Abstract
Background
Comorbidities including cardiovascular diseases are very common in chronic obstructive pulmonary disease (COPD) secondary to tobacco smoking and contribute to the overall severity of the disease. In non-smoking COPD, which accounts for about 25% of COPD cases worldwide, current knowledge on the frequency and determinants of comorbidities remains scarce. The aims of the current study were to assess the frequency of major comorbidities and to evaluate their determinants in a group of non-selected patients with mild-to-moderate COPD who were exposed to organic dust (dairy farmers), to tobacco smoking, or to both, and in controls without COPD who were exposed to organic dust (dairy farmers), or to tobacco smoking, or to both, or who were without exposure.
Patients and Methods
A total of 4665 subjects (2323 dairy farmers and 2342 non-farmers) including 355 patients with COPD and 4310 controls with normal spirometry were recruited through a large COPD screening program. Self-reported physician-diagnosed diseases with plausible links to COPD were recorded in this cross-sectional study.
Results
Whatever the exposure, cardiovascular comorbidities were not more frequent in patients with COPD than their counterparts without airflow limitation. A higher risk of major cardiovascular comorbidities was associated with tobacco smoking and a lower risk was associated with exposure to organic dusts.
Conclusion
Tobacco smoking (but not COPD) is associated with higher frequency of cardiovascular comorbidities. By contrast, being a dairy farmer exposed to organic dusts is associated with a lower frequency of the same comorbidities. This reinforces the crucial need for controlling established cardiovascular risk factors even in patients with mild-to-moderate COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is an important cause of morbidity and mortality worldwide.Citation1 Progressive respiratory failure accounts for only one third of all causes of mortality in patients with COPD, especially in those with severe disease, suggesting that many patients die from other causes.Citation2 COPD comorbidities are conditions associated with COPD that contribute to the overall severity of the disease, impairing quality of life and increasing mortality.Citation3,Citation4 Among these, coronary artery disease (CAD), diabetes mellitus and hypertension are very common in COPD.Citation5 Screening and developing intervention strategies for these major comorbidities in patients with COPD may be of importance given their impact on prognosis.Citation5
The prevalence of COPD-associated comorbidities varies according to several factors such as gender, age, body mass index and type of exposure.Citation3,Citation6,Citation7 Cigarette smoking is the main risk factor for COPD in developed countries, and a large majority of the current knowledge on comorbidities in COPD comes from analyses made in smoking-related COPD. Nevertheless, about 25% of COPD cases worldwide can involve other factors, including indoor and outdoor air pollutants as well as occupational hazards.Citation7,Citation8 Among occupational causes, exposure to organic dusts has been associated with accelerated impairment in lung function and with increased risk of COPD.Citation9–Citation11
Comorbidities have been rarely studied in mild-to-moderate COPD. Diagnosing COPD at mild-to-moderate stages (ie, in patients with preserved or moderately altered pulmonary function) and, if possible, at early stages (ie, at the beginning of pulmonary function deterioration) has however been identified as a priority for secondary prevention.Citation12 Theoretically, making a diagnosis of COPD at early and/or mild-to-moderate stages may also trigger the earlier introduction of medications for comorbidities associated with COPD.Citation13
COPD screening programs provide unique opportunities to study various aspects of early and mild-to-moderate COPD. In 2012, a large joint academic and public health screening program called BALISTIC was launched to detect and characterize COPD in dairy farmers compared with smokers in a largely agricultural region of France (see details below).Citation14
Here, we sought to assess the frequency of comorbidities in a group of non-selected patients with mild-to-moderate COPD who were exposed to organic dust (dairy farmers), to tobacco smoking, or to both, and in controls without COPD who were exposed to organic dust (dairy farmers), or to tobacco smoking, or to both, or who were without exposure, all detected by screening through the BALISTIC program. In addition, we sought to determine the respective contribution of COPD, tobacco smoking and organic dust exposure to these comorbidities.
Patients and Methods
Study Design and Subjects
Patients with COPD and control subjects without COPD were consecutively recruited between January 2012 and November 2015 through a regional COPD screening program involving general practitioners and occupational physicians (BALISTIC study; ClinicalTrials.gov Identifier: NCT2540408). This program was conducted at the University Hospital of Besançon in collaboration with the French national social security system for all agricultural workers (Mutualité Sociale Agricole, MSA) and with the care homes federation of the Franche-Comté region (Fédération des Maisons de Santé Comtoises, FéMaSaC).Citation14,Citation15 The study was set up primarily to obtain data on the prevalence of COPD and on characteristics of the disease occurring in dairy farmers (ie COPD secondary to organic dust exposure) by comparison with COPD patients who were not exposed to organic dusts.Citation16
Every five years, all persons affiliated to the MSA are invited to attend a free health check-up, and around 20% of those invited usually attend.Citation17 Among all persons aged 40–74 years who attended the health check-up between January 2012 and November 2015, participation in the screening phase of the BALISTIC study was offered to those who had been exposed either to organic dusts (dairy farmers) and/or to tobacco smoking and also to those with no identified exposure. Exclusion criteria were an established diagnosis of asthma, bronchiectasis and/or hypersensitivity pneumonitis. Patients with a documented diagnosis of severe COPD were also excluded from the screening program. In order to include subjects with an exposure as homogeneous as possible, subjects having or having had a history of occupational exposure other than dairy farming (including farmers with any other professional activity than dairy farming) were also excluded.
In parallel, general practitioners (GPs) working with the FéMaSaC proposed the participation in the screening phase of the BALISTIC study to their patients fulfilling the above inclusion and exclusion criteria. These patients were invited to attend free screening sessions during which spirometry was performed by their GPs after adequate training.
The criteria for retaining a diagnosis of mild-to-moderate COPD were (i) a history of exposure to risk factors (tobacco smoking and/or daily exposure to organic dusts) and (ii) evidence of persistent airflow limitation defined as a post-bronchodilator forced expiratory volume in 1 second (FEV1) on forced vital capacity (FVC) ratio <lower limit of normal (LLN) together with a FEV1 >50% of predicted value according to the GLI-2012 equations.Citation1,Citation18 COPD patients were classified into 3 groups: (i) COPD secondary organic dust exposure, ie, COPD patients with a history of at least 20 years of exposure to organic dust together with a smoking history of less than 1 pack-year; (ii) COPD secondary to tobacco smoking (more than 1 pack-year) with no history of organic dust exposure; and (iii) COPD secondary to both organic dusts and tobacco smoking exposures.
Subjects without airflow limitation participating in the screening program were also included in the current analysis.
This study was approved by the local ethics committee (CPP Est; P-2011-119), and written informed consent was obtained from all subjects. In addition, this trial was conducted in accordance with the Declaration of Helsinki.
Spirometry
Spirometry tests were performed using a pneumotachograph (MedGraphics; MSE Medical, Strasbourg, France). All spirometers were calibrated at least once daily using a 3-L syringe. Bronchodilation was performed by administering 400 μg of the short-acting β2-agonist salbutamol (Ventoline®, GlaxoSmithKline, Marly-le-Roi, France) and spirometry was repeated after a 10- to 15-min interval, as recommended. Only subjects with valid spirometry at screening were further included in the study.
Questionnaire
The study focused on self-reported physician-diagnosed diseases with possible or established links with COPD and tobacco or organic dust exposure: participants were asked whether they had been diagnosed with or treated for the following selected medical conditions (“comorbidities”): hypertension, heart disease (including coronary artery disease, chronic heart failure, atrial fibrillation and valvulopathy), peripheral artery disease (PAD), cerebrovascular disease, thromboembolic disease, obstructive sleep apnea syndrome (OSAS), diabetes mellitus, dyslipidemia, thyroid disorders, gastroesophageal reflux, osteoporosis, solid neoplasms (cancer), leukemia, lymphoma, chronic renal disease, liver disease, neurodegenerative disorder, epilepsy, anxiety-depression syndrome and connective tissue disease. Responses were dichotomous. The non-age-adjusted Charlson index and the COTE (COPD specific comorbidity test) index were calculated for every patient, as described elsewhere.Citation3,Citation19 Briefly, in the Charlson index, each condition is assigned a score of 1 point (CAD, chronic heart failure, PAD, dementia, cerebrovascular disease, chronic lung disease including COPD, connective tissue disease, ulcer, chronic liver disease, diabetes), 2 points (hemiplegia, moderate or severe renal disease, diabetes with end-organ damage, cancer, leukemia, lymphoma), 3 points (moderate or severe liver disease) or 6 points (cancer with metastasis, AIDS), depending on the risk of death associated with each one. In the COTE index, each condition is assigned a score of 6 points (anxiety (for female only), lung, esophageal, pancreatic, and breast cancer), 2 points (all other cancers, liver cirrhosis, atrial fibrillation, diabetes with neuropathy, pulmonary fibrosis) and 1 point (congestive heart failure, gastric/duodenal ulcers, CAD).
Statistical Analysis
Bivariate logistic regression analysis and chi-square tests were performed for categorical data and the t-test for quantitative data. Data were expressed as means (SD), numbers (percentages), or z scores, as appropriate. Continuous variables used for logistic regression were all categorized to obtain categorical variables. Age and tobacco habits were divided into three categories each (namely, age <55 years, 55–64 years, ≥65 years; and never smokers, former smokers, current smokers, respectively). Other variables were binary: sex (male versus female), BMI (<25 versus ≥25), and presence of comorbidity (yes versus no).
Multivariate logistic regressions were performed for each comorbidity of special interest (CAD, diabetes mellitus and hypertension) with the diagnosis of the comorbidity as a dependent variable and variables with a level of significance ≤0.20 in the bivariate analyses as explanatory variables. In addition, variables known to be usual confounding factors or variable of main interest were also included as explanatory variables. Multi-collinearity tests were carried out in the process of model building to identify correlated variables. When two variables were significantly correlated, only the variable most significantly linked to the comorbidity was included in the multivariate analysis. In order to obtain the models, a stepwise backward selection of variables was performed.
All analyses were performed with R version 3.5.0 and RStudio version 1.1.453 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Subject Characteristics
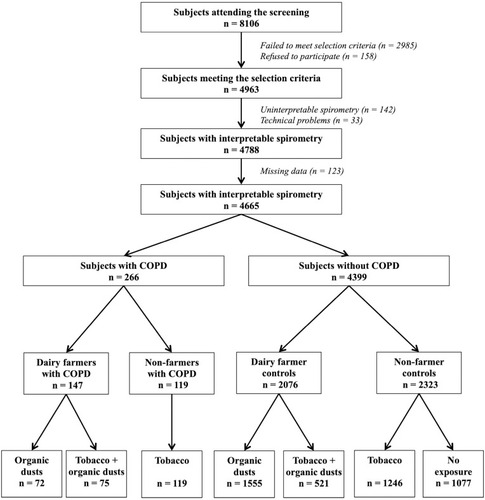
The flowchart of the participating population is displayed in . Of the 4963 subjects who underwent COPD screening, 4665 (94%) had a spirometry test of acceptable quality together with a complete report of comorbidities and were therefore included in the current analysis. Of these 4665 subjects, 266 patients (5.7%) had mild-to-moderate COPD and 4399 had normal spirometry (control subjects).
Figure 1 Flowchart of participants included in the study. Subjects were recruited through a screening program set up by two national health insurance organizations. Inclusion criteria in the screening programs were men or women aged 40 to 74 years, with no history of chronic respiratory disease including asthma and hypersensitivity pneumonitis.
On average, dairy farmers were slightly older, were more frequently men and were less often smokers than non-dairy farmers (Supplemental Table 1). The main characteristics of the three subgroups of COPD patients and their counterparts without airway obstruction separated according to exposure (ie, tobacco smoking, organic dusts and both exposures), as well as characteristics of control subjects without airway obstruction and without any exposure, are given in . All subgroups (patients with COPD and control subjects) had the same age. COPD patients were more frequently men than their counterparts with normal spirometry, except in the subgroup of subjects exposed only to tobacco smoking. In subgroups exposed to tobacco smoking (either exclusively or in association with organic dust exposure), smoking intensity was higher in COPD than in controls, and patients with COPD were more frequently active smokers (instead of former smokers) than controls. The subgroup of COPD patients with exclusive exposure to tobacco smoking had a significantly lower FEV1 compared with the other two exposure-defined subgroups. Of note, the mean ± standard deviation z-scores for FEV1 and FVC for all control subjects without COPD were very close to 0 ± 1, indicating a very good fit of our control population with the healthy population of the Global Lung Initiative for spirometry.Citation20
Table 1 Main Characteristics of Patients with COPD and Control Subjects, Separated into Three Subgroups According to Exposure to Tobacco Smoking, to Organic Dusts or Both
Comorbidities
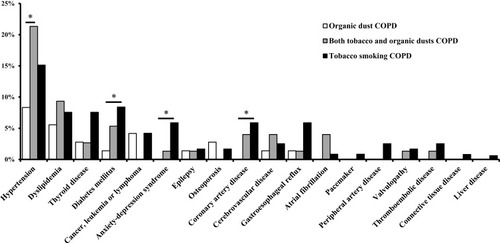
In the whole study population (patients with and without COPD), dairy farmers had a lower frequency of comorbidities including cardiovascular diseases (CVD), diabetes mellitus, anxiety-depression syndrome and cancer than non-dairy farmers (Supplemental Table 1). By contrast, in each exposure-defined subgroup, there were very few differences in reported comorbidities between COPD patients and their controls with normal spirometry (). Similarly, in each of the three exposure-defined categories, COPD patients had values of the non-age-adjusted Charlson comorbidity index that differed by less than 1 compared with their controls without COPD. As the item “COPD” counts 1 point in the Charlson comorbidity index, a difference of less than 1 between COPD and non-COPD indicates that values are in fact similar.
Table 2 Frequency of Main Comorbidities in the Different Exposure-Defined Subgroups
The non-age-adjusted Charlson comorbidity index and the COTE index were poorer for COPD patients exposed to tobacco smoking compared with COPD patients exposed to organic dust alone, while those exposed to both organic dusts and tobacco smoking had intermediate values. Nevertheless, after adjustment for FEV1, these two indexes were no longer different between these three exposure-defined subgroups of COPD. Comorbidities in each subgroups of COPD are is displayed in .
Factors Associated with 3 Selected Major Comorbidities
and and Supplemental Table 2 show the results of the univariate and multivariate analyses (made in the entire cohort: n=4665) for the association between three selected comorbidities (ie, coronary artery disease, diabetes mellitus and hypertension) and several selected variables. This analysis shows that COPD (at mild-to-moderate stages) was not associated with an increased risk of these three major comorbidities. By contrast, we found that exposure to organic dusts was associated with a lower risk of diabetes mellitus and of coronary artery disease. Conversely, a smoking intensity of more than 15 pack-years was associated with an increased risk of each of the three selected major comorbidities.
Table 3 Univariate and Multivariate Logistic Regression Analyses for Coronary Artery Disease
Table 4 Univariate and Multivariate Logistic Regression Analyses for Diabetes Mellitus
Discussion
The analysis of this large population of subjects comprising 5.7% of patients with mild-to-moderate COPD related to tobacco smoking and/or exposure to organic dusts (all identified by screening) reveals that (1) whatever the exposure, patients with COPD did not have a higher frequency of comorbidities than their counterparts without airflow limitation but with similar exposure; (2) after adjustment on airflow limitation severity, comorbidity indexes were similar in COPD secondary to tobacco smoking and in COPD secondary to organic dusts exposure; (3) tobacco smoking (but not COPD) was associated with a higher risk of major comorbidities and (4) exposure to organic dusts (here, to have been a dairy farmer for more than 20 years) was associated with a lower risk of major comorbidities.
Although contrasting with many previous studies that report an association between major cardiovascular comorbidities and COPD, the absence of such a relationship has also been previously reported in patients with mild-to-moderate stages of airflow obstruction.Citation5,Citation21,Citation22 As an example, Johnston et al observed a crude association between mild-to-moderate COPD and cardiovascular diseases (CVD), but this association was no longer present after adjusting for established CVD risk factors.Citation23 By analyzing the burden of obstructive lung disease (BOLD) study population, Triest et al very recently demonstrated that cardiovascular disease and hypertension were more prevalent in subjects with airflow obstruction, but this association was confounded by known cardiovascular risk factors, principally aging and smoking.Citation7 Our data provide further evidence that in patients with smoking-related COPD at mild or moderate stages, the relationship between COPD and CVD is mediated through established cardiovascular risk factors such as age and tobacco smoking rather than through COPD itself. These results are consistent with previously reported data from our team which demonstrated that COPD by itself is not sufficient to explain increased arterial stiffness (a predictor of cardiovascular events) and that tobacco smoking is a risk factor for elevated arterial stiffness in COPD.Citation24 These findings are of importance in order to plan and provide adequate care for patients. Indeed, in these patients at mild-to-moderate stages, it will be relevant to deal with the amenable CVD risk factors (and in particular tobacco smoking) rather than with COPD itself in order to prevent cardiovascular diseases to occur. This finding does not support the possibility of beneficial effects of drugs targeting COPD-associated inflammatory mechanisms on cardiovascular outcomes in patients with smoking-related mild-to-moderate COPD.
Few studies have investigated the prevalence of comorbidities among farmers. Regarding CVD, in a study conducted in Ireland, van Doorn et al have reported that livestock farmers were at high risk for cardiovascular disease because about half had hypertension and elevated total cholesterol.Citation25 By contrast, in our cross-sectional study, we found that dairy farmers, all from the French Franche-Comté region, were at lower risk for CVD compared with non-dairy farmers (all from Franche-Comté). Although intriguing, these different results may be explained by different types of exposure and/or lifestyle behaviors from one region/country to another. As an example, we recently reported that dairy farmers working in the Brittany region of France were not at higher risk for COPD while dairy farmers from Franche-Comté were, suggesting that meaningful differences in exposure and/or lifestyle behaviors and/or other uncontrolled factors exist between dairy farmers working in different regions and/or countries.Citation17 Identifying such factors would help to understand the lower frequency of CVD among our group of dairy farmers.
The finding that patients with COPD secondary to other causes than tobacco smoking may be at lower risk of comorbidities has been recently suggested elsewhere. By comparing the prevalence of comorbidities between subjects with COPD secondary to smoke of biomass combustion and COPD secondary to tobacco smoking, Golpe et al found that the prevalence of CVD was lower in the group of COPD exposed to biomass combustion.Citation6 In addition, the protective role of farming on comorbidities such as lung cancer has also been previously reported.Citation26 A survival bias is however not excluded in this population.
Strengths and Limitations
All subjects included in the current analysis came from the same region in a high-income country, and all data were collected in a relatively short period of time (between January 2012 and November 2015). In addition, the size of the analyzed population is large and its homogeneity can be regarded as a strength because it decreases the risk of uncontrolled parameters that could have influenced the results. Moreover, the fact that the control population had perfectly normal spirometry (ie, a mean ± standard deviation z-scores equal to 0 ± 1) further reduces the risk of bias.
Some limitations must be pointed out. First, as this is a cross-sectional study of disease frequency rather than incidence, there is uncertainty in the degree to which exposure preceded the outcomes observed. Second, as our population of interest results from a COPD screening program, there is an imbalance between cases and controls in the current study. Third, we did not quantify the level of organic dust exposure of all subjects included in the current analysis. However, this was assessed in a subset of our population, showing that exposure to molds and actinomycetes was higher in dairy farmers than in non-dairy farmers and was similar among dairy farmers with COPD and in those without.Citation16 Fourth, as the study was based on self-reported assessment of comorbidities and health-related variables, there may be misclassification in the outcome and covariate data. Undiagnosed and untreated cases may not have been reported, and there may have been misreporting of diagnoses or sensitive health-related characteristics such as weight, level of physical activity and alcohol use. Fourth, the cross-sectional design raises the possibility of survivor bias, subjects with more comorbidities being less likely to survive. Nevertheless, it is unlikely that such bias could account for the absence of relationship between COPD and comorbidities, as most previous studies reporting an association were also cross-sectional.
Conclusion
In conclusion, we found that tobacco smoking (but not COPD) was associated with higher frequency of cardiovascular comorbidities. By contrast, being a dairy farmer exposed to organic dusts was associated with a lower frequency of the same comorbidities. These results suggest that the systemic effects of organic dust exposure may be different from those of tobacco. However, these findings must be taken with caution as other factors such as lifestyle behavior could be potential confounders. Further studies are needed to confirm these results.
Abbreviations
BMI, body mass index; BOLD, burden of obstructive lung disease; CAD, coronary artery disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; COTE, COPD specific comorbidity test; CVD, cardiovascular diseases; FéMaSaC, Fédération des Maisons de Santé Comtoises; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GPs, general practitioners; LLN, lower limit of normal; MSA, Mutualité Sociale Agricole; OSAS, obstructive sleep apnea syndrome; PAD, peripheral artery disease.
Data Sharing Statement
Data underlying the findings described in this manuscript could not be shared as analysis and scientific promotion of these data have not been completed.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
N. R. reports grants and personal fees from Boehringer Ingelheim, Pfizer, and Novartis; and personal fees from Teva, GlaxoSmithKline, AstraZeneca, Chiesi, Mundipharma, Cipla, Sano, Sandoz, 3M, Trudell, and Zambon, outside the submitted work. S. J. received fees, funding or reimbursement for national and international conferences, boards, expert or opinion groups, and research projects over the past 5 years from Actelion, AIRB, Astra Zeneca, BMS, Boehringer, Chiesi, Gilead, GSK, LVL, Mundipharma, Novartis, Pfizer, Roche, Savara-Serendex. J.-C. D. reports grants, personal fees, and nonfinancial support from Novartis Pharma; personal fees and nonfinancial support from GlaxoSmithKline, Chiesi, InterMune, AstraZeneca, and Boehringer Ingelheim; and nonfinancial support from Stallergenes, outside the submitted work. The authors report no other conflicts of interest in this work.
Acknowledgments
We would like to express our appreciation to the patients who participated in the study. We also thank the clinical staff who contributed to the measurements. We are indebted to Antonin Grisey, Fanny Petitcuenot, Pauline Roux and Marc Laplante who performed most of the spirometric tests of the screening program. The authors thank Nina Crowte for editorial assistance.
References
- VogelmeierCF, CrinerGJ, MartinezFJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 Report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP28128970
- ZielinskiJ, MacNeeW, WedzichaJ, et al. Causes of death in patients with COPD and chronic respiratory failure. Monaldi Arch Chest Dis. 1997;52(1):43–47.9151520
- DivoM, CoteC, de TorresJP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–161. doi:10.1164/rccm.201201-0034OC22561964
- FearyJR, RodriguesLC, SmithCJ, HubbardRB, GibsonJE. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax. 2010;65(11):956–962. doi:10.1136/thx.2009.12808220871122
- ChenW, ThomasJ, SadatsafaviM, FitzGeraldJM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(8):631–639. doi:10.1016/S2213-2600(15)00241-626208998
- GolpeR, Martin-RoblesI, Sanjuan-LopezP, et al. Prevalence of major comorbidities in chronic obstructive pulmonary disease caused by biomass smoke or tobacco. Respiration. 2017;94(1):38–44. doi:10.1159/00047271828456807
- TriestFJJ, StudnickaM, FranssenFME, et al. Airflow obstruction and cardio-metabolic comorbidities. COPD. 2019;1–9.
- SalviSS, BarnesPJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374(9691):733–743. doi:10.1016/S0140-6736(09)61303-919716966
- MathesonMC, BenkeG, RavenJ, et al. Biological dust exposure in the workplace is a risk factor for chronic obstructive pulmonary disease. Thorax. 2005;60(8):645–651. doi:10.1136/thx.2004.03517016061705
- GuillienA, PuyraveauM, SoumagneT, et al. Prevalence and risk factors for COPD in farmers: a cross-sectional controlled study. Eur Respir J. 2015.
- BolundAC, MillerMR, SigsgaardT, SchlunssenV. The effect of organic dust exposure on long-term change in lung function: a systematic review and meta-analysis. Occup Environ Med. 2017;74(7):531–542. doi:10.1136/oemed-2016-10396328404791
- SorianoJB, ZielinskiJ, PriceD. Screening for and early detection of chronic obstructive pulmonary disease. Lancet. 2009;374(9691):721–732. doi:10.1016/S0140-6736(09)61290-319716965
- KaplanA, ThomasM. Screening for COPD: the gap between logic and evidence. Eur Respir Rev. 2017;26:143. doi:10.1183/16000617.0113-2016
- DeganoB, BouhaddiM, LaplanteJJ, et al. [COPD in dairy farmers: screening, characterization and constitution of a cohort. The BALISTIC study]. Rev Mal Respir. 2012;29(9):1149–1156. doi:10.1016/j.rmr.2012.08.00723200591
- SoumagneT, GuillienA, RouxP, et al. Quantitative and qualitative evaluation of spirometry for COPD screening in general practice. Respir Med Res. 2019;77:31–36. doi:10.1016/j.resmer.2019.07.00432035336
- BarreraC, RocchiS, DeganoB, et al. Microbial exposure to dairy farmers’ dwellings and COPD occurrence. Int J Environ Health Res. 2018;1–13.
- GuillienA, PuyraveauM, SoumagneT, et al. Prevalence and risk factors for COPD in farmers: a cross-sectional controlled study. Eur Respir J. 2016;47(1):95–103. doi:10.1183/13993003.00153-201526453630
- QuanjerPH, StanojevicS, ColeTJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi:10.1183/09031936.0008031222743675
- CharlsonME, PompeiP, AlesKL, MacKenzieCR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-83558716
- StanojevicS, GrahamBL, CooperBG, et al. Official ERS technical standards: global lung function initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J. 2017;50:3. doi:10.1183/13993003.00010-2017
- BatyF, PutoraPM, IsenringB, BlumT, BrutscheM. Comorbidities and burden of COPD: a population based case-control study. PLoS One. 2013;8(5):e63285. doi:10.1371/journal.pone.006328523691009
- LangeP, MogelvangR, MarottJL, VestboJ, JensenJS. Cardiovascular morbidity in COPD: a study of the general population. COPD. 2010;7(1):5–10. doi:10.3109/1541255090349950620214458
- JohnstonAK, ManninoDM, HaganGW, DavisKJ, KiriVA. Relationship between lung function impairment and incidence or recurrence of cardiovascular events in a middle-aged cohort. Thorax. 2008;63(7):599–605. doi:10.1136/thx.2007.08811218245145
- SoumagneT, RocheN, GuillienA, et al. Cardiovascular risk in COPD: deciphering the contribution of tobacco smoking. Chest. 2019.
- van DoornD, RichardsonN, OsborneA. Farmers have hearts: the prevalence of risk factors for cardiovascular disease among a subgroup of irish livestock farmers. J Agromedicine. 2017;22(3):264–274. doi:10.1080/1059924X.2017.131872828406370
- Ben KhedherS, NeriM, GuidaF, et al. Occupational exposure to endotoxins and lung cancer risk: results of the ICARE study. Occup Environ Med. 2017;74(9):667–679. doi:10.1136/oemed-2016-10411728490662