Abstract
Purpose
Fractional exhaled nitric oxide (FENO50) level and peripheral blood eosinophil count may serve as indicators of airway eosinophilia. The aim of this study was to estimate the diagnostic value of these markers for detecting airway eosinophilia in patients with stable chronic obstructive pulmonary disease (COPD) and those experiencing an acute exacerbation (AECOPD).
Patients and Methods
FENO50 levels, sputum and blood eosinophil counts were assessed in 53 clinically stable ex-smoker COPD patients and 67 ex-smoker COPD patients experiencing a severe exacerbation. In AECOPD, clinical variables were measured at the time of hospital admission and discharge following treatment.
Results
In stable COPD, blood eosinophil count but not FENO50 level was found to be a good predictor of airway eosinophilia (area under the receiver operating characteristic curve [ROC AUC]: ≥0.82). The sensitivity and the specificity of the test ranged between 75% and 98%, the negative predictive value (NPV) was high (>90%). In AECOPD, FENO50 was predictive for airway eosinophilia (ROC AUC: >0.8) with high NPV (>88%), but with lower sensitivity and specificity (64–70%). In contrast, the predictive accuracy of blood eosinophil count for airway eosinophilia in AECOPD was modest (ROC AUC: 0.54–0.63). The combined use of the two markers provided only limited additional benefit. Correlation analyses supported ROC curve findings.
Conclusion
In stable COPD the peripheral blood eosinophil count, while in AECOPD the FENO50 level is a good surrogate marker of airway eosinophilia.
Introduction
Several lines of evidence indicate that sputum eosinophil numbers are increased in a subset of patients with chronic obstructive pulmonary disease (COPD), both in stable diseaseCitation1 and acute exacerbation of COPD (AECOPD).Citation2 Studies have shown that this subgroup of patients respond favorably to inhaledCitation1 or oral corticosteroid treatment,Citation2–Citation5 have a reduced airway bacterial loadCitation6 and exhibit a more pronounced improvement in airflow limitation following treatment of AECOPDCitation7 suggesting that they may have a distinct disease phenotype. Therefore, differentiation between eosinophilic and noneosinophilic airway inflammation is of clinical importance.
In recent years both peripheral blood eosinophil countCitation8,Citation9 and fractional exhaled nitric oxide (FENO50) levelCitation10,Citation11 have been implicated as surrogate biomarkers to determine and quantify the degree of airway eosinophilia in COPD patients.
However, the predictive accuracy of these markers appears to be variable and often only modest. For example, Negewo et al found that blood eosinophil count can predict airway eosinophilia with a relatively high degree of accuracy in stable COPD patients,Citation12 while investigators of the SPIROMICS cohort, a large and well-characterized cohort of COPD patients, concluded that blood eosinophilia alone is not a reliable marker of airway eosinophilia (or phenotyping eosinophil COPD) despite the highly significant correlation between the two measures.Citation13
Concerning FENO50, a recent study reported a sensitivity of 62% and a specificity of 71% to identify stable COPD patients with eosinophilic airway inflammation.Citation14 Our previous data in patients with AECOPD assigned a better discriminatory power to FENO50;Citation7 however, a more recent study reported less promising data in this respect.Citation15 The inconsistent findings could be explained if the diagnostic accuracy of these markers were different between stable disease and AECOPD. Such a scenario is plausible since the local and systemic inflammatory responses, particularly the inflammatory cell ratios differ considerably between the two conditions.Citation16,Citation17 Nonetheless, this has not been systematically investigated to date.
Similarly, whether the combination of the two markers has additive value is poorly understood. This is again conceivable since there is evidence that exhaled nitric oxide and blood eosinophils are regulated by distinct inflammatory pathways and could be used as complementary biomarkers to estimate different aspects of an eosinophilic airway inflammation.Citation18
Thus, in this prospective study, we (i) determined the predictive accuracy of FENO50 levels and blood eosinophil counts for assessing airway eosinophilia in COPD patients, both in stable disease and AECOPD and (ii) tested whether the combination of these two markers provides additional diagnostic benefit.
Patients and Methods
Study Subjects
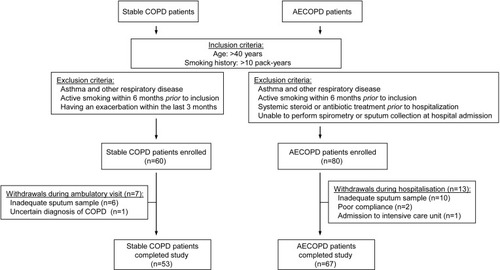
Ex-smoker, clinically stable COPD patients and patients hospitalized with AECOPD were recruited for the study between January 2017 and June 2019. Inclusion and exclusion criteria are summarized in . Diagnosis of COPD was established by chest physicians and all patients had documented airway obstruction (post-bronchodilator forced expiratory volume in one second [FEV1]/forced vital capacity [FVC] <0.7). AECOPD was defined as increased dyspnea, cough or sputum expectoration (quality or quantity) that led the subject to seek medical attention as specified in international guidelines.Citation19 The research protocol was approved by the Ethics Committee of the National Koranyi Institute of Pulmonology (No: 6/2017), and all subjects gave written informed consent to participate in the study. All procedures performed in the study involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethic standards.
Study Design
Stable COPD patients provided induced sputum samples during routine clinical visits following lung function, FENO50 and blood gas analysis. From patients with AECOPD spontaneously expectorated sputum was collected, and FENO50, blood gases and lung function parameters were measured at two time points: first at hospital admission, and again on the day of discharge. Routine chemistry and haematology from peripheral venous blood samples were performed on the day of sputum sample collection. Sputum induction, lung function and all other laboratory measurements were performed as previously described.Citation7,Citation20
Sputum Processing
Sputum samples were processed in PBS containing dithiothreitol as previously described.Citation7,Citation20 Cytospins were stained with May-Grunwald-Giemsa for differential cell counting. At least 400 inflammatory cells were counted for each cytospin slide. The number of inflammatory cells in sputum was recorded as a percentage of total non-squamous cells.
Measurement of FENO50
FENO50 levels were recorded using a chemiluminescence analyzer (Model LR2500, Logan Research, Rochester, UK) at an exhalation flow rate of 50 mL/s according to the procedure recommended by the European Respiratory Society.Citation21
Statistical Analysis
Data are presented as mean±SEM or median with interquartile range, as appropriate. Data distribution was analyzed by the Kolmogorov–Smirnov test. Paired Student’s t-test and the Wilcoxon signed rank test were used to compare variables measured at the time of hospital admission and discharge. Variables between eosinophilic and noneosinophilic patients were analyzed by the unpaired Student’s t-test or the Mann–Whitney test. Correlation coefficients were calculated by Spearman’s method. Receiver operator characteristic (ROC) curve analysis was performed to calculate the predictive accuracy and the optimum cut point of FENO50 and blood eosinophil count for assessing airway eosinophilia. The area under the ROC curve (AUC) was determined, and a value above 0.8 was considered as a good discriminator.Citation22,Citation23 Airway eosinophilia was defined as >2% sputum eosinophil cell count, but the ROC curve analysis was also performed using the higher cut point of 3%. Calculations were carried out in GraphPad Prism 4.0 (GraphPad Software Inc., San Diego, CA, USA). A p-value of <0.05 was considered significant.
Results
A total of 60 clinically stable COPD patients and 80 AECOPD patients were enrolled in the study, of which 7 and 13 were withdrawn, respectively, due to their inability to produce adequate sputum samples or unforeseen complications during hospitalization (). Demographic and clinical data of the 53 stable COPD and 67 AECOPD patients who completed the study are presented in and . Changes in clinical variables during the treatment of AECOPD are provided in Supplementary materials (Tables S1 and S2).
Table 1 Demographic and Clinical Characteristics of Stable COPD Patients
Table 2 Demographic and Clinical Characteristics of AECOPD Patients at the Time of Hospital Admission
Comparison of Patients with and without Airway Eosinophilia
Except for blood eosinophil counts, no clinical variables were significantly different between eosinophilic and noneosinophilic patients in the stable COPD group ().
In contrast, eosinophilic AECOPD patients had higher FENO50 levels but lower C-reactive protein (CRP) levels compared to noneosinophilic patients (). Additionally, sputum total cell counts, the number and the percentage of sputum neutrophils and the number of lymphocytes were also lower in these subjects.
Correlations Between FENO50, Sputum and Blood Eosinophil Counts
In stable COPD we found significant positive correlation between sputum eosinophil cell count and the number (r=0.557, p<0.0001) and percentage (r=0.498, p<0.001) of blood eosinophils. In contrast, FENO50 levels showed no relationship with eosinophil counts in the blood (r=0.197, p=0.161 for absolute number and r=0.182, p=0.196 for percentage) or in the sputum (r=0.086, p=0.554).
In AECOPD patients only FENO50 levels and sputum eosinophils showed significant association (r=0.362, p=0.003) and neither the number nor the percentage of blood eosinophils were related with either sputum eosinophils (r=0.182, p=0.15 and r=0.196, p=0.121, respectively) or FENO50 levels (r=0.205, p=0.11 and r=0.209, p=0.104, respectively) at hospital admission.
At discharge of AECOPD patients neither FENO50 (r=0.05, p=0.782) nor blood eosinophils (r=0.279, p=0.143 for absolute and r=0.221, p=0.241 for percentage) correlated significantly with sputum eosinophils. Similarly, FENO50 levels and blood eosinophils showed no correlation with each other (r=0.056, p=0.772 for absolute count and r=0.212, p=0.261 for percentage).
Predictive Accuracy of FENO50 and Blood Eosinophil Count for Assessing Airway Eosinophilia in Stable COPD
Based on ROC curve analysis, the number of eosinophils in the blood was a strong predictor (ROC AUC: ≥0.82) of airway eosinophilia in stable COPD patients, irrespective of whether the cut point of eosinophilia was set at >3% or >2% (, ). Notably, at the optimum cut points the negative predictive value (NPV) of the test was high (>90%). Although the percentage of blood eosinophils was also a good surrogate marker of airway eosinophilia (ROC AUC: ~0.8), its specificity and positive predictive value (PPV) were lower than those of the absolute number.
Table 3 Predictive Accuracy of FENO50 and Blood Eosinophil Measurements for Assessing Airway Eosinophilia in Stable COPD Patients
Figure 2 ROC curve for FENO50 and blood eosinophils (absolute count and percentage) to predict airway eosinophilia defined as >3% (A) or >2% (B) sputum eosinophil cell counts in stable COPD patients.
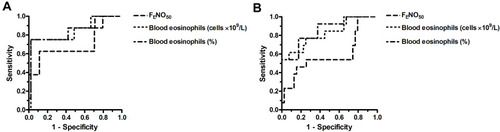
In contrast to blood eosinophil count, FENO50 was proven to be only a weak predictor (ROC AUC <0.70) of eosinophilic airway inflammation in stable COPD. Interestingly, with airway eosinophilia defined as >3% sputum eosinophils, FENO50 had high specificity and NPV. This, however, was associated with low sensitivity and PPV.
Predictive Accuracy of FENO50 and Blood Eosinophil Count for Assessing Airway Eosinophilia in AECOPD
In AECOPD, neither the absolute number nor the percentage of blood eosinophils was predictive (ROC AUC: <0.65) for airway eosinophilia irrespective of the definition of eosinophilia (, ). By contrast, FENO50 had good predictive accuracy (ROC AUC: ≥0.8) in these subjects with excellent NPV and relatively good sensitivity and specificity. Of note is, however, that the PPV of the test was low (29–36%).
Table 4 Predictive Accuracy of FENO50 and Blood Eosinophil Measurements for Assessing Airway Eosinophilia in AECOPD Patients at the Time of Hospital Admission
Figure 3 ROC curve for FENO50 and blood eosinophils (absolute count and percentage) to predict airway eosinophilia defined as >3% (A) or >2% (B) sputum eosinophil cell counts in AECOPD patients.
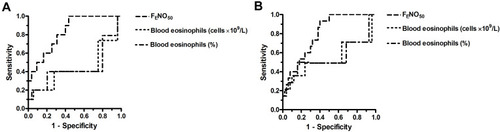
ROC curve analysis could not be performed for AECOPD patients at discharge, as none of the patients had >3% sputum eosinophil count and only one had >2%. A summary of the different sensitivity, specificity, PPV and NPV values of FENO50 and blood eosinophil measurements is provided in the Supplementary materials (Table S3).
Combining FENO50 and Blood Eosinophil Measurements for Assessing Airway Eosinophilia
In stable COPD, using both FENO50 and blood eosinophil values resulted in increased sensitivity but decreased specificity compared to using the blood eosinophil count alone (). These changes were associated with decreased PPV, particularly, when the cutoff was set at >3%. The NPV of the two tests combined remained high (>92%).
Table 5 Combined Use of FENO50 and Blood Eosinophil Measurements for Assessing Airway Eosinophilia in Stable COPD and AECOPD Patients
In AECOPD, the combination of the two measurements resulted in no significant improvement in diagnostic accuracy compared to the FENO50 test alone ().
Discussion
In this study we compared the diagnostic accuracy of FENO50 level and peripheral blood eosinophil count either used alone or in combination for detecting airway eosinophilia in patients with stable COPD and AECOPD. The main finding of the study was that in stable COPD the blood eosinophil count, while in AECOPD the FENO50 level was predictive for airway eosinophilia. Importantly, the NPV of both tests was high indicating that the clinical significance of using these markers could be the reliable identification of noneosinophilic subjects. The combination of the two markers on the other hand provided limited additional benefit.
As mentioned earlier, measurement of FENO50 may aid the distinction between eosinophilic and noneosinophilic airway inflammation.Citation10,Citation11 The measurement used aloneCitation24 or in combination with blood eosinophil countCitation25 has also been implicated in the differential diagnosis between asthma-COPD overlap and COPD, but neither studies involved sputum analysis. The results presented in our study extend the above findings by showing that FENO50 is a strong predictor of airway eosinophilia only in AECOPD, while in stable COPD it has only modest diagnostic value. In agreement with these results we only found a significant correlation between FENO50 levels and sputum eosinophil counts in the AECOPD group.
Although the utility of blood eosinophils in the management of COPD patients, in particular, to guide the use of inhaled corticosteroid (ICS) therapy for exacerbation prevention has been extensively investigated in recent years,Citation8,Citation9 the evidence for a relationship between local (airway) and systemic (blood) eosinophilia remains contradictory.Citation12,Citation13 We have shown here that the number of eosinophils in the blood is a good indicator of airway eosinophilia in stable COPD patients but not in those with an ongoing exacerbation where the sensitivity of the test was poor (20–40%). Again, the results of the correlation studies supported these findings. Based on our laboratory data we can speculate that the reason why FENO50 and not blood eosinophil count is the better marker of airway eosinophilia in exacerbation is that eosinophilic airway inflammation does not translate to systemic eosinophilia in AECOPD.
The percentage of blood eosinophil count had a slightly lower predictive value compared to the absolute eosinophil count in our study. However, the literature is equivocal whether the percentage or the absolute eosinophil count is more predictive for airway eosinophilia.Citation6,Citation12,Citation13 Importantly, the optimum cut points for blood eosinophil count (0.316 and 0.204 cells×109/L) found in our study were similar to the threshold for eosinophilia (300 cells/μL) proposed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) document.Citation26
In patients with AECOPD, Bafadhel and co-workers have shown recently that blood eosinophil count is a sensitive marker of airway eosinophilia (ROC AUC: 0.85),Citation2 while Gao et al reported that FENO50 is only modestly predictive (ROC AUC: 0.73) for eosinophils in sputum.Citation15 These findings are in discordance with the observations presented here. We speculate that differences in study populations or the severity of exacerbations could account for the discrepancies between the results. For example, in the study of Gao et alCitation15 smokers were also included in the study population (75%), even though it is well established that cigarette smoking is an important confounding factor in the measurement of FENO50.Citation10,Citation11 For this very reason, our recruitment strategy called for only ex-smokers, and therefore smoking status did not influence our results.
In our hands the combination of FENO50 and blood eosinophil measurements provided increased sensitivity but decreased specificity, while the PPV and the NPV did not alter. Thus, it appears that the overall benefit of the combined test is limited. A recent study in asthmatics also failed to detect improvements using the combination of three different eosinophilic biomarkers such as FENO50, blood eosinophils and periostin.Citation27
The definition of airway eosinophilia is inconsistent in the literature. When comparing the clinical parameters of our patients, the cut-off value was set at 2% sputum eosinophil cell count. To allow comparison with other studies, the ROC analysis, as the most important measurement in our study, was performed using a higher (3%) cut off value as well. Our main findings were independent of the various thresholds set for airway eosinophilia, however, the specificity and the PPV of blood eosinophil count in stable COPD patients considerably improved with the higher cut point (>3% eosinophils in sputum). Nonetheless, irrespective of the cutoff value, both blood eosinophil count and FENO50 (in stable COPD and in AECOPD, respectively) are suitable markers to rule out the presence of eosinophilic airway inflammation with high NPV.
As expected, the clinical characteristics of eosinophilic and noneosinophilic stable COPD patients were similar. By contrast, we observed lower sputum neutrophil count and CRP value in eosinophilic versus noneosinophilic AECOPD patients. These findings are in line with the general view that eosinophilic exacerbations are triggered by viral infections, while neutrophilic inflammation and elevation in systemic inflammatory marker levels may occur in exacerbations due to bacterial infection.Citation6,Citation28,Citation29 Nonetheless, the etiology of exacerbations has not been investigated in this study.
ICS therapy has been shown to influence FENO50 levels in someCitation30,Citation31 but not allCitation32 studies. Similarly, the relationship between ICS and blood and/or sputum eosinophil count appears to be ambiguous in COPD patients: some investigators documented no difference between ICS users and non-usersCitation6 while others found reduced eosinophil counts as a result of ICS treatment.Citation1 Retrospective analysis of a clinical trial that compared the effects of various bronchodilators has revealed that ICS has only a small effect on blood eosinophil count in steroid-naïve COPD patients.Citation33 In our current study the majority of patients were using ICS, and more importantly the proportion of patients on ICS therapy was similar between eosinophilic and noneosinophilic subjects. Therefore, we speculate that the effect of ICS, if any, would have been comparable in both subgroups of patients. In contrast, any possible confounding effect of systemic corticosteroid therapy on our results can be ruled out, since patients who received systemic steroids before hospitalization were excluded from the study, as indicated on the flow chart ().
Stability of FENO50 levels and blood eosinophil counts and the reproducibility of these measurements are important factors that may limit the use of these markers in the management of COPD patients. The issue has been intensively investigated in recent years, particularly the case of blood eosinophils. For example, Landis et al explored the reproducibility of blood eosinophil counts in a large cohort of stable COPD patients over 1 year and concluded that there is good reproducibility, although a subgroup of patients had variable eosinophil counts.Citation34 Similarly, Negewo et al investigated the stability of peripheral eosinophil counts between two measurements over a median period of 28 days and found a good agreement between the two measurements.Citation12 Nonetheless, other studies documented lower reproducibility and concluded that only a small proportion of patients remain persistently eosinophilic or noneosinophilic over a longer period.Citation35 Furthermore, there is evidence that at higher blood eosinophil counts greater variability can be observed.Citation36 Concerning FENO50, good repeatability has been reported in several studiesCitation37–Citation39 although it should be noted that in patients with severe disease difficulties may arise in maintaining the required flow rate and this could impair the repeatability of the measurement.Citation40 Nonetheless, the stability of the markers over time has not been investigated in our study.
Conclusion
In conclusion, our data collected by examination of previously diagnosed COPD patients suggest that surrogate markers to identify airway eosinophilia are not of equal value; instead their value depends on the status of the patient at the time of sample collection. In stable disease it is the peripheral blood eosinophil count, while in acute exacerbation it is the FENO50 level that predicts with good accuracy an increase in the number of eosinophil cells in the sputum. Thus, the difference in the utility of these markers to guide treatment decisions should be considered in clinical practice.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We are grateful to Maria Mikoss and Maria Hernadi (Department of Pathophysiology, National Koranyi Institute of Pulmonology) for technical assistance in FENO50 measurement, sputum collection and evaluation. The study was supported by the Hungarian Respiratory Society and the National Research, Development and Innovation Office (OTKA 124343).
References
- LeighR, PizzichiniMM, MorrisMM, MaltaisF, HargreaveFE, PizzichiniE. Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment. Eur Respir J. 2006;27:964–971. doi:10.1183/09031936.06.0007210516446316
- BafadhelM, McKennaS, TerryS, et al. Acute exacerbations of COPD: identification of biological clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184:662–671. doi:10.1164/rccm.201104-0597OC21680942
- FujimotoK, KuboK, YamamotoH, YamaguchiS, MatsuzawaY. Eosinophilic inflammation in the airway is related to glucocorticoid reversibility in patients with pulmonary emphysema. Chest. 1999;115:697–702. doi:10.1378/chest.115.3.69710084478
- PizzichiniE, PizzichiniMM, GibsonP, et al. Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am J Respir Crit Care Med. 1998;158:1511–1517. doi:10.1164/ajrccm.158.5.98040289817701
- BrightlingCE, MonteiroW, WardR, ParkerD, MorganMD, WardlawAJ. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2000;356:1480–1485. doi:10.1016/S0140-6736(00)02872-511081531
- KolsumU, DonaldsonGC, SinghR, et al. Blood and sputum eosinophils in COPD; relationship with bacterial load. Respir Res. 2017;18:88. doi:10.1186/s12931-017-0570-528482840
- SoterS, BartaI, AntusB. Predicting sputum eosinophilia in exacerbations of COPD using exhaled nitric oxide. Inflammation. 2013;36:1178–1185. doi:10.1007/s10753-013-9653-823681903
- SinghD. Predicting corticosteroid response in chronic obstructive pulmonary disease. Blood eosinophils gain momentum. Am J Respir Crit Care Med. 2017;196:1098–1100. doi:10.1164/rccm.201703-0592ED29090962
- KostikasK, BrindicciC, PatalanoF. Blood eosinophils as biomarkers to drive treatment choices in asthma and COPD. Curr Drug Targets. 2018;19:1882–1896. doi:10.2174/138945011966618021212001229437007
- BarnesPJ, DweikRA, GelbAF, et al. Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest. 2010;138:682–692. doi:10.1378/chest.09-209020822990
- TaylorDR, PijnenburgMW, SmithAD, De JongsteJC. Exhaled nitric oxide measurements: clinical application and interpretation. Thorax. 2006;61:817. doi:10.1136/thx.2005.05609316936238
- NegewoNA, McDonaldVM, BainesKJ, et al. Peripheral blood eosinophils: a surrogate marker for airway eosinophilia in stable COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1495–1504. doi:10.2147/COPD.S10033827445469
- HastieAT, MartinezFJ, CurtisJL, et al. SPIROMICS investigators. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5:956–967. doi:10.1016/S2213-2600(17)30432-029146301
- ChouKT, SuKC, HuangSF, et al. Exhaled nitric oxide predicts eosinophilic airway inflammation in COPD. Lung. 2014;192:499–504. doi:10.1007/s00408-014-9591-824816967
- GaoJ, ZhangM, ZhouL, et al. Correlation between fractional exhaled nitric oxide and sputum eosinophilia in exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1287–1293. doi:10.2147/COPD.S13499828490872
- FujimotoK, YasuoM, UrushibataK, HanaokaM, KoizumiT, KuboK. Airway inflammation during stable and acutely exacerbated chronic obstructive pulmonary disease. Eur Respir J. 2005;25:640–646. doi:10.1183/09031936.05.0004750415802337
- BathoornE, LieskerJJ, PostmaDS, et al. Change in inflammation in out-patient COPD patients from stable phase to a subsequent exacerbation. Int J Chron Obstruct Pulmon Dis. 2009;4:101–109. doi:10.2147/COPD.S485419436694
- ÇolakY, AfzalS, NordestgaardBG, MarottJL, LangeP. Combined value of exhaled nitric oxide and blood eosinophils in chronic airway disease: the Copenhagen General Population Study. Eur Respir J. 2018;52:pii:1800616. doi:10.1183/13993003.00616-2018
- RabeKF, HurdS, AnzuetoA, et al. Global initiative for chronic obstructive lung disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi:10.1164/rccm.200703-456SO17507545
- DrozdovszkyO, BartaI, AntusB. Sputum eicosanoid profiling in exacerbations of chronic obstructive pulmonary disease. Respiration. 2014;87:408–415. doi:10.1159/00035809924714447
- HorváthI, BarnesPJ, LoukidesS, et al. A European respiratory society technical standard: exhaled biomarkers in lung disease. Eur Respir J. 2017;49:1600965. doi:10.1183/13993003.00965-201628446552
- HanleyJA, McNeilBJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi:10.1148/radiology.143.1.70637477063747
- FabbriLM, RomagnoliM, CorbettaL, et al. Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:418–4124. doi:10.1164/rccm.200203-183OC12426229
- ChenFJ, HuangXY, LiuYL, LinGP, XieCM. Importance of fractional exhaled nitric oxide in the differentiation of asthma-COPD overlap syndrome, asthma, and COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2385–2390. doi:10.2147/COPD.S11537827713629
- TakayamaY, OhnishiH, OgasawaraF, OyamaK, KubotaT, YokoyamaA. Clinical utility of fractional exhaled nitric oxide and blood eosinophils counts in the diagnosis of asthma-COPD overlap. Int J Chron Obstruct Pulmon Dis. 2018;13:2525–2532. doi:10.2147/COPD.S16760030174422
- Global Initiative for Chronic Obstructive Lung Disease. Available from: https://goldcopd.org/. Accessed 219, 2020.
- WagenerAH, de NijsSB, LutterR, et al. External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2015;70:115–120. doi:10.1136/thoraxjnl-2014-20563425422384
- KimVL, CoombsNA, StaplesKJ, AERIS study group, et al. Impact and associations of eosinophilic inflammation in COPD: analysis of the AERIS cohort. Eur Respir J. 50;2017:1700853. doi:10.1183/13993003.00853-201729025891
- PapiA, BellettatoCM, BraccioniF, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–1121. doi:10.1164/rccm.200506-859OC16484677
- HögmanM, ThornadtssonA, BrömsK, et al. Different relationships between F(E)NO and COPD characteristics in smokers and ex-Smokers. COPD. 2019;16:227–233. doi:10.1080/15412555.2019.163835531357875
- LimCS, RaniFA, TanLE. Response of exhaled nitric oxide to inhaled corticosteroids in patients with stable COPD: a systematic review and meta-analysis. Clin Respir J. 2018;12:218–226. doi:10.1111/crj.1251827328740
- LiuJ, SandriniA, ThurstonMC, YatesDH, ThomasPS. Nitric oxide and exhaled breath nitrite/nitrates in chronic obstructive pulmonary disease patients. Respiration. 2007;74:617–623. doi:10.1159/00010637917643055
- KreindlerJL, WatkinsML, LettisS, Tal-SingerR, LocantoreN. Effect of inhaled corticosteroids on blood eosinophil count in steroid-naïve patients with COPD. BMJ Open Respir Res. 2016;3:e000151. doi:10.1136/bmjresp-2016-000151
- LandisSH, SurukiR, HiltonE, ComptonC, GalweyNW. Stability of blood eosinophil count in patients with COPD in the UK clinical practice research datalink. COPD. 2017;14:382–388. doi:10.1080/15412555.2017.131382728569614
- GreulichT, MagerS, LuckeT, et al. Longitudinal stability of blood eosinophil count strata in the COPD COSYCONET cohort. Int J Chron Obstruct Pulmon Dis. 2018;13:2999–3002. doi:10.2147/COPD.S16590930319247
- SouthworthT, BeechG, FodenP, KolsumU, SinghD. The reproducibility of COPD blood eosinophil counts. Eur Respir J. 2018;52:1800427. doi:10.1183/13993003.00427-201829724922
- BhowmikA, SeemungalTA, DonaldsonGC, WedzichaJA. Effects of exacerbations and seasonality on exhaled nitric oxide in COPD. Eur Respir J. 2005;26:1009–1015. doi:10.1183/09031936.05.0004730516319329
- BrindicciC, ItoK, RestaO, PrideN, BarnesPJ, KharitonovSA. Exhaled nitric oxide from lung periphery is increased in COPD. Eur Respir J. 2005;26:52–59. doi:10.1183/09031936.04.0012530415994389
- AntusB, HorvathI, BartaI. Assessment of exhaled nitric oxide by a new hand-held device. Respir Med. 2010;104:1377–1380. doi:10.1016/j.rmed.2010.06.00520594818
- RouhosA, KainuA, PiiriläP, et al. Repeatability of exhaled nitric oxide measurements in patients with COPD. Clin Physiol Funct Imaging. 2011;31:26–31. doi:10.1111/j.1475-097X.2010.00975.x21143751