Abstract
Purpose
Alpha-1-antitrypsin deficiency (AATD) is a rare hereditary condition characterized by low circulating levels of alpha-1antitrypsin (AAT). While the association between AATD and COPD/emphysema is undisputed, the association between AATD and asthma or bronchiectasis is still a matter of debate.
Aims and Objectives
Our study aimed to investigate the distribution of AAT genotypes between patients with COPD/emphysema, asthma and bronchiectasis. To back up the diagnostic labels, we described symptoms associated with the diagnosis.
Methods
Between September 2003 and March 2020, 29,465 testing kits (AlphaKit®) were analyzed in the AAT laboratory, University of Marburg, Germany. The diagnosis of AATD has been made based on the measurements of AAT serum levels, followed by genotyping, phenotyping or whole gene sequencing depending on the availability and/or the need for more detailed interpretation of the results. The respiratory symptoms were recorded as well.
Results
Regarding the distribution of the wild type allele M and the most frequent mutations S (E264V) and Z (E342K), no significant differences could be found between COPD/emphysema [Pi*MM (58.24%); Pi*SZ (2.49%); Pi*ZZ (9.12%)] and bronchiectasis [Pi*MM (59.30%) Pi*SZ (2.81%); Pi*ZZ (7.02%)]. When COPD/emphysema and bronchiectasis were recorded in the same patient, the rate of Pi* ZZ (14.78%) mutations was even higher. Asthma patients exhibited significantly less deficient genotypes [Pi*MM (54.81%); Pi*SZ (2%); Pi*ZZ (2.77%)] than two other groups. Associated respiratory symptoms confirmed the diagnosis.
Conclusion
COPD/emphysema and bronchiectasis, but not asthma patients, exhibit higher frequency of AATD genotypes. Our data suggest that AATD testing should be offered to patients with COPD/emphysema and bronchiectasis.
Introduction
Alpha-1 antitrypsin deficiency (AATD) is an autosomal codominant condition caused by mutations of the SERPINA1 gene characterized by low serum level of AAT.Citation1 Since the discovery of AATD in early 1960s has become apparent that the emphysematous form of chronic obstructive pulmonary disease (COPD) is the most frequent clinical manifestation in individuals with AATD. Other common clinical manifestations of AATD include hepatic diseases, and less frequently panniculitis and other skin diseases.Citation2 Despite of being one of the most common inherited conditions worldwide (AATD occurs in approximately 1:3,000–5,000 people), it remains significantly under-diagnosed. Data show that less than 10% of individuals with AATD identified and with delays of more than 5 years between initial symptoms and diagnosis.Citation3–Citation5 This is also in part related to the fact that many people with AATD are asymptomatic during the life time.
The clinical spectrum of AATD-related lung diseases is variable. AATD pulmonary involvement might include emphysema with variable functional and radiological impairments,Citation6 asthmaCitation7 and bronchiectasis.Citation8 In addition, the unusual associations between AATD and idiopathic pulmonary fibrosis have also been reported.Citation9 Therefore, an early detection of AATD is a crucial to define indicator diseases that should prompt physicians to screen for AATD. While according to recent guidelines-all patients with COPD and/or emphysema recommended for AATD screening, the importance of routinely screening of patients with asthma and bronchiectasis remains controversial. This is due to the conflicting epidemiological results concerning the association between AATD and those diseases. Regarding asthma, several investigators have suggested an increased risk of asthma among certain genotypes of AATD whereas others did not find an association.Citation10–Citation13 Nevertheless, few studies suggested that AATD patients with concomitant asthma have a worse prognosis.Citation14,Citation15 The recent ERS Statement of few years ago refer to the WHO recommendation to test all patients with COPD and all patients with adult-onset asthma. Thus also asthma patients (at least those with adult onset) should be tested.Citation16 Likewise, the causal relationship between AATD and bronchiectasis is controversial. Some studies have found a high prevalence of bronchiectasis among AATD patients while others found no association.Citation17–Citation19 Although data document the role of AATD as an underlying etiology of bronchiectasis, the majority of patients are not tested for AATD. It is likely that the estimation of AATD prevalence in bronchiectasis population remains poor. Because of the high prevalence of COPD/emphysema, asthma, and bronchiectasis and the common pathophysiological pathways, some AATD patients with different diseases may present with similar symptoms.Citation20 For example, common pathophysiological features of AATD-related bronchiectasis and emphysema include elevated elastin degradation as an indicator of elevated levels of desmosine and isodesmosine in both diseases.Citation21,Citation22 Considering latter, the investigations for the presence of chronic respiratory conditions, as asthma or bronchiectasis, in AATD are of great clinical importance. Hence, to elucidate the importance of COPD/emphysema, asthma, and bronchiectasis as indicator diseases for AATD, we compared the frequency and distribution of the different AAT alleles in patients with each of those three conditions.
Materials and Methods
Patients and Methods
Between September 2003 and March 2020, 29,465 testing kits (AlphaKit®) were analyzed in the AAT laboratory, University of Marburg, Germany. The diagnosis of AATD was made through a measurement of AAT serum levels, followed by genotyping, phenotyping or whole gene sequencing depending on availability and/or the need for more detailed interpretation of the AAT results.Citation23 Respiratory symptoms were recorded.
Clinical Samples
We used serum samples and dried blood spot (DBS) samples (AlphaKits® GE Healthcare Ltd, Cardiff, CF147YT, UK) for screening and testing for AATD. The AlphaKits® consists of an instruction manual that outlines the purpose of the testing and the procedure how to obtain a blood specimen. Blood is applied to a filter paper, dried and shipped to our laboratory at the University of Marburg for the routine diagnosis of AATD. The AlphaKits® includes a text field for patient and physician’s identification. Clinical characteristics are recorded on the AlphaKit® (acute bronchitis, asthma, bronchiectasis, COPD, dyspnoea on exertion, dyspnea attacks, emphysema, chronic bronchitis, cough, sputum, wheezing), and also the smoking history and substitution therapy for AAT are asked for. The physician’s signature on the AlphaKit® confirms that patients approved and signed informed consent for the samples shipped to our laboratory for genetic analysis. Because our study reflects a retrospective analysis of those routine data, a formal ethics approval for this summary statistics of anonymized data was not necessary.
Nephelometry was used for the semi-quantitative determination of the plasma AAT levels and polymerase chain reaction (PCR) for genotyping, which was eventually followed by isoelectric focusing (IEF) (phenotype) and gene sequencing. Two independent readers validated the results of PCR and IEF. For the sequencing, the DBS samples were shipped to a reference laboratory (Eurofins Genomics Germany GmbH or Progenika Biopharma, S.A. A Grifols Company). Details regarding the laboratory methods have been described before.Citation23,Citation24
Clinical characteristics were recorded on the AlphaKit® (acute bronchitis, asthma, bronchiectasis, COPD, dyspnea on exertion, dyspnea attacks, emphysema, chronic bronchitis, cough, sputum, wheezing) and were documented in a database.
Statistical Analysis
For the comparison of continuous variables Kruskal–Wallis test with Dunn’s test was applied (data were not normally distributed). For the comparison of categorical variables, chi-square test was applied. Microsoft Excel 2010 (Microsoft Corporation, Redmond, Washington, USA), IBM SPSS statistics version 24 (IBM, Armonk, New York, USA) and GraphPad version 7 (GraphPad, San Diego, CA, USA) were used for all calculation, graphs were constructed using Microsoft Excel and BioVenn.Citation25
Results
Study Population
In total 29,465 AlphaKits® were analyzed between September 2003 and March 2020. According to the transferred information, 18,736 patients had been diagnosed with COPD/emphysema, asthma, bronchiectasis or for the combination of these diseases. Further analysis was restricted to this population. Baseline characteristics are presented in .
Table 1 Baseline Characteristics of Probands
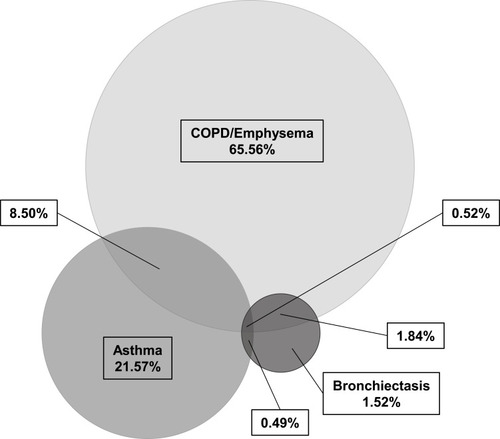
We identified patient having only COPD/Emphysema 12,283 (65.56%), asthma 4,041 (21.57%) or bronchiectasis 285 (1.52%). Additionally, we found 1,592 (8.50%) cases with combined COPD/emphysema and asthma, 345 (1.84%) COPD/emphysema and bronchiectasis, 92 (0.49%) asthma and bronchiectasis, and 98 (0.52%) COPD/emphysema, asthma and bronchiectasis (). The baseline characteristics of the respective groups well reflect reports in the literature. Patients with COPD/emphysema were older and smoked more often as compared to patients with asthma or bronchiectasis. Asthmatic patients were younger than in the other two groups ().
Figure 1 Frequency of 18,736 patients among chronic respiratory disorders without consideration on AATD. A three-way Venn diagram was plotted to investigate the relationship between patients of COPD/Emphysema, asthma and bronchiectasis and their overlaps. COPD/Emphysema only: 12,283 (65.56%); asthma only: 4,041 (21.57%); bronchiectasis only: 285 (1.52%); COPD/Emphysema and asthma overlap: 1,592 (8.50%); COPD/Emphysema and bronchiectasis overlap: 345 (1.84%); asthma and bronchiectasis overlap: 92 (0.49%); COPD/Emphysema and asthma and bronchiectasis overlap: 98 (0.52%). BioVenn – a web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. T. Hulsen, J. de Vlieg and W. Alkema, BMC Genomics 2008, 9 (1): 488.
Symptoms
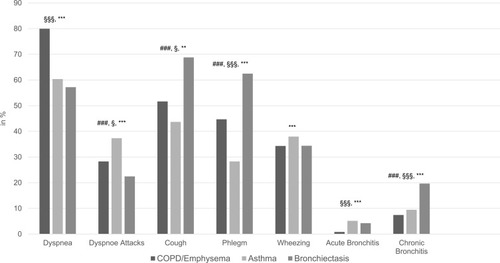
Based on the AlphaKit® recordings, the frequency of clinical symptoms such as dyspnea, dyspnea attacks, cough, phlegm, wheezing, acute bronchitis and chronic bronchitis, supported the recorded diagnosis: dyspnea was most often recorded in COPD/emphysema, wheezing was most often recorded in asthma, and productive cough (cough, phlegm, chronic bronchitis) were the leading symptoms in bronchiectasis ().
Figure 2 Frequency of symptoms among the chronic respiratory diseases: COPD/emphysema, asthma and bronchiectasis. Figure 2 displays the frequency of clinical symptoms (dyspnea, dyspnea attacks, cough, phlegm, wheezing, acute bronchitis and chronic bronchitis) among the chronic respiratory diseases (COPD/emphysema, asthma and bronchiectasis). Chi-square test was used for statistical analyses. This information has been evaluated on the basis of the information on the AlphaKits®. Dyspnea appeared most frequently in patients with COPD/emphysema, whereas dyspnea attacks were more often in patients with asthma. Cough and phlegm could be observed most frequently in patients with bronchiectasis. Acute and chronic bronchitis occurred more often in patients with asthma or bronchiectasis than in patients with COPD/emphysema. ###p<0.001 significant between bronchiectasis and asthma; §§§p<0.001, §p<0.05 significant between bronchiectasis and COPD/emphysema; ***p<0.001, **p<0.01 significant between asthma and COPD/emphysema.
Distribution of Genotypes
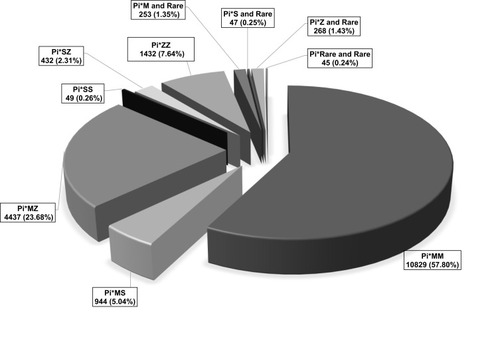
The distribution of the AAT alleles of the 18,736 individuals is illustrated in . In total 10,829 (57.80%) Pi*MM, 944 (5.04%) Pi*MS, 4,437 (23.68%) Pi*MZ, 49 (0.26%) Pi*SS, 432 (2.31%) Pi*SZ, 1,432 (7.64%) Pi*ZZ, 253 (1.35%) Pi*M and rare, 47 (0.25%) Pi*S and rare, 268 (1.43%) Pi*Z and rare, 45 (0.24%) Pi*rare and rare have been identified.
The distribution of the AAT alleles among patients with COPD/emphysema or asthma, or bronchiectasis yielded the following results (illustrated in and ): Regarding the distribution of the wild type allele M and the most frequent mutations S (E264V) and Z (E342K), no significant differences could be found between COPD/emphysema [Pi*MM (58.24%); Pi*SZ (2.49%); Pi*ZZ (9.12%)] and bronchiectasis [Pi*MM (59.30%) Pi*SZ (2.81%), Pi*ZZ (7.02%)]. In contrast, asthma patients exhibited significantly less deficient genotypes [Pi*MM (54.81%); Pi*SZ (2%); Pi*ZZ (2.77%)] than two other groups.
Table 2 Distribution of the AAT Alleles Among Patients with COPD/Emphysema or Asthma, or Bronchiectasis
Figure 4 Distribution of AAT alleles among the chronic respiratory diseases: COPD/emphysema, asthma and bronchiectasis. Figure 4 displays the distribution of AAT alleles among the chronic respiratory diseases bronchiectasis, asthma and COPD/emphysema. The distribution of the wild type (Pi*MM) was roughly comparable between the three groups (significant only between asthma and COPD/emphysema). The same applied to the distribution of the genotypes Pi*MS, Pi*M/rare and Pi*Z/rare, while the genotypes Pi*SS, Pi*SZ and Pi*S/rare were not distributed significantly different. A higher percentage of asthma patients exhibited the Pi*MZ genotype, whereas more COPD/emphysema and bronchiectasis patients exhibited the Pi*ZZ genotype when compared to asthma patients. ###p<0.001, ##p<0.01 significant between bronchiectasis and asthma; ***p<0.001, *p<0.05 significant between asthma and COPD/emphysema.
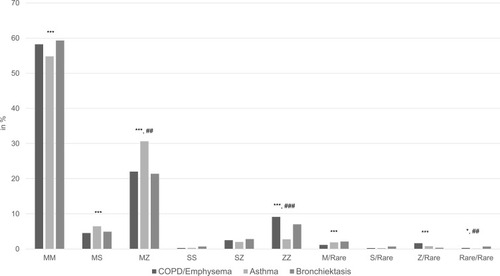
We also analyzed the distribution of these genotypes in a number of combinations of the three diagnostic groups (). Adding patients with the diagnostic label COPD/emphysema or bronchiectasis to any other group increases the frequency of Pi*ZZ samples while the addition of the diagnostic label asthma decreases the detection rate.
Table 3 Distribution of AAT Alleles Among Different Combinations of the Three Diagnostic Groups
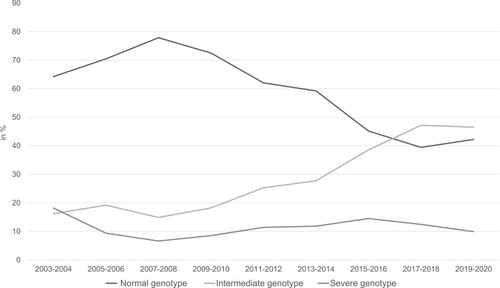
In the detection rate is shown over a period of 17 years of normal genotype, intermediate genotype and severe genotype of patients with COPD/emphysema, bronchiectasis, asthma. The genotypes have been grouped in: normal genotype (Pi*MM); intermediate genotype (Pi*MS, Pi*MZ, Pi*M/Rare) and severe genotype (Pi*ZZ, Pi*SS, Pi*SZ, Pi*Z/Rare, Pi*S/Rare, Pi*Rare/Rare). A decline of the detection rate of the normal genotype can be seen in . The detection rate of the intermediate genotype however increases. Over the 17 years, the detection rate of the severe genotypes remained unchanged.
Ten thousand seven hundred and twenty-nine samples were analyzed out of which none of the three indications (COPD/emphysema, bronchiectasis, asthma) were ticked off on the AlphaKit®. Dividing those samples into a group of samples with at least one symptoms and a second group without symptoms, the symptomatic patients exhibited a lower percentage of at least one mutation (43.44% vs 56.83%) and also a lower percentage of Pi*ZZ samples (4.87% vs 6.37%). We assume that the AlphaKits® have not been ticked off correctly. For this reason, we omitted further analyses of those samples.
Discussion
In our database of almost 30,000 samples, which had been tested for AATD for different reasons, we analyzed the distribution of three important respiratory diseases, namely COPD/emphysema, asthma, and bronchiectasis. Furthermore, we described the distribution of different AAT genotypes in this cohort. To the best of our knowledge, this is one of the largest cohorts comparing the distribution of AAT genotypes among different respiratory diseases. We found COPD/emphysema to be the most frequent reason for testing, followed by asthma and bronchiectasis. Remarkably, COPD/emphysema and bronchiectasis exhibited very similar distribution of the AAT genotypes. Both diseases exhibited higher percentages of severe deficient AAT genotypes as compared to asthma.
The COPD/emphysema was by far the most prevalent disease entity in our study population (65.56%), followed by asthma (21.57%) and bronchiectasis (1.52%). This is likely relates to the facts that: i) early onset emphysema known as a typical manifestation of AATDCitation26 and ii) COPD is a highly prevalent internationally and nationally.Citation27–Citation29 The bronchiectasis is gaining importance and awareness, however, it still remains a rare and underdiagnosed disease. Therefore, the low prevalence of samples in which bronchiectasis was the reason for testing, was expected.Citation30,Citation31 Furthermore, our analysis records 2.78% of all 29,465 subjects to be bronchiectatic (out of which 0.97% with bronchiectasis and absence of asthma or COPD/emphysema), and is in line with what has been reported by the NHLBI registry (2% of 1,129 participants).
By analyzing the association between recorded symptoms and the leading diagnosis, we gained confirming findings. While in COPD/emphysema was most often recorded dyspnea, wheezing was most often in asthma, and productive cough (cough, phlegm, chronic bronchitis) was most often in bronchiectasis. The combination of symptoms and the main diagnosis made us confident that the reported diagnoses were correct, even though lung function data and/or CT data are not part of the information that is transferred with the AlphaKit®.
Most interesting in our analysis is that we found a similar distribution of AAT genotypes between bronchiectasis and COPD/emphysema. The Pi*ZZ genotype was found significantly more often in patients with COPD/emphysema or bronchiectasis (9.12% in COPD/emphysema, 7.02% in bronchiectasis) than in patients with asthma (2.77%). The literature on AAT genotypes in patients with bronchiectasis is scarce. In a case-control study of 2000, Cuvelier and colleagues recorded no difference in the distribution of AAT alleles between bronchiectatic patients and 1,030 control subjects (unrelated healthy blood donors).Citation19 Very recently, investigators from two hospitals in the UK screened 1600 patients with bronchiectasis, which resulted in the detection of only eight individuals (0.5%) with AATD. The authors concluded that routine screening for AATD in bronchiectasis in the UK has a low rate of detection.Citation32 One can agree that 0.5% is certainly a low number; however, it is in the same range as the percentage of AATD patients within the overall COPD population, in which screening for AATD is accepted.Citation27,Citation33 Furthermore, these numbers come from rather unselected disease populations, while our results are from a pre-filtered population. Nevertheless, it is reassuring that in our as well as in UK study the frequency of severe AATD in bronchiectasis was comparable to that in COPD.
The recommendation of the WHO, the ERS and the ATS about the need to test all COPD patients (and all patients with adult-onset asthma) have been emphasized repeatedly. Despite this, the recommendation is clearly not followed by any health-care system throughout the world. One could argue that adding bronchiectasis as another rare (or believed to be rare) condition to the reasons for AATD screening could “dilute” the message of testing for AATD in routine screening. In our opinion, the recommendations depend on the clinical setting: While COPD should be a reason for AATD screening in every clinical setting, screening for AATD in bronchiectasis should be implemented in the workup of bronchiectasis patients in specialist centers.
One could argue that COPD and emphysema we need to analyz separately. In a subgroup analysis, we looked at the symptom and genotype distribution in patients with “COPD only” patients and compared them to “emphysema only”. In general, COPD patients were more symptomatic (supplement Figure 1) while emphysema patients more often exhibited a genotype compatible with severe deficiency (supplement Figure 2), both confirming earlier findings.Citation26,Citation34
Discussing the limitations of our analysis, we have to acknowledge that targeting preselected individuals (which is the basis for the vast majority of our analyses) might result in missing asymptomatic subjects with severe AATD, because a significant proportion of severe AATD patients do not develop pulmonary diseases.Citation35,Citation36 Secondly, our laboratory population only reflects a small and preselected part of the overall populations of the three diseases that we looked at. Thirdly, the retrospective nature of our analysis resulted in a high number of samples in which the reason for the analysis was not recorded. The reasons for that remain speculative. Finally, only limited information about the clinical characteristics was available, and the diagnostic accuracy of the disease entity remains unknown. Nevertheless, our analysis provides important insights into the distribution of ATTD among diseases, which are regularly screened for AAT genotypes.
Overall, in our population the COPD/emphysema and bronchiectasis exhibited very similar distributions of AAT genotypes. Hence, screening of patients with bronchiectasis for the presence of AATD is of importance.
Abbreviations
AAT, alpha 1 antitrypsin; AATD, alpha 1 antitrypsin deficiency; COPD, chronic obstructive pulmonary disease; DBS, dried blood spot; IEF, isoelectric focussing; PCR, polymerase chain reaction.
Ethics Approval and Informed Consent
Because our study reflects a retrospective analysis of those routine data, an ethics approval is not necessary.
Disclosure
Martina Veith reports grants from Grifols. Prof. Dr. Robert Bals reports grants from BMBF, during the conduct of the study; grants from Schwiete Stiftung, Novartis, and Mukoviszidose e.V. and personal fees from AstraZeneca and CSL Behring, outside the submitted work. Prof. Dr. Claus Franz Vogelmeier reports grants, personal fees from AstraZeneca, Boehringer Ingelheim, Grifols, GlaxoSmithKline, and Novartis; personal fees from CSL Behring, Chiesi, Menarini, Nuvaira, MedUpdate, outside the submitted work. Dr Timm Greulich reports grants from Grifols, during the conduct of the study; personal fees from AstraZeneca, serves as a lecturer and part of the advisory board for Berlin Chemie, Boehringer Ingelheim, Chiesi, CSL Behring, Grifols, GSK, Novartis, received grants from German Centre for Lung Research, outside the submitted work. The authors report no other conflicts of interest in this work.
Acknowledgments
We thank all employees of the alpha-1 antitrypsin laboratory at the University of Marburg for their continuous effort they put into this project. The alpha-1 antitrypsin laboratory at the University of Marburg has been supported by Grifols and Progenika Biopharma (A Grifols Company).
References
- JanciauskieneSM, BalsR, KoczullaR, VogelmeierC, KohnleinT, WelteT. The discovery of alpha1-antitrypsin and its role in health and disease. Respir Med. 2011;105(8):1129–1139.21367592
- European RS, American Thoracic Society. American thoracic society/European respiratory society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med. 2003;168(7):818–900.14522813
- BrantlyML, PaulLD, MillerBH, FalkRT, WuM, CrystalRG. Clinical features and history of the destructive lung disease associated with alpha-1-antitrypsin deficiency of adults with pulmonary symptoms. Am Rev Respir Dis. 1988;138(2):327–336. doi:10.1164/ajrccm/138.2.3273264124
- StollerJK, BrantlyM. The challenge of detecting alpha-1 antitrypsin deficiency. Copd. 2013;10(Suppl 1):26–34.23527684
- StollerJK, SmithP, YangP, SprayJ. Physical and social impact of alpha 1-antitrypsin deficiency: results of a survey. Cleve Clin J Med. 1994;61(6):461–467. doi:10.3949/ccjm.61.6.4617828337
- SilvermanEK, SandhausRA. Alpha1-antitrypsin deficiency. N Engl J Med. 2009;360(26):2749–2757. doi:10.1056/NEJMcp090044919553648
- SiriD, FarahH, HogarthDK. Distinguishing alpha1-antitrypsin deficiency from asthma. Ann Allergy Asthma Immunol. 2013;111(6):458–464.24267358
- StockleyRA. The multiple facets of alpha-1-antitrypsin. Ann Transl Med. 2015;3(10):130.26207223
- CottinV, NunesH, BrilletPY, et al. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J. 2005;26(4):586–593. doi:10.1183/09031936.05.0002100516204587
- EdenE. Asthma and COPD in alpha-1 antitrypsin deficiency. Evidence for the Dutch hypothesis. COPD. 2010;7(5):366–374. doi:10.3109/15412555.2010.51015920854052
- McGeeD, SchwarzL, McClureR, et al. Is PiSS alpha-1 antitrypsin deficiency associated with disease? Pulm Med. 2010;2010:570679. doi:10.1155/2010/57067921687342
- van VeenIH, Ten BrinkeA, van der LindenAC, RabeKF, BelEH. Deficient alpha-1-antitrypsin phenotypes and persistent airflow limitation in severe asthma. Respir Med. 2006;100(9):1534–1539.16476537
- DavilaI, ValeroA, EntrenasLM, ValvenyN, HerraezL. Relationship between serum total IgE and disease severity in patients with allergic asthma in Spain. J Investig Allergol Clin Immunol. 2015;25(2):120–127.
- DemeoDL, SandhausRA, BarkerAF, et al. Determinants of airflow obstruction in severe alpha-1-antitrypsin deficiency. Thorax. 2007;62(9):806–813. doi:10.1136/thx.2006.07584617389752
- DowsonLJ, GuestPJ, StockleyRA. Longitudinal changes in physiological, radiological, and health status measurements in alpha(1)-antitrypsin deficiency and factors associated with decline. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1805–1809. doi:10.1164/ajrccm.164.10.210603611734427
- MiravitllesM, DirksenA, FerrarottiI, et al. European respiratory society statement: diagnosis and treatment of pulmonary disease in α1-antitrypsin deficiency. Eur Respir J. 2017;50(5):1700610. doi:10.1183/13993003.00610-201729191952
- ParrDG, GuestPG, ReynoldsJH, DowsonLJ, StockleyRA. Prevalence and impact of bronchiectasis in alpha1-antitrypsin deficiency. Am J Respir Crit Care Med. 2007;176(12):1215–1221.17872489
- GuestPJ, HansellDM. High resolution computed tomography (HRCT) in emphysema associated with alpha-1-antitrypsin deficiency. Clin Radiol. 1992;45(4):260–266. doi:10.1016/S0009-9260(05)80011-X1395384
- CuvelierA, MuirJF, HellotMF, et al. Distribution of α1-antitrypsin alleles in patients with bronchiectasis. Chest. 2000;117(2):415–419. doi:10.1378/chest.117.2.41510669684
- BarnesPJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8(3):183–192. doi:10.1038/nri225418274560
- ChalmersJD, MoffittKL, Suarez-CuartinG, et al. Neutrophil elastase activity is associated with exacerbations and lung function decline in bronchiectasis. Am J Respir Crit Care Med. 2017;195(10):1384–1393. doi:10.1164/rccm.201605-1027OC27911604
- TurinoGM, MaS, CantorJO, LinYY. Biomarkers in alpha-1 antitrypsin deficiency chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13(Suppl 4):S336–340. doi:10.1513/AnnalsATS.201509-574KV27564670
- GreulichT, NellC, HerrC, et al. Results from a large targeted screening program for alpha-1-antitrypsin deficiency: 2003–2015. Orphanet J Rare Dis. 2016;11(1):75. doi:10.1186/s13023-016-0453-827282198
- VeithM, KlemmerA, AntonI, et al. Diagnosing alpha-1-antitrypsin deficiency using A PCR/luminescence-based technology. Int J Chron Obstruct Pulmon Dis. 2019;14:2535–2542. doi:10.2147/COPD.S22422131819391
- HulsenT, de VliegJ, AlkemaW. BioVenn – a web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genomics. 2008;9(1):488. doi:10.1186/1471-2164-9-48818925949
- StrnadP, McElvaneyNG, LomasDA. Alpha1-antitrypsin deficiency. N Engl J Med. 2020;382(15):1443–1455. doi:10.1056/NEJMra191023432268028
- VogelmeierCF, CrinerGJ, MartinezFJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–582.28128970
- GeldmacherH, BillerH, HerbstA, et al. [The prevalence of chronic obstructive pulmonary disease (COPD) in Germany. Results of the BOLD study]. Dtsch Med Wochenschr. 2008;133(50):2609–2614. doi:10.1055/s-0028-110585819052996
- BuistAS, McBurnieMA, VollmerWM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–750. doi:10.1016/S0140-6736(07)61377-417765523
- RingshausenFC, de RouxA, DielR, HohmannD, WelteT, RademacherJ. Bronchiectasis in Germany: a population-based estimation of disease prevalence. Eur Respir J. 2015;46(6):1805–1807. doi:10.1183/13993003.00954-201526293498
- ChalmersJD, AlibertiS, PolverinoE, et al. The EMBARC European bronchiectasis registry: protocol for an international observational study. ERJ Open Res. 2016;2(1):00081–2015. doi:10.1183/23120541.00081-201527730179
- CarretoL, MorrisonM, DonovanJ, et al. Utility of routine screening for alpha-1 antitrypsin deficiency in patients with bronchiectasis. Thorax. 2020. doi:10.1136/thoraxjnl-2019-214195
- RahaghiFF, SandhausRA, BrantlyML, et al. The prevalence of alpha-1 antitrypsin deficiency among patients found to have airflow obstruction. COPD. 2012;9(4):352–358. doi:10.3109/15412555.2012.66943322506682
- ChaiCS, LiamCK, PangYK, et al. Clinical phenotypes of COPD and health-related quality of life: a cross-sectional study. Int J Chron Obstruct Pulmon Dis. 2019;14:565–573. doi:10.2147/COPD.S19610930880946
- ErikssonS. A 30-year perspective on α1-antitrypsin deficiency. Chest. 1996;110(6 Suppl):237S–242S. doi:10.1378/chest.110.6_Supplement.237S8989157
- ParrDG, StoelBC, StolkJ, StockleyRA. Pattern of emphysema distribution in α1-antitrypsin deficiency influences lung function impairment. Am J Respir Crit Care Med. 2004;170(11):1172–1178. doi:10.1164/rccm.200406-761OC15306534