Abstract
Background
COPD exacerbations occur more frequently with disease progression and are associated with worse prognosis and higher healthcare expenditure.
Purpose
To utilize a networked system, optimized with statistical process control (SPC), for remote patient monitoring (RPM) and to identify potential predictors of COPD exacerbations.
Methods
Seventeen subjects, mean (SD) age of 69.7 (7.2) years, with moderate to severe COPD received RPM. Over 2618 patient-days (7.17 patient-years) of monitoring, we obtained daily symptom scores, treatment adherence, self-reported activity levels, daily spirometry (SVC, FEV1, FVC, PEF), inspiratory capacity (IC), and oxygenation (SpO2). These data were used to identify predictors of exacerbations defined using Anthonisen and other criteria.
Results
After implementation of SPC, concordance analysis showed substantial agreement between FVC (decrease below the 7-day rolling average minus 1.645 SD) and self-reported healthcare utilization events (κ=0.747, P<0.001) as well as between increased use of inhaled short-acting bronchodilators and exacerbations defined by two Anthonisen criteria (κ=0.611, P<0.001) or modified Anthonisen criteria (κ=0.622, P<0.001). There was a moderate agreement between FEV1 (decrease >1.645 SD below the 7-day rolling average) and self-reported healthcare utilization events (κ=0.475, P<0.001) and between SpO2 less than 90% and exacerbations defined by two Anthonisen criteria (κ=0.474, P<0.001) or modified Anthonisen criteria (κ=0.564, P<0.001).
Conclusion
Exacerbations were best predicted by FVC and FEV1 below the one-sided 95% confidence interval derived from SPC but also by increased use of inhaled short-acting bronchodilators and fall in oxygen saturation. An RPM program that captures these parameters may be used to guide appropriate interventions aimed at reducing healthcare utilization in COPD patients.
Introduction
Chronic obstructive pulmonary disease (COPD) is a debilitating condition and the fourth leading cause of death worldwide.Citation1–Citation3 The disease is characterized by chronic symptoms and episodes of exacerbation, often due to infection but also idiopathic.Citation4 Increasing frequency of exacerbations is a marker for disease progression, and is associated with significant morbidity, increased healthcare utilization, poor health status and death.Citation5 Evidence suggests that patients with lower forced expiratory volume in one second (FEV1) tend to have more exacerbations, and those with more frequent exacerbations have more rapid declines in lung function and susceptibility to future exacerbations.Citation6–Citation8 The need for early detection of COPD exacerbations is evident, because early intervention appears to be a key factor in improving outcomes and preserving quality of life.Citation9 Current interventions aimed at slowing disease progression and perhaps reducing exacerbation frequency include smoking cessation, vaccination, inhaled maintenance therapies and oxygen therapy. Focusing efforts on early detection of exacerbations could lead to earlier treatment and therefore improve outcomes and preserve health status.
Emerging technologies in telemedicine and remote patient monitoring (RPM) offer possibilities for the proactive management of patients at risk of COPD exacerbation. RPM has been shown to be feasible in chronic heart failure patients, improving adherence to prescribed medications.Citation10 Similar studies have attempted to reduce healthcare utilization and improve quality of life.Citation11–Citation13 Due to the complexity and variability of the changes in COPD exacerbations, RPM should include both symptom assessment and physiological measurements in order to detect these events. The challenge of RPM is determining which symptoms or physiological measures have the best predictive value.Citation11,Citation14 Previous remote monitoring studies in COPD patients demonstrated the reliability of daily spirometry performed at home.Citation15,Citation16 Forced vital capacity (FVC), FEV1 and peak expiratory flow (PEF) are known to decrease during exacerbations; therefore, these could be useful parameters for remote monitoring.Citation17,Citation18 Inspiratory capacity (IC) could be helpful to detect worsening air trapping but has not routinely been used for daily monitoring due to inadequate data on reliability and reproducibility. Commercial pulse oximeters are now relatively inexpensive and can be programmed to give not only oxygen saturation (SpO2) but also heart rate and breathing frequency from a detectable waveform called photoplethysmography (PPG).Citation19 More recently, machine learning methods have been used to associate airway volume from functional imagingCitation20 and history of previous hospital admission with COPD exacerbations.Citation21
Despite newer approaches and improved technologies for remote patient monitoring, the expectation of being able to impact healthcare utilization seems elusive. One reason is that instead of being able to detect exacerbations reliably, RPM can generate frequent alerts, or false positive signals, that actually result in unnecessary interventions.Citation22 In fact, there have been several systematic reviews over recent years concluding that remote patient monitoring can improve health status and potentially improve intervention strategies to prevent exacerbations. However, these reviews have typically found difficulty in drawing firm conclusions and usually state that more research is needed.Citation23,Citation24 Previously, we described a networked system for early detection of COPD exacerbations by RPM and optimized its performance using statistical process control (SPC).Citation25 We demonstrated feasibility in terms of data capture and data reliability. The goal of this paper is to explore the predictive value of the different parameters obtainable from RPM. We also aimed to evaluate the effects of RPM on healthcare utilization in comparison with non-monitored subjects; however, the relatively few exacerbation events in our patient cohort precluded this analysis.
Participants and Methods
Study Design
The design of this study, including the characteristics of the study population, inclusion and exclusion criteria, baseline assessments and detailed methods for measuring symptoms, medication adherence, physical activity, spirometry and oxygenation, have all been described in an earlier publication.Citation25 We enrolled 17 subjects who collectively provided 2618 days (7.17 patient-years) of data. This study was approved by the UCLA Institutional Review Board (IRB) in accordance with the Declaration of Helsinki. All subjects gave written informed consent.
Remote Patient Monitoring (RPM)
Daily Routine
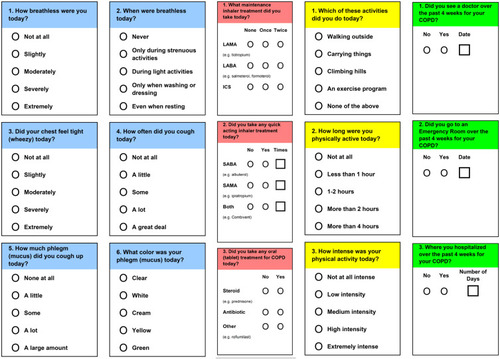
The subjects receiving RPM were trained in the use of novel software that recorded their daily symptoms, medication use and self-reported activity level. They were also trained to use the handheld spirometer (SpiroPro®, eResearch Technology, Philadelphia, PA) and pulse oximeter (Onyx II. Nonin Medical, Plymouth, MN). Details of the standard operating procedure for spirometry are shown in Supplementary Appendix A including error messages and quality improvement messages seen by the patients. The handheld spirometer was also programmed with electronic diaries, questionnaires and the capability for recording pulse oximetry. This device included questions on daily symptoms such as cough, dyspnea, wheezing, sputum volume and purulence (CLEAR-Sx), daily physical activities (CLEAR-Ex), and questions on daily medications (CLEAR-Rx). Details of these questionnaires are shown in . Measured physiological values were FEV1, FVC, PEF, IC and pulse oximetry (SpO2). Statistical process control (SPC), an approach which is used in engineering for quality assurance, allowed subjects to perform only one or two forced maneuvers each day of monitoring provided that one of the maneuvers was technically acceptable and could be evaluated along with sequential maneuvers performed on preceding days. The details of the SPC have been previously publishedCitation25 but are shown again in Supplementary Appendix B. Subjects transferred the collected data via modem every 1–3 days through a secure UCLA network system to the UCLA Exercise Physiology Research Laboratory.
Monthly Telephone Contact
During the study, a research coordinator from the UCLA Exercise Physiology Research Laboratory made a monthly telephone call to each subject. Data obtained from these monthly telephone calls included the CAT questionnaire, changes of medication, and any self-reported exacerbation events which had necessitated healthcare utilization in terms of physician visits, emergency department visits and hospitalizations.
Variables of Interest
Predictors (Monitoring Events)
A clinically meaningful change was determined for each of the variables captured by RPM. These changes are summarized in . For spirometric measures, the end of positive predictor signal was identified if the value for each predictor had returned to within the prediction interval based on the previous 7-day average for two consecutive days.
Table 1 Predictors from Remote Patient Monitoring and Their Clinically Meaningful Changes
Outcomes (Exacerbations)
The outcomes of interest in this study were COPD exacerbations. These were captured in several different ways as shown in .
Table 2 Exacerbation Outcomes During Remote Patient Monitoring
Data Analysis
Concordance Method for Comparing Predictors and Outcomes
For each pair of a predictor and an outcome, a 2-by-2 table was constructed and Cohen’s kappa was calculated to express the level of agreement. This analysis was performed based on the daily data of the predictors and the outcomes. The calculated values for kappa were adjusted for imbalances caused by differences in prevalence and bias.Citation26 Details of the concordance analysis including the method for adjusting Cohen’s kappa for imbalances caused by differences in prevalence and bias are shown in Supplementary Appendix C along with an interpretation table for levels of agreement defined by the value for Cohen’s kappa.
Missing Data
Due to incomplete adherence, we encountered irregular occurrences of days with missing data for most of the subjects. We developed a standardized approach for handling these missing data which is shown in Supplementary Appendix D. For example, if a subject met criteria for a fall in FEV1 on two consecutive days, but then stopped monitoring, criteria would not be met on subsequent days even though the FEV1 could have remained below the threshold value associated with an event. In this situation, the days with missing data were eliminated from the sequential analysis so that an event would be considered ongoing until two values of FEV1 within the confidence interval previously defined as normal by SPC had been recorded.
Results
Baseline Assessment
The baseline characteristics for the subjects undergoing RPM showed that they were on average older, predominantly female (12 out of 17), with moderate COPD. The mean (SD) age was 71.1 (7.2) years, FEV1 was 1.54 (0.73) L or 56.8 (15.9) % of predicted, FVC was 3.04 (1.09) L or 86.8 (14.1) % of predicted, FEV1/FVC of 46.3%. Median CAT score was 17 (range 5–25), indicating that most of the subjects were highly symptomatic according to the GOLD 2017 grading.Citation27 Health status scores were 36.5 (4.4) by the SF-12 and 29.6 (14.2) by the SGRQ. These findings categorize the subjects as having mildly to moderately compromised health status.
Identification of Predictors (Monitoring Events)
For symptom scores and physiological measures, we counted events when these criteria were present for 2 consecutive days and the end of a prediction event was defined as when the parameter returned back to baseline for >2 consecutive days. The numbers of days meeting criteria for each of the chosen predictors are shown in . We detected 173 days when SpO2 fell below 90% and 6 days when SpO2 fell >4% below the 7-day average determined by SPC. These criteria resulted in 26 and 6 events, respectively. There were 18, 15, 23 and 17 days when FEV1, FVC, PEF and IC, respectively, fell below the 1-sided 95% confidence interval determined by SPC. These criteria resulted in 15, 13, 16 and 16 events, respectively. There were 13 occurrences of subjects not taking maintenance therapy for >2 days resulting in 2 prediction events. There were 117 occurrences of subjects increasing the use of their rescue inhaler by two puffs above baseline, resulting in 52 prediction events.
Table 3 Event Counts for Predictors
Based on 2618 patient-days (7.17 patient-years) of monitoring, the predictor event rates were as follows: SpO2 <90% = 3.63/patient-year, SpO2<7-day average minus 4% = 0.84/patient-year, FEV1<95% CI = 2.09/patient-year, FVC<95% CI = 1.81/patient-year, PEF<95% CI = 2.23/patient-year, IC<95% CI = 2.23/patient-year, inactive>2 days = 2.51/patient-year, non-compliant with maintenance therapy = 0.28/patient-year and increased use of rescue bronchodilator inhaler use = 7.25/patient-year. Predictor event rates for individual patients are shown in Appendix E.
Detection of Outcomes (Exacerbations)
We defined exacerbation events by self-reported exacerbation, Anthonisen Class 1 or 2 criteria and modified Anthonisen criteria based on daily questionnaires completed by subjects.Citation28 For telephone-reported exacerbations, antibiotic use, and systemic corticosteroid use, we counted only the day these criteria were first reported, indicating the onset of the event. The number of days meeting criteria for each of these outcomes are shown in . There were three exacerbation events reported from 11 subjects with 2618 patient-days of monitoring. Using daily questionnaires, 29 single event-days were identified by Anthonisen Class 1 criteria, 160 single event-days were identified by Anthonisen Class 2 criteria and 314 single event-days were identified by the modified Anthonisen criteria. The numbers of events detected (criteria met for ≥2 consecutive days) were 13, 48 and 63, respectively. Four events were captured by use of antibiotics and four events by use of systemic corticosteroids. Based on 7.17 patient-years of monitoring, the exacerbation event rates for telephone reported, Anthonisen Class 1, Anthonisen Class 2, modified Anthonisen, antibiotic use and systemic corticosteroid use were 0.42, 1.81, 6.69, 8.79, 0.56 and 0.56, respectively.
Table 4 Event Counts for Outcomes
Concordance Analysis
The concordance analyses between predictors and outcomes are shown in . Using statistical process control, there was substantial agreement between FVC (below the 7-day average minus 1.645 SD) and self-reported healthcare utilization events (κ=0.747, P<0.001) as well as between increased use of inhaled short-acting bronchodilators and two Anthonisen criteria (κ=0.611, P<0.001) or modified Anthonisen criteria (κ=0.622, P<0.001). There was also moderate agreement between FEV1 (below the 7-day average minus 1.645 SD) and self-reported healthcare utilization events (κ=0.475, P<0.001) and between SpO2 less than 90% and two Anthonisen criteria (κ=0.474, P<0.001) or modified Anthonisen criteria (κ=0.564, P<0.001). No single criterion showed more than fair concordance with exacerbations defined by three Anthonisen criteria, but there were relatively few of these events (n=29) in the database. We did not see statistically significant or clinically meaningful concordance between any of the symptomatic or physiological predictors and events defined by onset of antibiotic or systemic corticosteroid use.
Table 5 Concordance Analysis Between Predictors and Outcomes*
Discussion
Previously, we reported the feasibility of RPM using the methods developed for this study.Citation25 An additional goal was to explore the early detection of COPD exacerbations by performing a concordance analysis between predictors obtained by RPM with events using pre-defined exacerbation criteria. Several attempts have been made to accomplish this; however there is no consensus on what should be measured and what criteria warrant intervention. Previous studies have associated changes in breathing frequency, oxygen saturation, end-of-effort heart rate, and walking distance with exacerbation events.Citation19,Citation29-31 Other studies have used machine learning to associate image-based measures of airway volume and resistanceCitation20 or hospitalization historyCitation21 with exacerbation risk. Despite these different approaches, several systematic reviews have cast doubt on the benefits of telemonitoring interventions for COPD on mortality, quality of life, exercise capacity, and exacerbation-related outcomes.Citation22–Citation24
In the present analysis, exacerbation rates relating to self-reported healthcare utilization, including prescriptions of antibiotics and systemic corticosteroids (0.42–0.56/patient-year), were less frequent compared with exacerbations defined by the traditional three-out-of-three or two-out-of-three Anthonisen criteria (1.81 and 6.69/patient-year).Citation28 These rates are higher than typically reported exacerbation rates in patients with a similar severity of COPD who are taking combined long-acting inhaled bronchodilator maintenance therapy.Citation32,Citation33 Interestingly, as others have also reported, the exacerbation rate determined using the broader modified Anthonisen criteriaCitation34 was higher (6.79/patient-year) than the rate determined by the original Anthonisen criteria.Citation28 Our findings, therefore, suggest that inclusion of a broader range of symptoms along with physiological parameters presents a more detailed picture of COPD and the day-to-day fluctuations in disease activity.
The inclusion of statistical process control, described in our previous publication, led to promising results from a concordance analysis between predictors (events) and outcomes (exacerbations). Exacerbations defined by self-reported healthcare utilization were best predicted by falls in FVC and FEV1 below the 95% confidence interval derived from SPC. However, a fall in FVC was a more reliable predictor with stronger concordance (κ=0.747 versus κ=0.475). This observation concurs with other opinions that FEV1 alone has less predictive value for onset of exacerbations, and that due to the heterogeneity of the disease, a broader range of parameters should be measured.Citation17,Citation18,Citation35
For exacerbations defined by traditional or modified Anthonisen criteria, the most reliable predictors were increased use of inhaled short-acting bronchodilators and fall in SpO2 below 90%. In fact, as the defining criteria for exacerbations become broader and more symptom-based, increased use of inhaled short-acting bronchodilators becomes a stronger predictor, as did fall in SpO2 below 90%. These are fairly simple predictors, and this raises the question of how much value spirometry may add in predicting exacerbations. We suggest that these parameters could be used as simple means of daily monitoring to reflect the variability in disease control.
Use of inhaled short-acting bronchodilators and fall in SpO2 below 90% occurred most frequently out of our chosen predictors. All predictors, including decreases in oxygenation, spirometry, physical activity, and increases in inhaled short-acting bronchodilator use occurred more often than did exacerbations defined using conventional criteria. This spectrum of measures is likely more representative of the real-world-experience of the COPD patient than the conventional definitions of exacerbations. RPM, therefore, warrants further investigation to seek links between such daily predictors and longer-term outcomes such as disease progression, debility, and death.
Although, peak expiratory flow was monitored during this study, falls below the 95% confidence interval did not show concordance with any of the chosen outcome measures. This finding supports the view that peak expiratory flow is not a reliable measure in patients with COPD because of its increased variabilityCitation36 and the possibility of pressure-dependent airway collapse in the presence of emphysema.Citation37 Another interesting finding in our study was the absence of concordance between symptomatic and physiological measures and patient reporting of the commencement of antibiotic or systemic corticosteroid treatment. The reasons for this disparity are not clear, but one possibility is that patients provided with antibiotics or systemic corticosteroids for discretionary use might not know when they are best clinically indicated unless they are provided with strict criteria. If remote monitoring of COPD patients does gain popularity in the future, one potential benefit would be to enable the development of clearer guidelines for the introduction of these medications.
Our study has certain weaknesses. Firstly, due to logistical reasons, we ended up with a small number of patients, even though we were able to capture, collectively, over 7 patient-years of daily monitoring. The small number of subjects and the small number of outcomes did not allow us to subdivide the patients into different COPD phenotypes or to examine whether certain phenotypes had different patterns of predictors or outcomes.
Conclusion
Although remote monitoring of COPD patients holds promise for detection of exacerbation events, until now there has been insufficient reliable data to show how symptoms and physiological measures vary prior to these events.Citation38 Using RPM and concordance analysis, we showed that a range of symptoms and physiological measurements showed positive concordance with outcomes (COPD exacerbations). This was especially true for FVC, FEV1, increased use of short-acting bronchodilators and falls in oxygen saturation but not for falls of peak expiratory flow below the 95% confidence interval. Furthermore, these measurements give a more detailed picture into the daily clinical fluctuations of COPD. We have demonstrated that statistical process control (SPC) can improve the ability to predict exacerbations. Although remote monitoring is not yet feasible for everyone, we believe that there is value in refining remote monitoring techniques for high-risk patients such as those with a history of frequent exacerbations or those with comorbidities that may contribute to worse exacerbation outcomes.
Disclosure
This investigation was funded by eResearch Technologies, Inc (ERT). During this study, CBC received consulting fees from ERT for serving as Medical Director of their Clinical Services Division; he also reports grants from ERT, personal fees from GlaxoSmithKline, non-financial support from Equinox Fitness Clubs, personal fees from PulmonX, grants from NHLBI, grants from Foundation NIH, grants from COPD Foundation, personal fees from NUVAIRA, personal fees from MGC Diagnostics, outside the submitted work. MT reports previous employment by ERT whose equipment was used in the investigation. The authors report no other conflicts of interest in this study.
References
- American Lung Association. Trends in COPD (Chronic Bronchitis and Emphysema): Morbidity and Mortality. Epidemiology and Statistics Unit Research and Health Education Division; 2013 Available from http://www.lung.org/finding-cures/our-research/trend-reports/copd-trend-report.pdf. Accessed 16, 2014.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2020 Available from: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf. Accessed 812, 2020.
- World Health Organization. World health statistics 2008; 2008 Available from: http://www.who.int/whosis/whostat/EN_WHS08_Full.pdf. Accessed 16, 2014..
- LareauS, MosesonE, SlatoreCG. Exacerbation of COPD. Am J Respir Crit Care Med. 2018;198:21–22. doi:10.1164/rccm.19811P21
- CoteCG, DordellyLJ, CelliBR. Impact of COPD exacerbations on patient-centered outcomes. Chest. 2007;131(3):696–704. doi:10.1378/chest.06-161017356082
- HalpinDM, DecramerM, CelliB, KestenS, LiuD, TashkinDP. Exacerbation frequency and course of COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:653–661. doi:10.2147/COPD.S3418623055714
- AnzuetoA, LeimerI, KestenS. Impact of frequency of COPD exacerbations on pulmonary function, health status and clinical outcomes. Int J Chron Obstruct Pulmon Dis. 2009;4:245–251. doi:10.2147/copd.s486219657398
- WedzichaJA, DonaldsonGC. Exacerbations of chronic obstructive pulmonary disease. Respir Care. 2003;48(12):1204–1215.14651761
- WilkinsonTM, DonaldsonGC, HurstJR, SeemungalTA, WedzichaJA. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169(12):1298–1303. doi:10.1164/rccm.200310-1443OC14990395
- RahimiK, WoodwardM, KorshidiR, et al. Home monitoring with IT-supported specialist management versus home monitoring alone in patients with heart failure: design and baseline results of the SUPPORT-HF 2 randomized trial. Am Heart J. 2018;208:55–64.30553130
- BuiAL, FonarowGC. Home monitoring for heart failure management. J Am Coll Cardiol. 2012;59(2):97–104. doi:10.1016/j.jacc.2011.09.04422222071
- ClarkRA, InglisSC, McAlisterFA, ClelandJG, StewartS. Telemonitoring or structured telephone support programmes for patients with chronic heart failure: systematic review and meta-analysis. BMJ. 2007;334(7600):942. doi:10.1136/bmj.39156.536968.5517426062
- TseG, ChanC, GongM, et al. Telemonitoring and hemodynamic monitoring to reduce hospitalization rates in heart failure: a systematic review and meta-analysis of randomized controlled trials and real-world studies. J Geriatr Cardiol. 2018;15(4):298.29915620
- PandorA, GomersallT, StevensJW, et al. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta-analysis. Heart. 2013;99(23):1717–1726. doi:10.1136/heartjnl-2013-30381123680885
- MadsenF, UlrikCS, DirksenA, et al. Patient-administered sequential spirometry in healthy volunteers and patients with alpha 1-antitrypsin deficiency. Respir Med. 1996;90(3):131–138. doi:10.1016/S0954-6111(96)90154-78736204
- LindgrenBR, FinkelsteinSM, PrasadB, et al. Determination of reliability and validity in home monitoring data of pulmonary function tests following lung transplantation. Res Nurs Health. 1997;20(6):539–550. doi:10.1002/(SICI)1098-240X(199712)20:6<539::AID-NUR8>3.0.CO;2-N9397133
- SeemungalTA, DonaldsonGC, BhowmikA, JeffriesDJ, WedzichaJA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(5):1608–1613. doi:10.1164/ajrccm.161.5.990802210806163
- SundZM, PowellT, GreenwoodR, JaradNA. Remote daily real-time monitoring in patients with COPD–a feasibility study using a novel device. Respir Med. 2009;103(9):1320–1328. doi:10.1016/j.rmed.2009.03.01719375294
- ShahSA, VelardoC, FarmerA, TarassenkoL. Exacerbations in chronic obstructive pulmonary disease: identification and prediction using a digital health system. J Med Internet Res. 2017;19(3):e69. doi:10.2196/jmir.720728270380
- LanclusM, ClukersJ, Van HolsbekeC, et al. Machine learning algorithms utilizing functional respiratory imaging may predict COPD exacerbations. Acad Radiol. 2019;26(9):1191–1199. doi:10.1016/j.acra.2018.10.02230477949
- CavaillesA, MelloniB, MotolaS, et al. Identification of patient profiles with high risk of hospital re-admissions for Acute COPD Exacerbations (AECOPD) in France using a machine learning model. Int J Chron Obstruct Pulmon Dis. 2020;15:949–962. doi:10.2147/COPD.S23678732431495
- LiX, XieY, ZhaoH, ZhangH, YuX, LiJ. Telemonitoring interventions in COPD patients: overview of systematic reviews. Biomed Res Int. 2020;2020:5040521. doi:10.1155/2020/392028432016115
- CruzJ, BrooksD, MarquesA. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68(3):369–378. doi:10.1111/ijcp.1234524472009
- McCabeC, McCannM, BradyAM. Computer and mobile technology interventions for self-management in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;5:CD011425.28535331
- CooperCB, SirichanaW, NeufeldEV, TaylorM, WangX, DolezalBA. Statistical process control improves the feasibility of remote physiological monitoring in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2019;14:2485–2496. doi:10.2147/COPD.S20762632009781
- ByrtT, BishopJ, CarlinJB. Bias, prevalence and kappa. J Clin Epidemiol. 1993;46(5):423–429. doi:10.1016/0895-4356(93)90018-V8501467
- VogelmeierCF, CrinerGJ, MartinezFJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP28128970
- AnthonisenNR, ManfredaJ, WarrenCP, HershfieldES, HardingGK, NelsonNA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106(2):196–204. doi:10.7326/0003-4819-106-2-1963492164
- YañezAM, GuerreroD, de AlejoRP, et al. Monitoring breathing rate at home allows early identification of COPD exacerbations. Chest. 2012;142(6):1524–1529. doi:10.1378/chest.11-272822797131
- Gálvez-BarrónC, Villar-ÁlvarezF, RibasJ, et al. Effort oxygen saturation and effort heart rate to detect exacerbations of chronic obstructive pulmonary disease or congestive heart failure. J Clin Med. 2019;8(1):42. doi:10.3390/jcm8010042
- RubioN, ParkerRA, DrostEM, et al. Home monitoring of breathing rate in people with chronic obstructive pulmonary disease: observational study of feasibility, acceptability, and change after exacerbation. Int J Chron Obstruct Pulmon Dis. 2017;12:1221–1231. doi:10.2147/COPD.S12070628458534
- DecramerM, CelliB, TashkinDP, et al. Clinical trial design considerations in assessing long‐term functional impacts of tiotropium in COPD: the UPLIFT trial. COPD. 2004;1(2):303–312. doi:10.1081/COPD-20002693417136995
- WedzichaJA, DecramerM, FickerJH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1(3):199–209. doi:10.1016/S2213-2600(13)70052-324429126
- BurgeS, WedzichaJA. COPD exacerbations: definitions and classifications. Eur Respir J Suppl. 2003;41:46s–53s. doi:10.1183/09031936.03.0007800212795331
- O’DonnellDE, ParkerCM. COPD exacerbations 3: pathophysiology. Thorax. 2006;61(4):354–361. doi:10.1136/thx.2005.04183016565268
- SoJY, LastraAC, ZhaoH, MarchettiN, CrinerGJ. Daily peak expiratory flow rate and disease instability in chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. 2015;3(1):398–405. doi:10.15326/jcopdf.3.1.2015.014228848862
- WhiteP. Spirometry and peak expiratory flow in the primary care management of COPD. Prim Care Respir J. 2004;13(1):5–8. doi:10.1016/j.pcrj.2003.11.00916701628
- Al RajehAM, HurstJR. Monitoring of physiological parameters to predict exacerbations of chronic obstructive pulmonary disease (COPD): a systematic review. J Clin Med. 2016;5(12):108. doi:10.3390/jcm5120108