Abstract
Background
COPD is characterized by a persistent inflammatory response, especially against cigarette smoke. COPD patients may develop varying degrees of emphysematous destruction of the lungs. A pathophysiological role for miRNAs in COPD has been suggested in several studies. We examined changes in microRNAs expression profile during 10 years follow-up in relation to COPD progression.
Methods
Clinical and lung function parameters were registered from every subject included in the study. miRNAs expression was determined in 14 serum samples from 7 patients in two moments (4 smokers with COPD (BODE cohort) and 3 smokers without COPD) by next generation sequencing (NGS) at baseline and after 10 years follow-up. A validation study was performed by qPCR in 20 patients with COPD (13 emphysema-diagnosed by CTscan) and 10 smoker controls at baseline and after 10 years follow-up. hsa-miRNA-20a-5p and hsa-let-7d-5p were used as endogenous controls.
Results
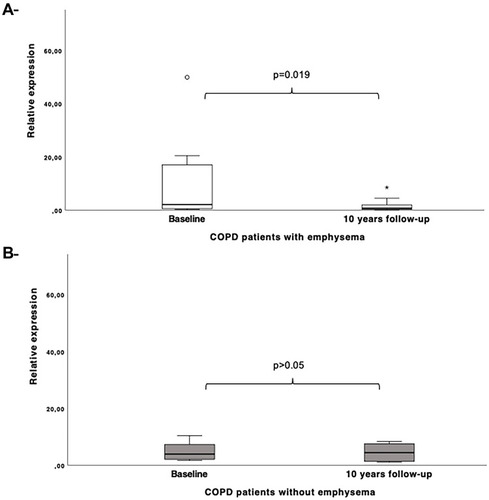
A total of 198 miRNAs (≥10TPM) were identified by NGS. Between these, hsa-miR-1246 was found significantly downregulated in COPD patients after 10 years when compared to baseline (p<0.0001, FDR=0.05). Seventy-five percent of these patients had an emphysema diagnose. In the validation analysis, when analyzed longitudinally, hsa-miR-1246 was significantly downregulated in COPD patients with emphysema after 10 years (p= 0.019). However, no association was found between the expression of miR-1246 and any other lung function parameters (FEV1, PaO2, DLCO, IC/TLC) within the follow-up period. GO and KEGG enrichment analysis revealed miR-1246 to be associated with target genes in several pathways involved in COPD/emphysema development.
Conclusion
Our findings suggest that hsa-miR-1246 may act as a biomarker of emphysema in COPD. Functional analysis is guaranteed to elucidate its role in COPD.
Keywords:
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by a progressive airflow obstruction and a persistent inflammatory response, especially against cigarette smoke.Citation1,Citation2 COPD is the third leading cause of chronic morbidity and mortality worldwide.Citation3
Recent reports suggest that cigarette smoke induces oxidative stress-mediated DNA damage and triggers cellular senescence in the lungs, which results in several pathophysiological changes in the lung.Citation2,Citation3 The infiltration of small airways by neutrophils, macrophages and T lymphocytes is associated with further release of cytokines and proteolytic enzymes, which amplifies the inflammation and release proteases and reactive oxidative species that damage the parenchymal lung tissue, results in the destruction of alveolar structure and increased mucus secretion, contributing to the development of emphysema in COPD patients.Citation4 This impaired tissue regeneration activates matrix remodeling and tissue repair mechanism such as cellular proliferation and senescence that are however insufficient to counteract its effect.
miRNAs are non-coding 18–24 nucleotide long RNA molecules. miRNAs play a critical role in several biological processes such as apoptosis, cell differentiation, proliferation, DNA damage repair, angiogenesis, stress response and stem cell division. They act primarily by suppressing mRNA translation and thereby reducing the levels of the proteins involved.Citation5,Citation6 Deregulation of miRNAs can be caused by various mechanisms, either genetic or epigenetic.Citation7 It has been reported that miRNA expressions dysregulation is associated with the development and progression of several human diseases like cancer, neurological, cardiovascular diseasesCitation8 and pulmonary ones.Citation9
A pathophysiological role for miRNAs in COPD has been suggested in several studies. Previous studies showed a differential expression of certain miRNAs in different types of samples such as sputum, bronchial and lung tissueCitation10–Citation12 or even serum or plasmaCitation13,Citation14 in smokers and COPD cases. It has also been described how tobacco smoke affects the expression of miRNAs of the bronchial epithelium.Citation15 It has been suggested several deregulated miRNAs to be involved in the development of tobacco-associated diseases such as COPD and its progression.Citation16 A study by Xie et al demonstrated that the serum ratio of miR-21 to miR-181a could predict the risk of suffering COPD in asymptomatic smokers with a significant smoking burden.Citation17 Moreover, Sato et al found that the expression of miR-146a was correlated with the severity of COPD.Citation18 However, all research designs until now were cross-sectional. Longitudinal studies are needed to evaluate potentially useful miRNAs as biomarkers for early detection of disease-related molecular and genetic changes, as well as a risk marker of the disease evolution by early detection of pulmonary function loss, exacerbations and/or mortality.
The aim of the present study was to examine changes in a microRNA expression profile after 10 years-follow-up in relation to COPD progression serum samples in a very well characterized cohort of patients.
Methods
Study Individuals
Patients
Twenty-four individuals with a diagnosis of COPD recruited from the Hospital Universitario N/S de Candelaria, Tenerife, Spain, were included in this study. They are part of a cohort of 362 individuals with annual clinical follow-up since 2002, part of the BODE multicenter study.Citation19 From these, four individuals were included in the screening step and other 20 were included in the subsequent validation step. Inclusion criteria: age >40 years, post-bronchodilator FEV1/FVC ratio <0.70 and clinically stable for at least 6 weeks at the time of evaluation. Spirometry lung volumes, pulmonary function test, and exercise capacity were measured according to ATS-ERS guidelinesCitation20–Citation22 and severity was graded by the Global Initiative for Obstructive Lung Disease (GOLD).Citation21 Dyspnoea was evaluated by mMRC scaleCitation23 and the BODE Index was calculated as previously described.Citation19 Co-morbidities were quantified using the Charlson index.Citation24 A pulmonologist visually scored the baseline for emphysema presence, using validated criteria established by the Fleischner Society.Citation25 Exclusion criteria included any other respiratory diseases and uncontrolled comorbidities such as malignancy at baseline.
Controls
Based on the availability of tobacco-smoker controls without COPD (>44 years, smoking history of >15 pack-year and FEV1% pred >0.80; FEV1/FVC >0.70), with blood sample and with a follow-up of ten years, 13 individuals could be included in this study (3 smoker controls for screening step and 10 for the validation study in the second stage of this research).
The study was approved by the ethical committee board of Hospital Universitario N/S de Candelaria and written informed consent was obtained from all participants (PI 55/17). This study was conducted in accordance with the Declaration of Helsinki.
Sample Collection
A total of 74 serum samples were collected from participants (24 stable COPD patients and 13 control smokers without COPD at two moments: baseline and after 10 years of follow-up) between the screening and validation steps. Serum samples were isolated within 1 h after receiving whole blood and immediately stored at −80 °C until further use.
miRNA Screening in COPD Patients
RNA was isolated from 7 participants (4 smokers with COPD and 3 control smokers without COPD) at baseline and after 10 years follow-up (14 serum samples). Isolation of RNA from 200 µL serum of patients was performed using a miRNeasy Serum/Plasma Advanced Kit (Qiagen). miRNAs expression was determined by next generation sequencing (NGS) in an Illumina NextSeq500 platform at Exiqon A/S company (Denmark). After mapping the data and counting to relevant entries in miRBase v20 software (http://mirbase.org)Citation26 the numbers of known microRNAs were calculated. miRNAs expression is expressed as Tags Per Million (“TPM”, the number of reads for a particular microRNA). microRNA stably expressed across all samples were identified using NormFinder software.Citation27
Quantitative RT-PCR for miRNA Expression. Validation Assay
A validation study was performed in 20 patients with COPD (from them 13 had emphysema-diagnosed by CT scan) and 10 control smokers without COPD, at two moments: baseline and after 10 years follow-up (a total of 60 serum samples). Isolated RNA from serum samples was used in a retro transcription reaction to synthesize cDNA using miRCURY LNA RT kit (Qiagen Inc., Germany), according to the manufacturer instructions. We also used RNA spike-ins (UniSp2, UniSp4 and UniSp5, as an RNA isolation control. And cel-miR-39-3p in conjunction with the UniSp6 spike-in as cDNA synthesis and amplification controls) (miRCURY LNA RT Kit and RNA Spike-in Kit, Qiagen Inc., Germany). A qPCR analysis was used to quantify the expression of the resulting significant miRNAs from the NGS screening study at the first stage. In this case, hsa-miR-1246 expression was determined by using miRCURY LNA Sybr Green PCR Master Mix (Qiagen Inc., Germany). The reactions were performed in a final volume of 10 µL and contained 1X Sybr Green Master Mix, 200 nmol/L specific primer set (miRCURY LNA miRNA PCR assay, Qiagen Inc., Germany), using 3 ng cDNA per reaction. The conditions included an initial denaturation at 95 °C for 15 min., followed by 40 cycles of 94 °C for 15 s, 55 °C for 30 s, and 72 °C for 30 s. All the samples were performed in triplicates. Experiments were performed on a StepOnePlus real-time PCR system (Applied Biosystem, Foster city, CA, USA). hsa-miRNA-20a-5p and hsa-let-7d-5p, that resulted as the best candidates for normalization control in the first stage screening by the NormFinder software, were also used for normalization as the reference genes in this assay. A non-template control (NTC) was carried out in each experiment, crucial to identify any contamination that may increase the background.
The relative expression analysis of the target miRNAs was performed using the comparative threshold method 2−ΔΔCt.Citation28
In silico Analysis
We also integrated the miRNA and the differentially expressed putative target mRNA using the miRBase v20 (http://www.mirbase.org/),Citation26 TargetScan (http://www.targetscan.org/vert_72/)Citation29 and the DIANA-microT_CDS softwares (http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=MicroT_CDS/index).Citation30 In the next step, the g:Profiler software was used to contrast the resulting genes proposed as targets of miR-1246.Citation31
We performed functional and enrichment analyses through the Kyoto Encyclopedia of Genes and Genomes (KEGG) (https://www.kegg.jp/) and the Gene-ontology (GO) termsCitation32,Citation33 by using the miRTar.Human software (http://mirtar.mbc.nctu.edu.tw/human/index.php).
Statistical Analysis
For the cross-sectional analysis, a t-Student, ANOVA, Chi2, Fisher Exact, Kruskal–Wallis test were used to test differences in means and proportions of clinical and lung function characteristics between smoker patients with COPD and smokers without the disease.
miRNAs differential expression analysis resulting from NGS was performed using the EdgeR statistical software package (Bioconductor, http://bioconductor.org/).Citation34 For normalization, the trimmed mean of M-values method based on log-fold and absolute gene-wise changes in expression levels between samples (TMM normalization) was used. The Benjamini-Hochberg false discovery rate (FDR) algorithm was used to corrected p-values for multiple testing.Citation35
The association between baseline miRNA expression with clinical and/or pulmonary function variables was explored using Pearson’s correlation coefficients. χ2 Pearson test was used for comparisons of differential miRNAs expression between cases and the control group.
A multiple logistic regression was performed to test the association of telomere length with COPD adjusting for age, sex and pack years. General linear model (GLIM) for repeated measures was used to assess disease progression from longitudinal clinical and lung function data. For genetic analysis, SPSS 21.0 IBM Co software was used for all statistical analyses and two-tailed p-values <0.05 were considered significant.
Results
Screening by NGS
A total of seven individuals (COPD cases and smoker controls) were included in the screening study in two moments. Both groups were similar in age, gender and smoking habit. Three COPD patients had emphysema diagnosed by CT scan. The main clinical characteristics of these individuals are shown in Supplemental Table 1.
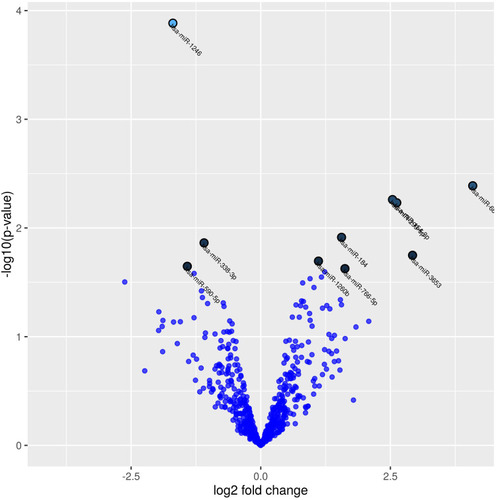
One hundred and ninety-eight miRNAs (≥10TPM) were identified by Next Generation Sequencing (NGS) in the 7 patients (14 samples analyzed) (). Between these, hsa-miR-184 was found differentially expressed between cases and controls at baseline (fold change=1.89, p=0.004) although it was discarded for further analysis as FDR>0.05 (). Interestingly, hsa-miR-1246 was found significantly downregulated in COPD patients after 10 years when compared to baseline (fold change=1.7; p<0.0001; FDR=0.05) (). miR-1246 expression level in the control smoker group after 10-year follow-up was similar to the one observed at baseline (p=0.704). Comparison between smokers without COPD at baseline and after the follow-up resulted in hsa-miR-203a downregulated with a log fold change of 1.89 (p=0.0012), but FDR>0.05 so discarded for further analysis.
Table 1 Main Dysregulated miRNAs in Serum from Patients with COPD vs. Smokers Without the Disease at Baseline
Figure 1 Heat Map showing the expression profiling of circulating miRNAs in patients with COPD and control smokers without COPD. miRNA assay by NGS was performed in 14 serum samples (7 corresponds to baseline (named COPD-1 to 4_bas and Controls-1 to 3_bas) and 7 to the sample individuals after 10 years of follow-up (named COPD-1 to 4_10y and Controls-1 to 3_10y)). Red represents a high level of gene expression while green represents a low level of expression.
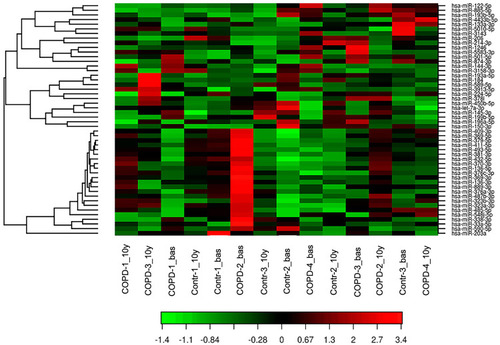
Figure 2 Volcano plot showing differentially expressed miRNAs in terms of the fold change in normalized expression in COPD patients at baseline compared to COPD patients after 10 years follow-up. The 10 microRNAs with the lowest p-values are marked with names on the plot (p-values <0.02 and log fold change (LogFC) >1).
Validation Study
shows the main clinical characteristics of the 30 individuals included in the validation study. Patients with COPD and smoker controls were similar in age, gender and body mass index (BMI). Although COPD cases had a higher number of pack-years smoked, this was not significant and neither existed differences between individuals who continued smoking in both groups. Patients with COPD presented worse lung function and walked less. More than a half of the cases presented emphysema diagnosed by CT scan.
Table 2 Baseline Clinical and Lung Function Characteristics of the COPD Patients and Control Smokers Included in the Validation Study
Cross Sectional
In the validation analysis, we did not find any significant difference between the expression of miR-1246 and the groups of patients with COPD and control smokers at baseline (Supplemental Figure 1). When we investigate the relation between the clinical and pulmonary function parameters or the presence of emphysema with differential expression of this miRNA in COPD patients, no significant differences were found between groups. When we compared the expression of miR-1246 in COPD with emphysema vs. COPD without emphysema at baseline, we did not find any difference (p=0.817).
Longitudinal Analysis
In addition, in the longitudinal analysis, miR-1246 was found to be less expressed although no significant, in patients with COPD after 10 years of follow-up when compared to baseline (fold change=1.73). The smokers without COPD did not show any differential expression for this miRNA during the monitoring period. Clinical and lung function differences achieved in COPD patients during the monitoring time are shown in , as well as for controls in Supplemental Table 1. Interestingly, when we analyzed only patients with emphysema, this miRNA was significantly less expressed (p= 0.019) in this group after the 10-year follow-up period in contrast to baseline (). However, no association was found between lung function parameters (FEV1, PaO2, IC/TLC) including the diffusion capacity (DLCO), a subrogate marker of emphysema, and miR-1246 when evaluating emphysema progression during monitoring follow-up.
Table 3 Baseline and 10-Years Follow-Up Clinical and Lung Function Characteristics of the COPD Patients Included in the Validation Study
In silico Analysis
Our functional analysis, through GO enrichment of the DEGs on the biological process (BP), molecular function (MF) and cellular component (CC) categories and the KEGG enrichment pathways revealed miR-1246 to be significantly associated with target genes enriched in several functions and signaling pathways. Between them, are of particular interest, the vascular smooth muscle signaling pathway, the MAPK signaling cascade, the chemokine signaling pathway and the Wnt cascade (), all reported to be involved in COPD/emphysema development.
Table 4 The Enriched KEGG Pathway of Predicted Genes
Discussion
The main finding of this study is the significant downregulation of miR-1246 observed in COPD patients with emphysema after a follow-up of 10 years. GO ontology and KEGG enrichment analysis revealed miR-1246 to be associated with target genes in several pathways involved in COPD/emphysema development.
Altered miRNAs expression was reported in several studies performed on different samples as sputum, serum and lung tissue of patients with COPD when compared to smokers without the disease.Citation10,Citation12,Citation17,Citation36,Citation37 Dysregulated miRNAs were observed in relation to COPD, and in relation clinical severityCitation18 and to increase the risk of acute exacerbations in stable patients with COPD.Citation37
Other authors investigated potential miRNAs to be implicated in the destruction of the emphysematous lung in COPD. Christenson et al found several miRNAs to be dysregulated in patients with severe emphysema and between them, demonstrated a role for miR-638 in promoting the maturity of emphysematous lung tissue and lung fibroblasts.Citation38 Savarimuthu et al found miR-34b and miR-34c to be less significantly expressed in lung tissue of patients with moderate emphysema when compared to patients with mild forms of the disease, suggesting this miRNA to modulate SERPINE gene expression.Citation39 Another study on lung tissue from patients with COPD showed increased expression of miR-15b in areas of emphysema as well as fibrosis.Citation12 A recent study that analyzed dysregulated miRNAs in PBMCs found miR-335 to be downregulated in patients with severe emphysema with PiZZ AATD when compared to mild ones suggesting a role for this miRNA in the activation of pathways related to inflammation and angiogenesis.Citation40 miR-1246 expression has also been reported to be altered in relation to several cancer: breast cancerCitation41 hepatocellular carcinoma,Citation42 pancreatic cancer,Citation43 and lung cancer developmentCitation44,Citation45 and therapeutics.Citation46
In our study a diminished expression of miRNA-1246 was observed particularly in COPD patients with emphysema after 10 years. Although this novel relationship, our longitudinal analysis over the follow-up period did not find an association between the progression of emphysema monitored by the diffusion capacity (DLCO). Further studies in a larger cohort that may include a longitudinal evaluation of emphysema by CT scan could contribute to understand the possible role of this miRNA in this type of patients.
To our knowledge, a prognostic value of miRNAs that may be involved in a worse progression of COPD has not been investigated by previous studies. Furthermore, there are no previous longitudinal studies exploring deregulated miRNAs in relation to the progression of COPD. Our research expanded over the past ones in studying miRNA expression dysregulation in patients suffering COPD and smoker controls during 10 years of follow-up.
Our functional in silico analysis suggests miR-1246 to be linked to genes mediators in various signaling pathways that may be related to emphysema. Our results showed mir-1246 to target genes like AKT1 in the insulin growth factor (IGF) pathway. A recent study by Cottage et al (2019) reveals that levels of pro-growth mediators IGF1 and Akt are diminished in COPD lung compared with normal subjects, suggesting that a defective IGF1 pathway may mediate in part, the compromised tissue regeneration seen in COPD lungs.Citation47 The age-related loss of the replicative and regenerative signaling is known to be associated with the diminution of the insulin/insulin like growth factor (IGF1) pathway. The IGF1 pathway is involved in various mechanisms of the cell cycle, including proliferation, survival, and differentiation through downstream Akt activity.Citation48 In this sense miR-1246 may have interesting role in this pathway that is to be unravel in relation to emphysema and COPD.
It was proposed that imbalance between protease and antiprotease process that results on emphysema could also have direct implication on chemokines degradation. The CXCL12-CXCR4 axis in chemokine-mediated signaling pathway has been the focus target of many strategies based on its neutralizationCitation49 and targeting the CXCL12-CXCR4 axis may be promising for COPD treatment. We found miR-1246 to be associated with CXCL12 in this pathway. Barbiwska et al (2018) demonstrated that in a mouse model of cigarette smoke exposure, the intermittent administration of plerixafor (FDA-approved CXCR4 antagonist) decreases emphysema damages, without affecting CXCL12 level and inflammation in BALF.Citation50
Our predictive analysis also suggested a link between miR-1246 and genes in the Wnt signaling pathway. This cascade has been deeply studied and its known to serve as a critical regulator in lung development and also in physiological and pathophysiological processes of lung in adulthood.Citation51 A recent research by Yang et al (2019) demonstrated in an in vitro study that miR-1246 regulates Wnt/β-catenin pathway through targeting GSK-3β/β-catenin.Citation52
This study has several limitations: firstly, we used a small number of participants in the screening phase to identify key miRNAs to be later validated in the total sample. Although, this is a common limitation inherent to this type of analysis due to its complexity and economic cost. Second, we did not study the expression of miR-1246a in lung tissue. However, circulating miRNAs has demonstrated to be feasible biomarkers in the diagnosis of several diseases. For example, miRNA profiles in some biological fluids have been shown to adequately reflect tumor characteristics, without the need for tissue biopsies.Citation53 Third, the lack of longitudinal evaluation of emphysema by CT scan. However, we used instead of the annuals records of DLCO, a recognized subrogate marker of emphysema.
In conclusion, our longitudinal observational study on COPD showed a novel link with hsa-miR-1246 consistent with a differential downregulated expression pattern in COPD patients with emphysema after 10 years of follow-up. Functional analyses are guaranteed to elucidate its role in emphysema progression in COPD.
Abbreviations
COPD, chronic obstructive pulmonary disease; miRNA, microRNA; hsa-miRNA, homo sapiens microRNA; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; BMI, body mass index; PaO2, partial oxygen tension; DLCO, diffusion capacity for carbon monoxide; IC/TLC, inspiratory capacity to total lung capacity ratio; 6MWD, six-minute walking distance test; CT scan, computed tomography scanner; NGS, next generation sequencing; TPM, tags per million; FDR, false discovery rate; qPCR, quantitative real time polymerase chain reaction; GLIM, general linear modelling for repeated measures test.
Data Sharing Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as Supplemental Table 1 and Supplemental Figure 1.
Ethics Approval
Ethical approval was obtained from Hospital Universitario La Candelaria, Tenerife, Spain (PI 55/17).
Patient Consent
Written informed consent was obtained from all participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Sara Cazorla-Rivero, Glorian Mura-Escorche, Francisca Gonzalvo-Hernández, Delia Mayato, and Elizabeth Córdoba-Lanús, declare not to have any financial or personal conflict of interests. Ciro Casanova Macario reports grants from Astra-Zeneca, GSK, and Menarini, advisory board for Astra-Zeneca and GSK, financial support for congress from Boehringer, Novartis and Menarini, personal fees from Bial, and lectures for Novartis, outside the submitted work; and declares to have received lectures and/or scientific advice from Laboratorios Bial, Boehringer-Ingelheim, AstraZeneca, GSK, Esteve, Menarini, Novartis and Rovi in the last three years. The authors report no other potential conflicts of interest for this work.
Acknowledgments
We will like to thank Hilaria González Acosta for her excellent technical assistance and the Instituto Universitario de Enfermedades Tropicales y Salud Pública de Canarias (IUETSPC) for the use of laboratory equipment. Elizabeth Córdoba-Lanús is supported by Cabildo de Tenerife (Agustín de Betancourt programme).
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2018 report). Available from: https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdf. Accessed 1128, 2017.
- John-SchusterG, GünterS, HagerK, et al. Inflammaging increases susceptibility to cigarette smoke-induced COPD. Oncotarget. 2016;7(21):30068–30083. doi:10.18632/oncotarget.402726284585
- LeeJ, SandfordA, ManP, SinDD. Is the aging process accelerated in chronic obstructive pulmonary disease? Curr Opin Pulm Med. 2011;17(2):90–97. doi:10.1097/MCP.0b013e328341cead21365793
- SethiS, MahlerDA, MarcusP, et al. Inflammation in COPD: implications for management. Am J Med. 2012;125(12):1162–1170. doi:10.1016/j.amjmed.2012.06.02423164484
- WuL, FanJ, BelascoJG. MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci U S A. 2006;103(11):4034–4039. doi:10.1073/pnas.051092810316495412
- MeisterG. miRNAs get an early start on translational silencing. Cell. 2007;131(1):25–28. doi:10.1016/j.cell.2007.09.02117923084
- CroceCM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10(10):704–714. doi:10.1038/nrg263419763153
- DaiR, AhmedSA. MicroRNA, a new paradigm for understanding immunoregulation, inflammation, and autoimmune diseases. Transl Res. 2011;157(4):163–179. doi:10.1016/j.trsl.2011.01.00721420027
- RupaniH, Sanchez-ElsnerT, HowarthP. MicroRNAs and respiratory diseases. Eur Respir J. 2013;41(3):695–705. doi:10.1183/09031936.0021201122790917
- Van PottelbergeGR, MestdaghP, BrackeKR, et al. MicroRNA expression in induced sputum of smokers and patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183(7):898–906. doi:10.1164/rccm.201002-0304OC21037022
- SchembriF, SridharS, PerdomoC, et al. MicroRNAs as modulators of smoking-induced gene expression changes in human airway epithelium. Proc Natl Acad Sci USA. 2009;106(7):2319–2324. doi:10.1073/pnas.080638310619168627
- EzzieME, CrawfordM, ChoJH, et al. Gene expression networks in COPD: microRNA and mRNA regulation. Thorax. 2012;67:122–131. doi:10.1136/thoraxjnl-2011-20008921940491
- AkbasF, CoskunpinarE, AynaciE, et al. Analysis of serum micro-RNAs as potential biomarker in chronic obstructive pulmonary disease. Exp Lung Res. 2012;38(6):286–294. doi:10.3109/01902148.2012.68908822686440
- SoedaS, OhyashikiJH, OhtsukiK, et al. Clinical relevance of plasma miR-106b levels in patients with chronic obstructive pulmonary disease. Int J Mol Med. 2013;31(3):533–539. doi:10.3892/ijmm.2013.125123338559
- GraffJ, PowersL, DicksonA, et al. Cigarette smoking decreases global microRNA expression in human alveolar macrophages. PLoS One. 2012;7(8):e44066. doi:10.1371/journal.pone.004406622952876
- BanerjeeA, LuettichK. MicroRNAs as potential biomarkers of smoking-related diseases. Biomark Med. 2012;6(5):671–684. doi:10.2217/bmm.12.5023075247
- XieL, WuM, LinH, et al. An increased ratio of serum miR-21 to miR-181a levels is associated with the early pathogenic process of chronic obstructive pulmonary disease in asymptomatic heavy smokers. Mol Biosyst. 2014;10(5):1072–1081. doi:10.1039/C3MB70564A24556821
- SatoT, LiuX, NelsonA, et al. Reduced miR-146a increases prostaglandin E 2 in chronic obstructive pulmonary disease fibroblasts. Am J Respir Crit Care Med. 2010;182(8):1020–1029. doi:10.1164/rccm.201001-0055OC20522791
- CelliBR, CoteC, MarinJM, et al. The body mass index, airflow obstruction, dyspnea, exercise performance (BODE) index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi:10.1056/NEJMoa02132214999112
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test [published correction appears in Am J Respir Crit Care Med. 2016 May 15;193(10):1185]. Am J Respir Crit Care Med. 2002;166(1):111–117.12091180
- American Thoracic Society Statement. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144(5):1202–1218. doi:10.1164/ajrccm/144.5.12021952453
- MacintyreN, CrapoRO, ViegiG, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720–735. doi:10.1183/09031936.05.0003490516204605
- MahlerDA, WellsCK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. doi:10.1378/chest.93.3.5803342669
- CharlsonM, SzatrowskiTP, PetersonJ, GoldJ. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi:10.1016/0895-4356(94)90129-57722560
- LynchDA, AustinJH, HoggJC, et al. CT-definable subtypes of chronic obstructive pulmonary disease: a statement of the fleischner society. Radiology. 2015;277(1):192–205. doi:10.1148/radiol.201514157925961632
- KozomaraA, BirgaoanuM, Griffiths-JonesS. miRBase: from microRNA sequences to function. Nucleic Acids Res. 2019;47(D1):D155–D162. doi:10.1093/nar/gky114130423142
- AndersenCL, JensenJL, ØrntoftTF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64(15):5245–5250. doi:10.1158/0008-5472.CAN-04-049615289330
- LivakKJ, SchmittgenTD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402‐408. doi:10.1006/meth.2001.1262
- AgarwalV, BellGW, NamJW, BartelDP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4:e05005. doi:10.7554/eLife.05005
- ParaskevopoulouMD, GeorgakilasG, KostoulasN, et al. DIANA-microT web server v5.0: service integration into miRNA functional analysis workflows. Nucleic Acids Res. 2013;41(Web Server issue):W169–W173. doi:10.1093/nar/gkt39323680784
- RaudvereU, KolbergL, KuzminI, et al. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019;47(W1):W191–W198. doi:10.1093/nar/gkz36931066453
- AshburnerM, BallCA, BlakeJA, et al. Gene ontology: tool for the unification of biology. The gene ontology consortium. Nat Genet. 2000;25(1):25‐29. doi:10.1038/75556
- MiH, MuruganujanA, EbertD, et al. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019;47(D1):D419–D426. doi:10.1093/nar/gky103830407594
- HuberW, CareyV, GentlemanR, et al. Orchestrating high-throughput genomic analysis with bioconductor. Nat Methods. 2015;12(2):115–121. doi:10.1038/nmeth.325225633503
- StoreyJD, TibshiraniR. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100(16):9440–9445. doi:10.1073/pnas.153050910012883005
- DangX, QuX, WangW, et al. Bioinformatic analysis of microRNA and mRNA regulation in peripheral blood mononuclear cells of patients with chronic obstructive pulmonary disease. Respir Res. 2017;18(1):4. doi:10.1186/s12931-016-0486-528057018
- ChenB-B, LiZ-H, GaoS. Circulating miR-146a/b correlates with inflammatory cytokines in COPD and could predict the risk of acute exacerbation COPD. Medicine (Baltimore). 2018;97(7):e9820. doi:10.1097/MD.000000000000982029443743
- ChristensonSA, BrandsmaCA, CampbellJD, et al. miR-638 regulates gene expression networks associated with emphysematous lung destruction. Genome Med. 2013;5:114. doi:10.1186/gm51924380442
- Savarimuthu FrancisSM, DavidsonMR, TanME, et al. MicroRNA-34c is associated with emphysema severity and modulates SERPINE1 expression. BMC Genomics. 2014;15(1):88. doi:10.1186/1471-2164-15-8824479666
- EsquinasC, JanciauskieneS, GonzaloR, et al. Gene and miRNA expression profiles in PBMCs from patients with severe and mild emphysema and PiZZ alpha1-antitrypsin deficiency. Int J Chron Obstruct Pulmon Dis. 2017;12:3381–3390. doi:10.2147/COPD.S14544529238183
- DvingeH, GitA, GräfS, et al. The shaping and functional consequences of the microRNA landscape in breast cancer. Nature. 2013;497(7449):378–382. doi:10.1038/nature1210823644459
- MoshiriF, SalviA, GramantieriL, et al. Circulating miR-106b-3p, miR-101-3p and miR-1246 as diagnostic biomarkers of hepatocellular carcinoma. Oncotarget. 2018;9(20):15350–15364. doi:10.18632/oncotarget.2460129632649
- XuY-F, HannafonBN, ZhaoYD, et al. Plasma exosome miR-196a and miR-1246 are potential indicators of localized pancreatic cancer. Oncotarget. 2017;8(44):77028–77040. doi:10.18632/oncotarget.2033229100367
- ZhangWC, ChinTM, YangH, et al. Tumour-initiating cell-specific miR-1246 and miR-1290 expression converge to promote non-small cell lung cancer progression. Nat Commun. 2016;7(1):11702. doi:10.1038/ncomms1170227325363
- KimG, AnH-J, LeeM-J, et al. Hsa-miR-1246 and hsa-miR-1290 are associated with stemness and invasiveness of non-small cell lung cancer. Lung Cancer. 2016;91:15–22. doi:10.1016/j.lungcan.2015.11.01326711929
- YuanD, XuJ, WangJ, et al. Extracellular miR-1246 promotes lung cancer cell proliferation and enhances radioresistance by directly targeting DR5. Oncotarget. 2016;7(22):32707–32722. doi:10.18632/oncotarget.901727129166
- BottA, ErdemN, LerrerS, et al. miRNA-1246 induces pro-inflammatory responses in mesenchymal stem/stromal cells by regulating PKA and PP2A. Oncotarget. 2017;8(27):43897–43914. doi:10.18632/oncotarget.1491528159925
- TranD, BergholzJ, ZhangH, et al. Insulin-like growth factor-1 regulates the SIRT 1-p53 pathway in cellular senescence. Aging Cell. 2014;13(4):669–678. doi:10.1111/acel.1221925070626
- HenrotP, PrevelR, BergerP, et al. Chemokines in COPD: from implication to therapeutic use. Int J Mol Sci. 2019;20(11):2785. doi:10.3390/ijms20112785
- BarwinskaD, OueiniH, PoirierC, et al. AMD3100 ameliorates cigarette smoke-induced emphysema-like manifestations in mice. Am J Physiol Lung Cell Mol Physiol. 2018;315(3):L382–L386. doi:10.1152/ajplung.00185.201829745251
- Skronska-WasekW, GosensR, KonigshoffM, et al. WNT receptor signalling in lung physiology and pathology. Pharmacol Ther. 2018;187:150–166. doi:10.1016/j.pharmthera.2018.02.00929458107
- YangF, XiongH, DuanL, et al. MiR-1246 promotes metastasis and invasion of A549 cells by targeting GSK-3β‒mediated Wnt/β-catenin pathway. Cancer Res Treat. 2019;51(4):1420–1429. doi:10.4143/crt.2018.63830913872
- PassigliaF, BronteG, CastigliaM, et al. Prognostic and predictive biomarkers for targeted therapy in NSCLC: for whom the bell tolls? Expert Opin Biol Ther. 2015;15(11):1553–1566. doi:10.1517/14712598.2015.107134826360115