA was treated by continuous positive airway pressure in time, the mortality and morbidity were significantly decreased in OVS patients.Citation11 Therefore, it is very essential to screen out OSA from COPD patients.
Diagnosis of OSA requires overnight recordings with portable limited-channel recorders (respiratory polygraph) or full polysomnography (PSG), either at home or in a sleep laboratory,Citation12 which are expensive, time-consuming, and not suitable for routine screening. Therefore, different questionnaires have been widely used as screening tools for OSA in clinical practice, such as Berlin,Citation13 modified Berlin questionnaire,Citation14 and STOP-Bang score.Citation15 Berlin and modified Berlin Questionnaire combine information about snoring, apnea, daytime sleepiness or fatigue, obesity, and hypertension, while STOP-Bang questionnaire collects information about snoring, tiredness, observed apnea, hypertension, age, sex, body mass index (BMI), and neck circumference.
However, few studies have reported the diagnostic performance of these questionnaires in screening OVS among COPD subjects.Citation16,Citation17 Moreover, no study has investigated whether COPD-related paraments would exert effects on the performance of these diagnostic questionnaires. Therefore, this study was conducted to evaluate the value of Berlin Questionnaire (BQ), modified Berlin Questionnaires (MBQ), and STOP-Bang score in screening OVS from COPD subjects, and investigate whether the pulmonary function parameters affect the diagnostic accuracy of these questionnaires in COPD population.
Subjects and Methods
Study Design
It is a cross-section study approved by the ethics committees of Zhongshan Hospital affiliated to Fudan University, Shanghai, China.
When patients were recruited, written informed consent was obtained, and data were collected by questionnaire survey. Demographic information (age, sex, state of smoking, and drinking), comorbidities, and acute exacerbation of COPD were obtained by questionnaire. BMI (a person’s weight in kilograms divided by the square of your height in meter) and neck circumference was measured and calculated by two nurses before patients filled out questionnaires. Modified Medical Research Council (mMRC) scale was used to assess dyspnea,Citation18 and health status impairment was assessed by the COPD assessment test (CAT).Citation19 The prior probability of OSA was investigated using the Berlin,Citation13 modified Berlin,Citation14 and STOP-Bang questionnaire.Citation15
When questionnaires were completed, we performed pulmonary function test and polysomnography (PSG) test to confirm their diagnosis.
Based on Berlin, modified Berlin, and STOP-Bang questionnaire diagnosis, we compared lung function parameters of patients correctly diagnosed and misdiagnosed by questionnaires, and analyzed the correlation between lung function parameters and diagnostic questionnaire scores in COPD subjects without OSA and OVS subjects. Univariate logistic regression analysis was used to assess whether pulmonary function parameters affected the accuracy of questionnaires’ diagnosis.
Thereafter, to explore the mechanism by which lung function affected diagnostic questionnaire scores, we analyzed the correlation between lung function parameters and BMI or neck circumference separately, for BMI is an important scoring element in all three questionnaires, while neck circumference is a scoring element in STOP-Bang questionnaire. Based on the analyses above, we draw the conclusions that pulmonary functions of COPD subjects do exert effect on the diagnosis of Berlin, modified Berlin, and STOP-Bang questionnaire by their correlation with BMI and neck circumference.
Subjects
Patients from the Pneumology Department of Zhongshan hospital were invited, screened, and enrolled into this study from September 2015 to October 2019.
The inclusion criteria were (1) Age ≥40 years, ≤80; (2) Diagnosis of COPD by GOLD guidelines.Citation1,Citation2
The exclusion criteria were (1) Sleep less than 4 hours tested by PSG; (2)Patients on home oxygen therapy or mechanical ventilation; (3) Acute exacerbation of COPD in the preceding month; (4) Other lung diseases; (5) Sleep disorders other than OSA; (6) Active or unstable cardiovascular diseases; (7) Non-controlled arterial hypertension; (8) Severe dementia; (9) Severe untreated psychiatric conditions; (10) Neuromuscular disease; (11) Unwilling or undisciplined patient.
OSA-Related Questionnaires
Berlin questionnaire (BQ) comprises three categories including 10 questions. Part (category) 1 of BQ includes information on snoring and apnea, part 2 reflects daytime sleepiness or fatigue, and part 3 combines information about obesity and hypertension. BMI cut-off point was adjusted from 30.0 to 25.0 in MBQ compare to BQ. High risk of OSA is defined as ≥2 positive results of the three categories of BQ or MBQ. STOP-BANG questionnaire is a tool involving 3 subjective items (snoring, tiredness, and observed apnea) and 5 objective items (hypertension, age, sex, body mass index (BMI), and neck circumference), a score ≥ 3 is regarded as having a moderate to severe risk of OSA.
Pulmonary Function Examination
Spirometry was performed by Jaeger machine (Master Screen PFT, Hochberg, Germany) in Pulmonary Function Laboratory of Zhongshan Hospital according to the guidelines of the American Thoracic Society.Citation20 COPD was diagnosed if forced expiratory volume in 1 second and forced vital capacity ratio (FEV1/FVC) below 70%, after inhalation of bronchodilators.Citation1
Polysomnography
PSG was tested in Sleep Center of Zhongshan Hospital by a PSG recorder (Respironics, Alice-5 Respironics, Pittsburgh, Pennsylvania, USA) within 1 week after pulmonary function examination, including electromyogram, electrocardiogram, electrooculogram, oronasal flow, thoracoabdominal movements, arterial oxygen saturation, body position, and snoring sounds. Breathing was recorded with nasal pressure transducer. PSG reports were analyzed by two skilled specialists followed by guideline.Citation21 Apnea was defined as a decrease of at least 90% of airflow from baseline, lasting 10 s or longer, and hypopnea was defined as ≥30% decrease of airflow lasting at least 10 s, associated with either an arousal or a ≥3% O2 saturation according to American Academy of Sleep Medicine criteria.Citation22 The mean number of apneas and hypopneas per hour of sleep (Apnea–Hypopnea Index [AHI]) was calculated, and OSA was diagnosed if the Apnea–Hypopnea Index (AHI) was ≥5 events per hour.Citation12
Statistical Analysis
Consecutive data are presented as the mean ± SD (standard deviation) or median (interquartile range [IQR]) depending on whether they were normally distributed.
Categorical data were shown as number (percentage). The differences of continuous variables between groups were compared by Student’s t-tests or Mann–Whitney rank-sum tests, and Chi-square tests or Fisher’s exact test were used for analyzing categorical variables. Spearman test or Pearson test was used to determine correlations between variables. Multivariate regression model was established to investigate relationships between AHI (dependent variable) and possible determinants with OSA. Pulmonary function parameters were analyzed by univariate logistical analysis to show whether they were associated with an increased risk of misdiagnosis with questionnaires. The sensitivities, specificities, positive predictive value (PPV), negative predictive value (NPV), and area under the curve (AUC) values were calculated to compare the diagnosed efficacy of a STOP-Bang score (≥3), BQ, and MBQ (high-risk) classification with PSG results (AHI ≥5) in predicting OSA. The accepted significance level for all tests was set as 5%, and statistical analyses were performed using Stata software (version 13.0, stataCorp LP, Texas, USA).
Results
Demographic Characteristics
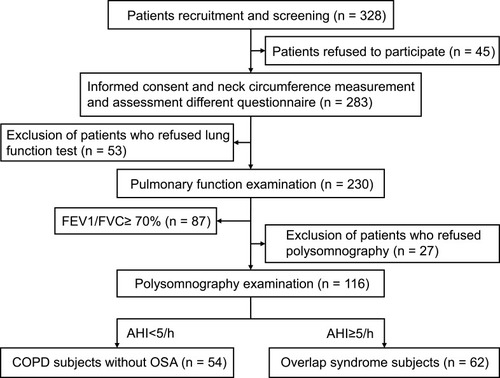
Flow diagram for participants included in this study is displayed in .
presents the descriptive information on demographics, disease characteristics, and the scores of questionnaires for all subjects (n = 116), and for those with COPD alone (n = 54) and concomitant OSA (n = 62).
Table 1 Demographic Data and Characteristics of the COPD Subjects
OVS was more common in men and obese subjects. Minimum and mean nocturnal SaO2 are significantly lower in the subjects with OVS compared with the subjects with COPD alone. Individuals with OVS also had higher (worse) scores of STOP-Bang (P<0.0001), Berlin Questionnaire (P<0.0001), and modified Berlin Questionnaire (P< 0.0001). However, OVS subjects are less likely to suffer from dyspnea since their mMRC scores (P= 0.015) are lower (better) than COPD subjects.
Parameters Associated with Apnea–Hypopnea Index in COPD Subjects
shows the correlation between AHI and potential-related parameters. Later, a multivariate regression model was established to investigate independent relationships between AHI (dependent variable) and possible determinants with OSA ().
Table 2 Correlation Between Potentially Risk Factors and Apnea–Hypopnea Index
Table 3 Multivariate Regression for Parameters Associated with Apnea–Hypopnea Index
These analyses showed that BMI was positively correlated with AHI in the subjects with OVS (P= 0.0002), and it was an independent risk factor of OSA in COPD patients. These analyses also indicated that pulmonary function parameters were not correlated with AHI in COPD subjects.
Performance of Berlin, Modified Berlin Questionnaire, and STOP-Bang Score
The diagnostic performance of three questionnaires in identifying OSA from COPD patients are summarized in . All three questionnaires showed a good performance in COPD subjects for screening OSA. Compared to STOP-Bang, BQ and MBQ had higher specificity and PPV, but lower sensitivity and NPV. The accuracy of MBQ was slightly higher than other two, but the difference was not significant (BQ vs MBQ: P= 0.1148, MBQ vs SB: P= 0.4452). Therefore, BQ, MBQ, and STOP-Bang are all suitable for screening OSA patients from COPD subjects in clinical application, among which MBQ has the best diagnostic efficacy.
Table 4 Performance of Berlin Questionnaire, Modified Berlin Questionnaire, and STOP-Bang in Identifying OSA Among COPD Subjects
Pulmonary Function Parameters Affected the Risk of Questionnaire Misdiagnosis
Patients with the overlap syndrome, who are correctly identified by BQ, MBQ, and STOP-Bang among all the COPD subjects, had significantly lower spirometric parameters than those of the misdiagnosed (S-Table 1). In COPD patients without OSA, the scores of BQ, MBQ, and STOP-Bang were positively correlated with FEV1%pred, while questionnaire scores were negatively correlated with FVC%pred in patients with OVS (S-Table 2).
Therefore, univariate logistic regression analysis was used to assess the impact of spirometric parameters on the risk of questionnaires misdiagnosis (). FEV1%pred and FVC%pred of all COPD patients were statistically associated with the risk of misdiagnosis of BQ (FEV1%pred: OR= 1.028, P= 0.0194; FVC%pred: OR= 1.036, P= 0.0130), MBQ (FEV1%pred: OR= 1.034, P= 0.0084; FVC%pred: OR= 1.044, P= 0.0052), and STOP-Bang (FEV1%pred: OR= 1.031, P= 0.0120; FVC%pred: OR= 1.040, P= 0.0080).
Table 5 Univariate Logistic Regression Analysis of Pulmonary Function Parameters Affecting the Risk of Misdiagnosis with Questionnaires
In COPD patients without OSA, FEV1/FVC and FEV1%pred were statistically associated with the risk of misdiagnosis of BQ (FEV1/FVC: OR= 4.016, P= 0.0330; FEV1%pred: OR= 1.112, P= 0.0091) and MBQ (FEV1/FVC: OR= 1.617, P= 0.0472; FEV1%pred: OR= 1.075, P= 0.0143), while FEV1%pred and FVC%pred were statistically associated with the risk of STOP-Bang misdiagnosis (FEV1%pred: OR= 1.035; P= 0.0453; FVC%pred: OR= 1.048, P= 0.0450).
In addition, FVC%pred was statistically associated with the risk of misdiagnosis by BQ (OR= 1.032 P= 0.0413), MBQ (OR= 1.041, P= 0.0150), and STOP-Bang (OR= 1.053, P= 0.0241) in patients with the overlap syndrome.
These evidence suggested that COPD patients with higher pulmonary function parameters had more chances to get a misdiagnosis by BQ, MBQ, and STOP-Bang questionnaire.
Pulmonary Function Parameters Were Correlated with BMI and Neck Circumference
Correlation analyses were conducted to explore the relationship between spirometric parameters and BMI or neck circumference.
For COPD subjects without OSA, BMI was positively correlated with FEV1/FVC (P= 0.0141) and FEV1%pred (P= 0.0391), while in OVS group BMI was negatively correlated with FEV1%pred (P= 0.0454) and FVC%pred (P= 0.0018) (). The analyses also showed, in subjects whose BMI<25kg/m2, FEV1/FVC (P= 0.0027) and FEV1%pred (P= 0.0215) were positively correlated with BMI, while in BMI≥25, FEV1%pred (P= 0.0280) and FVC%pred (P= 0.0251) was negatively correlated with BMI ().
Table 6 Pulmonary Function Parameters Were Correlated with Body Mass Index and Neck Circumference
Table 7 Correlation Between Body Mass Index and Pulmonary Function Parameters
Neck circumferences of OVS subjects are negatively correlated with FEV1%pred (P= 0.0230) and FVC%pred (P= 0.0367) ().
Discussion
This study evaluated the performance of BQ, MBQ, and STOP-Bang in identifying OSA patients from COPD in the study and investigated how pulmonary function affects the accuracy of questionnaire diagnosis. We found all the three questionnaires were suitable for screening OSA patients from COPD subjects in clinical application, among which MBQ has the best diagnostic efficacy. The diagnosis of the three questionnaires was more accurate in subjects with lower FEV1%pred or FVC%pred value, and pulmonary function exert influence on the diagnosis efficacy of the three questionnaires through BMI and neck circumference.
The co-existence of COPD and OSA was defined by OVS and is associated with an increased risk of comorbid cardiovascular disease and diabetes, mortality and hospitalization if OSA was left untreated.Citation7,Citation9-11 Therefore, it is very essential to have OVS screened out from COPD patients in time. Since the standard diagnosis of OSA is expensive and time-consuming,Citation12 different questionnaires have been used as screening tools for OSA.
An ideal sleep-disordered breathing screening score should have a high sensitivity to avoid false-negative results, but also be specific enough to avoid referral of low-risk patients for costly and time-consuming sleep monitoring.Citation23 Analyses of this study showed the specificity of BQ was higher than STOP-Bang, while the sensitivity was lower in BQ, which was in accordance with the previous study reported the performance of questionnaires in subjects without COPD.Citation23–Citation25 The performance of MBQ is slightly better than BQ, for they both displayed a high specificity moderate, but BQ showed a relatively poor sensitivity. According to the Asia-Pacific obesity definition, the cut-off point of BMI was adjusted to 25.0 in MBQ compare to BQ, which might be the reason for the superiority of MBQ in our study population.
In order to investigate how pulmonary function affected the accuracy of questionnaire diagnosis, analyses were conducted to discover the relationship between pulmonary function parameters and BMI or neck circumference.
In COPD subjects without OSA, we assume the positive correlation between BMI and pulmonary could be explained by the disease characteristic of COPD itself. COPD is a chronic wasting disease associated with significant loss of weight, body water compartments, and muscle mass.Citation28,Citation29 Studies have reported body weight is negatively correlated with FEV1.Citation30–Citation32 According to a series of articles on the obesity paradox in COPD,Citation33 compared with normal BMI, low BMI is a risk factor for accelerated lung function decline, whilst high BMI has a protective effect.Citation34 These research results were in accordance with our finding in COPD subjects without OSA. Therefore, we assumed the mechanism behind the association between the accuracy of the three questionnaires and FEV1%pred was related to the correlation between BMI and the severity of COPD. Patients with severe COPD had more severe weight loss, and low BMI accelerated the decline in lung function. As a result, subjects with severe COPD were inclined to receive lower questionnaire scores. Therefore, in COPD without OSA group, subjects were more likely to get a correct diagnosis by BQ, MBQ, and STOP-Bang score.
Our study demonstrated an interesting result that the correlation between BMI and pulmonary function was reversed in COPD subjects with and without OSA. The correlation between pulmonary function and BMI in COPD patients without OSA was consistent with that in non-obese patients, while the correlation in patients with overlap syndrome was consistent with that in obese patients. Therefore, we assumed the reversed correlation was led by the difference in the proportion of obesity in patients with COPD alone and patients with OVS. Our analyses indicated BMI was an independent risk factor for the prevalence of OSA in COPD subjects, and the proportion of obese subjects in overlap group was significantly higher than COPD subject without OSA, which is in accordance with the results from other studies.Citation26,Citation27
Many studies have demonstrated an association between excess weight or weight gain and pulmonary dysfunction.Citation35–Citation38 Boriek AM has reported the reduced curvature of the diaphragm muscle fibers in obese subjects suggesting that obesity leads to respiratory muscle dysfunction in patients with COPD and reduces the value of FEV1%pred and FVC%pred.Citation39 This finding was consistent with the overlap group of our study. Therefore, we assumed in OVS subjects FVC%pred exerted interference impact on diagnosis of the questionnaires through its negative interaction with BMI and neck circumference. Since high BMI raised the score of all three questionnaires and neck circumference upregulated STOP-Bang score, the higher BMI and neck circumference were, the subjects were more likely to get a correct diagnosis by BQ, MBQ, and STOP-Bang score in OVS subjects.
Our study also demonstrated the severity of OSA and COPD were not correlated. However, mMRC-assessed dyspneaCitation18 was more severe in COPD subjects without OSA than the overlap subjects, and the reason behind it remains to be investigated.
To our knowledge, this is the first article to compare the performance of BQ, MBQ, and STOP-Bang score for screening OSA in subjects with COPD and investigate how pulmonary function affects the accuracy of questionnaire diagnosis. However, our study was limited by the sample size and all the included subjects were Asian. Also, because of the open invitation for participation in the study, patients with more frequent symptoms and greater concern about having OSA may have accepted the invitation. Therefore, our sample failed to represent the actual prevalence of overlap syndrome in patients with COPD.
Conclusion
In conclusion, our study demonstrated that BQ, MBQ, and STOP-Bang score showed a good performance in COPD subjects for screening OSA, and the accuracy of MBQ was slightly higher than the other two. In COPD subjects without OSA, patients with low FEV1%pred were associated with increased diagnosis accuracy of BQ, MBQ, and STOP-Bang score, while OVS patients with low FVC%pred had more chances to get a correct diagnosis by the three questionnaires. Pulmonary function could exert influence on the diagnostic efficacy of the three questionnaires through its association with BMI and neck circumference. BMI was positively correlated with FEV1/FVC and FEV1%pred in COPD subjects without OSA, while negatively correlated with FEV1%pred and FVC%pred in OVS subjects. However, more studies are needed to investigate the effects of lung function on BQ, MBQ, and STOP-Bang score in the whole population or other specific populations.
Abbreviations
COPD, chronic obstructive pulmonary disease; OSA, obstructive sleep apnea syndrome; BMI, body mass index; AHI, Apnea–Hypopnea Index; REMS, rapid eye movement sleep; SaO2, oxygen saturation; mMRC, Modified Medical Research Council; CAT, COPD assessment test; BQ, Berlin Questionnaire; MBQ, modified Berlin Questionnaire; AECOPD, acute exacerbations of chronic obstructive pulmonary disease; FEV1/FVC, forced expiratory volume in 1 second and forced vital capacity ratio; FEV1%pred, percentage of predicted forced expiratory volume in 1 second; FVC%pred, percentage of predicted forced vital capacity ratio; SD, standard deviation; IQR, interquartile range; PPV, positive predictive value; NPV, negative predictive value; CI, confidence interval; OR, odds ratio.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study received ethical approval from the Zhongshan Hospital Institutional Review Board. The objective of the study and its requirements was explained to the subjects, and all participants provided written informed consent. This study was conducted according to the Helsinki Declaration.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This work was supported by grants from the National Key Research and Development Program of China (NO. 2018YFC1313600) and the National Natural Science Foundation of China (No. 81570081, 81770083).
References
- VogelmeierCF, CrinerGJ, MartinezFJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP28128970
- VestboJ, HurdSS, AgustiAG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi:10.1164/rccm.201204-0596PP22878278
- Diaz-GuzmanE, ManninoDM. Epidemiology and prevalence of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35(1):7–16. doi:10.1016/j.ccm.2013.10.00224507833
- GuilleminaultC, TilkianA, DementWC. The sleep apnea syndromes. Annu Rev Med. 1976;27:465–484. doi:10.1146/annurev.me.27.020176.002341180875
- SenaratnaCV, PerretJL, LodgeCJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi:10.1016/j.smrv.2016.07.00227568340
- WeitzenblumE, ChaouatA, KesslerR, CanuetM. Overlap syndrome: obstructive sleep apnea in patients with chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(2):237–241. doi:10.1513/pats.200706-077MG18250217
- FlenleyDC. Sleep in chronic obstructive lung disease. Clin Chest Med. 1985;6(4):651–661.2935359
- ChaouatA, WeitzenblumE, KriegerJ, IfoundzaT, OswaldM, KesslerR. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med. 1995;151(1):82–86. doi:10.1164/ajrccm.151.1.78125777812577
- MarinJM, SorianoJB, CarrizoSJ, BoldovaA, CelliBR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325–331. doi:10.1164/rccm.200912-1869OC20378728
- HangLW, HsuJY, ChangCJ, et al. Predictive factors warrant screening for obstructive sleep apnea in COPD: a Taiwan National Survey. Int J Chron Obstruct Pulmon Dis. 2016;11:665–673. doi:10.2147/COPD.S9650427099484
- MachadoMC, VollmerWM, TogeiroSM, et al. CPAP and survival in moderate-to-severe obstructive sleep apnoea syndrome and hypoxaemic COPD. Eur Respir J. 2010;35(1):132–137. doi:10.1183/09031936.0019200819574323
- KapurVK, AuckleyDH, ChowdhuriS, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy Of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(3):479–504. doi:10.5664/jcsm.650628162150
- NetzerNC, StoohsRA, NetzerCM, ClarkK, StrohlKP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. doi:10.7326/0003-4819-131-7-199910050-0000210507956
- SharmaSK, VasudevC, SinhaS, BangaA, PandeyRM, HandaKK. Validation of the modified Berlin questionnaire to identify patients at risk for the obstructive sleep apnoea syndrome. Indian J Med Res. 2006;124(3):281–290.17085831
- ChungF, YegneswaranB, LiaoP, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812–821. doi:10.1097/ALN.0b013e31816d83e418431116
- FariaAC, da CostaCH, RufinoR. Sleep Apnea Clinical Score, Berlin Questionnaire, or Epworth Sleepiness Scale: which is the best obstructive sleep apnea predictor in patients with COPD? Int J Gen Med. 2015;8:275–281. doi:10.2147/IJGM.S8647926345497
- SolerX, LiaoSY, MarinJM, et al. Age, gender, neck circumference, and Epworth sleepiness scale do not predict obstructive sleep apnea (OSA) in moderate to severe chronic obstructive pulmonary disease (COPD): the challenge to predict OSA in advanced COPD. PLoS One. 2017;12(5):e0177289. doi:10.1371/journal.pone.017728928510598
- MahlerDA, HarverA. A factor analysis of dyspnea ratings, respiratory muscle strength, and lung function in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1992;145(2 Pt 1):467–470. doi:10.1164/ajrccm/145.2_Pt_1.4671736759
- JonesPW, HardingG, BerryP, WiklundI, ChenWH, Kline LeidyN. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.0010250919720809
- MillerMR, HankinsonJ, BrusascoV, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.0003480516055882
- RedlineS, SandersMH, LindBK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21(7):759–767. doi:10.1093/sleep/21.7.75911300121
- BerryRB, BudhirajaR, GottliebDJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi:10.5664/jcsm.217223066376
- Marti-SolerH, HirotsuC, Marques-VidalP, et al. The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med. 2016;4(9):742–748. doi:10.1016/S2213-2600(16)30075-327321086
- PengM, ChenR, ChengJ, LiJ, LiuW, HongC. Application value of the NoSAS score for screening sleep-disordered breathing. J Thorac Dis. 2018;10(8):4774–4781. doi:10.21037/jtd.2018.07.4630233849
- HongC, ChenR, QingS, et al. Validation of the NoSAS score for the screening of sleep-disordered breathing: a hospital-based retrospective study in China. J Clin Sleep Med. 2018;14(02):191–197. doi:10.5664/jcsm.693029394959
- ZhuJ, ZhaoZ, NieQ, et al. Effect of lung function on the apnea-hypopnea index in patients with overlap syndrome: a multicenter cross-sectional study. Sleep Breath. 2019. doi:10.1007/s11325-019-01961-w
- BednarekM, PlywaczewskiR, JonczakL, ZielinskiJ. There is no relationship between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome: a population study. Respiration. 2005;72(2):142–149. doi:10.1159/00008404415824523
- WilsonDO, RogersRM, HoffmanRM. Nutrition and chronic lung disease. Am Rev Respir Dis. 1985;132(6):1347–1365. doi:10.1164/arrd.1985.132.6.13473907446
- BaarendsEM, ScholsAM, van Marken LichtenbeltWD, WoutersEF. Analysis of body water compartments in relation to tissue depletion in clinically stable patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 1997;65(1):88–94. doi:10.1093/ajcn/65.1.888988918
- KaradagF, KarulAB, CildagO, AltunC, GurgeyO. Determinants of BMI in patients with COPD. Respirology. 2004;9(1):70–75. doi:10.1111/j.1440-1843.2003.00533.x14982605
- WilsonDO, RogersRM, WrightEC, AnthonisenNR. Body weight in chronic obstructive pulmonary disease. The National Institutes of Health Intermittent Positive-Pressure Breathing Trial. Am Rev Respir Dis. 1989;139(6):1435–1438. doi:10.1164/ajrccm/139.6.14352658702
- MarquisK, DebigareR, LacasseY, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(6):809–813. doi:10.1164/rccm.210703112231489
- GrubergL, WeissmanNJ, WaksmanR, et al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol. 2002;39(4):578–584. doi:10.1016/S0735-1097(01)01802-211849854
- SunY, MilneS, JawJE, et al. BMI is associated with FEV1 decline in chronic obstructive pulmonary disease: a meta-analysis of clinical trials. Respir Res. 2019;20(1):236. doi:10.1186/s12931-019-1209-531665000
- HeederikD, MillerBG. Weak associations in occupational epidemiology: adjustment for exposure estimation error. Int J Epidemiol. 1988;17(4):970–974. doi:10.1093/ije/17.4.9703225115
- BandeJ, ClementJ, Van dWKP. The influence of smoking habits and body weight on vital capacity and FEV1 in male Air Force personnel: a longitudinal and cross-sectional analysis. Am Rev Respir Dis. 1980;122(5):781–790.6969563
- CotesJE, GilsonJC, JohnC. Effects of inactivity, weight gain and antitubercular chemotherapy upon lung function in working coal-miners. Ann Occup Hyg. 1967;10(4):327–335.6063962
- ChenY, HorneSL, DosmanJA. Body weight and weight gain related to pulmonary function decline in adults: a six year follow up study. Thorax. 1993;48(4):375–380. doi:10.1136/thx.48.4.3758511735
- BoriekAM, LopezMA, VelascoC, et al. Obesity modulates diaphragm curvature in subjects with and without COPD. Am J Physiol Regul Integr Comp Physiol. 2017;313(5):R620–r629. doi:10.1152/ajpregu.00173.201728903915