Abstract
Background
To estimate the potential cost savings by following the current Global Initiative for Chronic Obstructive Lung Disease (GOLD) guideline recommendations in patients being treated for chronic obstructive pulmonary disease (COPD) with the combination of long-acting β2-agonist (LABA), long-acting muscarinic antagonist (LAMA) or inhaled corticosteroids (ICS).
Methods
The Geisinger Health System (GHS) database was utilized to identify subjects between January 1, 2004 to March 12, 2007. The index date was based on the first prescription of a LAMA plus LABA, LAMA plus LABA/ICS, or LABA plus ICS. Patients were included in the study if they: had a COPD diagnosis; had data representative of treatment 12 months prior to and 12 months post index date; were 40 years of age or over; had no prior diagnosis for asthma; and had pulmonary function test (PFT) data. We examined the baseline characteristics of these patients along with their healthcare resource utilization. Based on PFT data within 30 days of the index date, a subgroup was classified as adhering or non-adhering to GOLD guidelines.
Results
A total of 364 subjects could be classified as adhering or non-adherent to current GOLD guidelines based on their PFT results. The adherent subgroup received COPD medications consistent with current GOLD guidelines. Of the LAMA plus LABA cohort, 25 patients adhered and 39 patients were non-adherent to current GOLD guidelines. In the cohort of LABA plus ICS, 74 patients were adherent and 180 patients non-adherent to current GOLD guidelines. In the cohort of LAMA plus LABA/ICS, 21 patients were adherent and 25 patients non-adherent to current GOLD guidelines. GOLD adherence was associated with mean total cost of all services savings of $5,889 for LAMA plus LABA, $3,330 for LABA + ICS, and $10,217 for LAMA plus LABA/ICS cohorts.
Conclusion
Staging of COPD with a PFT and adherence to current GOLD guidelines was associated with lower costs in subjects with moderate to severe COPD. Appropriate use of LAMA plus LABA, LABA plus ICS, and LAMA plus LABA/ICS has economic as well as clinical benefits for patients and payers.
Keywords:
Introduction
Chronic obstructive pulmonary disease (COPD), characterized by shortness of breath and other breathing related problems, is the fourth leading cause of death in the United States (US), with more than 120,000 deaths every year.Citation1 Although the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH) reports that 24 million people are affected with COPD, only 10 million adults were estimated to be diagnosed with COPD in 2000.Citation2 The current prevalence rate of COPD is 12 million, with an another likely 12 million individuals not knowing that they have disease.Citation1 The economic burden (direct and indirect costs) of COPD to the US was estimated to be $30.4 billion in 2000 which increased to nearly $32.1 billion in 2002.Citation3,Citation4 In 2002, the direct medical costs constituted $18 billion and indirect costs (related to morbidity and mortality) were $14.1 billion.Citation3 Prescription drug costs accounted for $2.7 billion (20.6%) of the total direct COPD costs.
According to the current Global Initiative for Chronic Obstructive Lung Disease (GOLD) report, COPD is a disease state characterized by airflow limitation that is not fully reversible.Citation5 Both small airway disease (obstructive bronchiolitis) and parenchymal destruction lead to chronic airflow limitations in COPD that varies from individual to individual.Citation6 Long-term smoking is the most common risk factor associated with COPD and accounts for 80% to 90% of the cases.Citation7,Citation8 In addition, a current smoker is ten times more likely to die from COPD compared to a non-smoker. Other COPD risk factors include heredity, second-hand smoke, exposure to air pollution at work and in the environment, and a history of childhood respiratory infections.Citation9
Spirometry is recommended by the current GOLD guidelines to measure airflow limitation as this is the most widely available, reproducible test of lung function. Spirometry measures an individual’s forced expiratory volume in 1 second (FEV1) and forced expiratory vital capacity (FVC) based on which COPD is classified into four stages of severity.Citation5 The current GOLD guidelines’ approach to the management of disease is driven by the need to control symptoms. Bronchodilators are central to such a therapeutic approach. The main bronchodilators are β2-agonists, anticholinergics, theophylline and a combination of these medications. The choice among these medications depends on individual response, availability, and cost. Short-acting inhaled bronchodilators are recommended from Stage I onward, on an as-needed basis. Long-acting inhaled bronchodilators should be added from Stage II onward, on a regular basis, for subjects whose symptoms are not controlled with as-needed short-acting bronchodilators. For stage III to IV, with a history of repeated exacerbations, regular treatment with inhaled corticosteroids is recommended in addition to long-acting bronchodilators.Citation5 Also, the current GOLD guidelines’ caution that chronic treatment with oral glucocorticosteroids should be avoided.
The lack of association between FEV1 and other elements of functional status has increased attention in the multi-systematic evaluation of COPD to determine severity.Citation5,Citation10
Many subjects with COPD continue to be undiagnosed or undertreated, suggesting that the guidelines are not being fully utilized.Citation10,Citation11 There is scant empirical evidence in the literature that investigates disparity between current guidelines and practice. The objective of this study is to estimate the potential cost savings by following current GOLD guideline recommendations in patients being treated with the combination of long-acting β2-agonist (LABA), long-acting muscarinic antagonist (LAMA) or inhaled corticosteroids (ICS). The strength of this study is that it utilized lung function measured by spirometry to define severity.
Methods
Data source
The study utilized data from the Geisinger Health System (GHS), which was founded in 1915 and is a physician-led organization comprising of 650 plus physicians, 75 medical and surgical specialties, and 42 pediatric medical and surgical subspecialties. GHS, which also has one of the largest not-for-profit rural health maintenance organizations in the US, has three existing hospitals (primary to quaternary care) and 41 community practice offices. The GHS service area is limited to the state of Pennsylvania. The core of the data originates from an electronic health record (EHR) infrastructure that contains longitudinal clinical patient data including lab results for over 3 million subjects from 1996 to 2008. In addition to the electronic health record core, the database has Geisinger incurred medical-service cost, charge, and payment data dating back to 2003.
Study design and time frame
A retrospective cohort study design was utilized and the study duration ranged from January 2004 to December 2007. The date when the subjects were first prescribed COPD therapies of interest – LAMA plus LABA or LABA plus ICS, or LAMA plus LABA/ICS – was identified as the index date during the study period. Patient data for the 12 months prior to the index date was evaluated to identify baseline characteristics. Patient data representative of 12 months after the index date were examined to compare the healthcare utilization costs of combination therapies, LAMA plus LABA to LABA plus ICS, and LAMA plus LABA/ICS based on GOLD guidelines.
Study population
Subjects were eligible for this study if they had a diagnosis of COPD (identified by International Classification of Diseases, Clinical Modification Ninth Revision [ICD9-CM] codes for COPD: 491, 492, and 496), did not have an asthma diagnosis (ICD-9-CM: 493.0), were at least 40 years of age and continuously enrolled for at least 12 months pre- and post-index date. In observational, retrospective COPD research, 40 years of age or older is routinely used as an inclusion criteria to correctly identify the COPD population and rule-out miscoding or misdiagnosis of asthma, which is typically a younger population.
In addition, the subjects were required to have a prescription for combination therapies, LAMA plus LABA, LABA plus ICS, or LAMA plus LABA/ICS. Based on these treatments, subjects were categorized into three mutually exclusive cohorts. The subjects were excluded if their prescribed therapy was switched from the index date therapy to another within 90 days of start of the therapy. For the subgroup analysis, subjects were also required to have pulmonary function test (PFT) data (available from two clinics of GHS).
Identification of demographic and clinical characteristics
The demographic characteristics that were identified in the study are age, gender, race (white, black, Hispanic), smoking history (current, passive, former, never, unknown), employment status (employed, unemployed), and insurance status (Medicaid, Medicare, commercial, self-pay). The clinical characteristics that were assessed are duration of COPD prior to index date and through index day, number of office visits, emergency room (ER) visits, hospitalizations, number of exacerbations defined as sum of length of stay during hospitalizations, and number of prescriptions for antibiotics and prednisone, concomitant medications (related to COPD only and excluding the study drugs) prior to index date, mean baseline Deyo–Charlson’s comorbidity index (DCI),Citation12 total encounter cost to Geisinger for COPD including hospital and physician costs, and PFT data (FEV1% predicted and FEV1/FVC% predicted). For LAMA plus LABA cohort, subjects were classified as adhering to GOLD guideline group, if the medications were prescribed from stage II COPD onwards and similarly stage III onwards for LABA plus ICS and LAMA plus LABA/ICS cohorts. All the costs (encounters) were adjusted to 2006 US dollars based on the medical care component of the consumer price index (CPI).Citation13 PFT data within 30 days of the index date were used to classify subjects into adhering and non-adhering to current GOLD guidelines () groups.
Table 1 GOLD treatment guidelines
Statistical analysis
Descriptive statistics were utilized to depict the study population characteristics at baseline. To determine if there were any differences in baseline characteristics (both demographic and clinical) among the three drug therapy cohorts, Chi-square tests for categorical variables, and t-tests for continuous variables (Wilcoxon-Mann-Whitney test was used if n < 30) were utilized. The total cost for adhering and nonadhering groups were compared by using t-test at baseline and follow-up. All results were measured at the 5% significant level (P ≤ 0.05) and SAS version 9.1 (SAS Institution Inc, Cary, NC) was used for the analyses.
Results
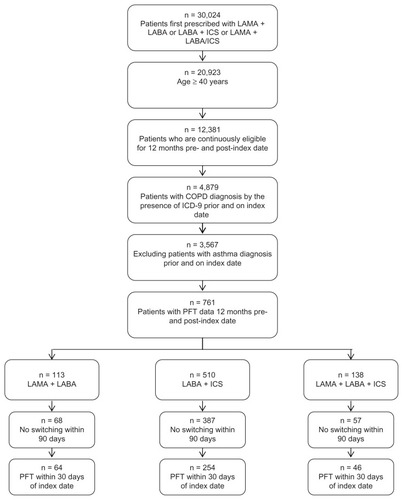
A total of 30,024 subjects initially received one of the study drug combinations between 2004 and 2007 and of this group 70% (n = 20,923) were ≥40 years old. After applying the other inclusion criteria, 68 subjects were identified in LAMA plus LABA cohort, 387 in LABA plus ICS, and 57 in LAMA plus LABA/ICS cohorts. A total of 364 subjects had PFT data permitting assessment of compliance with GOLD guidelines based on their stage of COPD ().
Figure 1 Population identification.
Baseline characteristics (demographic and clinical)
A summary of baseline characteristics is provided in . The mean age of subjects ranged from 66 to 68 years across all the cohorts of the study. The proportion of males (range: 60%–71%) was higher than females (range: 29%–40%) across the study cohorts with predominant race (99%) being white. The majority of study subjects were former smokers (64%), and unemployed (88%) with a commercial insurance (60%).
Table 2 Overall patient characteristics at baseline
A greater number of subjects (51%) in all the treatment cohorts had chronic airway obstruction, not otherwise specified as a COPD diagnosis as opposed to chronic bronchitis and emphysema. The mean DCI ranged from 3.06 to 3.82 and mean number of concomitant medications ranged from 8.18 to 16 across the cohorts. The duration of COPD prior to index date ranged from 139 to 203 days among the cohorts of the study (). The mean DCI ranged from 3.06–3.82 for the three cohorts with a significant difference between LAMA + LABA and LABA/ICS cohorts. The mean number of concomitant medications ranged from 8.18–16 across the cohorts with a significant difference between LAMA + LABA and LAMA + LABA/ ICS cohorts. The duration of COPD prior to index date ranged from 139–203 days among the cohorts of the study with a significant difference between LAMA + LABA and LABA/ICS cohorts.
Subjects prescribed LAMA plus LABA differed significantly (P < 0.05) by race, smoking history, COPD diagnosis, mean DCI and duration of COPD prior to index date compared to LABA plus ICS. There was a statistically significant difference (P < 0.05) observed in only mean number of concomitant medications for LAMA plus LABA cohort when compared with LAMA plus LABA/ICS. A summary of baseline health care utilization and total Geisinger encounter costs (in 2006 US dollars) are presented in . In the pre-index date period (baseline), subjects prescribed LAMA plus LABA differed significantly (P < 0.05) in mean number of outpatient visits (5 vs 3.5 or 8.3), oxygen use (62% vs 24% or 37%) as compared to LABA plus ICS or LAMA plus LABA/ICS groups respectively. Compared to subjects with LAMA plus LABA, the mean number of office visits (2.8 vs 2.4 or 4.7) and mean number of hospitalizations (2.1 vs 1.9 or 3.1) were found to be significantly different (P < 0.05) in only LAMA plus LABA/ICS group. There were no statistically significantly differences identified in mean hospital costs ($5,661 vs $4,701 or $10,553), mean physician costs ($1,371 vs $995 or $2,110), and total encounter costs to Geisinger ($6,948 vs $4,538 or $12,077).
Table 3 Health care utilization and total Geisinger encounter costs for overall COPD subjects at baseline
The baseline predicted values of FEV1%, FEV1/FVC%, and FVC% ranged from 41.9 to 55.41, 70.44 to 78.46, and 61.44 to 73.51 respectively with statistically significant differences (P < 0.05) in FEV1% and FVC% predicted values in LAMA plus LABA/ICS cohort as compared to LAMA plus LABA cohort. Also, significant differences were determined in mean change of FVC% predicted values between baseline and follow-up periods in LABA plus ICS and LAMA plus LABA/ICS cohorts. The mean change of FEV1% predicted values was found to be significantly different (P < 0.05) in only LAMA plus LABA/ICS cohort (). This is noteworthy as anything greater than 5% in terms of predicted values is regarded as being clinically significant.
Table 4 PFT values of the study population at baseline and mean change from baseline at follow-up
Of the overall cohort, 364 subjects had PFT data within 30 days of the index date and thus could be classified as adhering or not adhering to current GOLD guidelines. Of 68 patients in the LAMA plus LABA cohort, 25 patients adhered and 39 patients were non-adherent to current GOLD guidelines. There are 387 patients in the cohort of LABA plus ICS with 74 patients adhering and 180 patients non-adherent to current GOLD guidelines. There are 57 patients in the cohort of LAMA plus LABA/ICS with 21 patients adhering and 25 patients non-adherent to current GOLD guidelines. Overall, 33% of the 364 subjects received medication therapy consistent with current GOLD guidelines. The mean cost savings associated with adherence to GOLD guidelines were: $5,889 for LAMA plus LABA cohort, $3,330 for LABA plus ICS cohort, and $10,217 for LAMA + LABA/ICS cohort respectively ().
Table 5 Total cost savings of adhering to the GOLD guidelines
Discussion
According to current GOLD guidelines, COPD medications if prescribed according to the stages of severity will prevent disease progression, relieve symptoms, improve health status, prevent exacerbations, and eventually reduce mortality. The classification of subjects according to their pulmonary function data as outlined in current GOLD guidelines has been depicted as an independent predictor for exacerbation-related hospitalizations, which implies that the severe subjects (Stage IV) tend to have more hospitalizations as a result of an exacerbation.Citation14 In addition, it has been established that failure to use medications in accordance with guidelines increases the burden of the disease.Citation15–Citation19 However, there exists a gap among primary care physicians and pulmonologists in implementing or being consistent with the guidelines for diagnosing and treating COPD subjects.Citation20 There is evidence that mild to moderate COPD subjects are not being treated optimally and that ICS were frequently prescribed by both general practitioners and pulmonologists contrary to current guideline recommendations.Citation21,Citation22 Furthermore, improved survival, health status, and lung function with fewer exacerbations were observed with combination therapy as compared to monotherapy.Citation23,Citation24 Interestingly, a study found that hospitalization leads to improved adherence to GOLD guidelines.Citation25 This study is unique in characterizing the cost savings associated with adherence to current GOLD guidelines.
Our study found that 66% of subjects with PFT data were prescribed medications, inconsistent with their spirometry results and current GOLD guideline recommendations. Adherence with current GOLD guidelines was associated with cost savings in all three cohorts. Subjects prescribed LAMA plus LABA/ICS in accordance with current GOLD guidelines had the highest mean cost saving of $10,217 followed by LAMA plus LABA ($5,889) and LABA plus ICS ($3,330). These are interesting findings as LAMA plus LABA and LABA plus ICS groups are similar at baseline except in the case of LABA plus ICS cohort having had COPD for approximately 1 year less and as a result they are on fewer medications. As such, it is difficult to determine if the cost difference we are observing is attributable to the baseline differences.
Limitations
The GHS database, like any other electronic medical record (EMR) database, may not capture the entire medical history of a patient, specifically if the patient is seen by non-network providers. In addition, an EMR does not provide any information as to whether a service was delivered or a prescription was purchased or refilled by the patient. Hence, the study assumed that patients adhered perfectly to the prescribed therapies of interest. Moreover, an EMR database has missing data related to out of network healthcare utilizations including hospitalizations, ER visits, office visits etcetera, and no data were identified for actual drug costs, whereas the drug administering costs were present. Thus the true cost of treatment may be higher than reported here. Furthermore, a relatively small number of subjects that met the other inclusion/ exclusion criteria had a record of PFT data.
Conclusion
Adherence with current GOLD guidelines is associated with lower healthcare utilization costs in subjects with moderate to severe COPD treated with LAMA plus LABA, LABA plus ICS or LAMA plus LABA/ICS therapies.
Disclosure
CA, SR, MY, XY, and DY report no conflict of interest in this work. SL was an independent consultant to Novartis Pharmaceuticals Corporation during this study. CP is an employee of Novartis Pharmaceuticals Corporation, who provided funding to conduct the study.
References
- The National Heart, Lung, and Blood Institute Health Information CenterLung information http://www.nhlbi.nih.gov/health/public/lung/index.htmAccessed March 25, 2010
- Center for Disease Control and PreventionFacts about chronic obstructive pulmonary disease (COPD) http://www.cdc.gov/copd/copdfaq.htmAccessed March 23, 2010
- SkrepnekGHSkrepnekSVEpidemiology, clinical and economic burden, and natural history of chronic obstructive pulmonary disease and asthmaAm J Manag Care7200410Suppl 5S12913815354678
- TovarJMGumsJGMonitoring pulmonary function in asthma and COPD: point-of-care testingAnn Pharmacother200438112613314742806
- Global Initiative for Chronic Obstructive Lung Disease (GOLD)Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2008 update http://www.goldcopd.comAccessed March 22, 2010
- DaimonTFujimotoKTanakaKVolume of pulmonary lobes and segments in chronic obstructive pulmonary diseases calculated using newly developed three-dimensional softwareJpn J Radiol4200927311512219412678
- BresnitzEAEpidemiology of advanced lung disease in the United StatesClin Chest Med19971834214339329867
- KabirZConnollyGNKohHKClancyLChronic Obstructive Pulmonary Disease hospitalization rates in Massachusetts: a trend analysisQJM2010103316316820123682
- EdelmanNHKaplanRMBuistASChronic obstructive pulmonary disease. Task Force on Research and Education for the Prevention and Control of Respiratory DiseasesChest19921023 Suppl243S256S1516454
- BuistASGuidelines for the management of chronic obstructive pulmonary diseaseRespir Med200296Suppl CS111612199486
- RamseySDSuboptimal medical therapy in COPD: exploring the causes and consequencesChest20001172 Suppl33S37S10673472
- DeyoRACherkinDCCiolMAAdapting a clinical comorbidity index for use with ICD-9-CM administrative databasesJ Clin Epidemiol19924566136191607900
- United States Department of LaborConsumer Price Index for Medical Care http://www.bls.gov/CPI/Accessed October 15, 2009
- LusuardiMLucioniCDe BenedettoFMazziSSanguinettiCMDonnerCFGOLD severity stratification and risk of hospitalisation for COPD exacerbationsMonaldi Arch Chest Dis200869416416919350838
- MiravitllesMMayordomoCArtésMSánchez-AgudoLNicolauFSegúJLTreatment of chronic obstructive pulmonary disease and its exacerbations in general practice. EOLO Group. Estudio Observacional de la Limitacion Obstructiva al Flujo aEreoRespir Med199993317317910464874
- EstebanCMorazaJQuintanaJMAburtoMCapelasteguiAUse of medication and quality of life among patients with COPDRespir Med2006100348749516039840
- López VarelaMVMuinoAPérez PadillaRTreatment of chronic obstructive pulmonary disease in 5 Latin American cities: the PLATINO studyArch Bronconeumol2008442586418361870
- RennardSDecramerMCalverleyPMImpact of COPD in North America and Europe in 2000: subjects’ perspective of Confronting COPD International SurveyEur Respir J200220479980512412667
- RocheNLepageTBourcereauJTerriouxPGuidelines versus clinical practice in the treatment of chronic obstructive pulmonary diseaseEur Respir J200118690390811829094
- GlaabTBanikNRutschmannOTWenckerMNational survey of guideline-compliant COPD management among pneumologists and primary care physiciansCOPD20063314114817240616
- DecramerMBartschPPauwelsRYernaultJCManagement of COPD according to guidelines. A national survey among Belgian physiciansMonaldi Arch Chest Dis2003591628014533285
- Izquierdo-AlonsoJLde Miguel-DíezJEconomic impact of pulmonary drugs on direct costs of stable chronic obstructive pulmonary diseaseCOPD20041221522317136989
- SorianoJBVestboJPrideNBKiriVMadenCMaierWCSurvival in COPD patients after regular use of fluticasone propionate and salmeterol in general practiceEur Respir J200220481982512412670
- CalverleyPMAndersonJACelliBSalmeterol and fluticasone propionate and survival in chronic obstructive pulmonary diseaseN Engl J Med2007356877578917314337
- IncalziRACorsonelloAPedoneCFrom Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines to current clinical practice : an overview of the pharmacological therapy of stable chronic obstructive pulmonary disorderDrugs Aging200623541142016823994