Abstract
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive disease with deteriorating cardiopulmonary function that decreases the health-related quality of life (HRQL) and exercise capacity. Patients with COPD often have cardiovascular and muscular problems that hinder oxygen uptake by peripheral tissues, resulting in poor oxygen consumption efficiency. It is important to develop new physiological parameters to evaluate oxygen consumption efficiency during activities and to evaluate its association with exercise capacity and HRQL. Work efficiency (WE) measures oxygen consumption efficiency during exercise. We hypothesize that patients with poor WE should have exercise intolerance and poor HRQL. Therefore, we aimed to evaluate the association between WE and exercise capacity, HRQL and other cardiopulmonary parameters.
Patients and Methods
Seventy-eight patients with COPD were evaluated with spirometry, cardiopulmonary exercise testing, and assessment of dyspnea score and HRQL (using the St. George’s Respiratory Questionnaire [SGRQ]). Cardiopulmonary exercise testing was performed using a cycle ergometer with an incremental protocol and exhaled breath analysis to assess oxygen consumption. WE was defined as the relationship between oxygen consumption and workload.
Results
There were 31 patients with normal WE (group I) and 47 patients (group II) with poor WE. Patients with poor WE had lower exercise capacity (maximal oxygen consumption, group I vs II as 1050±53 vs 845 ±34 mL/min, p=0.0011), poorer HRQL (SGRQ score 41.1±3.0 vs 55±2.2, p=0.0002), higher exertional dyspnea score (5.1±0.2 vs 6.1±0.2, p= 0.0034) and early anaerobic metabolism during exercise (anaerobic threshold, 672±27 vs 583 ±18 mL/min, p=0.0052).
Conclusion
WE is associated with exercise capacity and HRQL. Here, patients with poor WE also had exercise intolerance, poorer HRQL, and more exertional dyspnea.
Introduction
Chronic obstructive pulmonary disease (COPD) is an inexorable progressive disease with worsening cardiopulmonary function that decreases the endurance to perform activities of daily living.Citation1,Citation2 According to the World Health Organization (WHO), 64 million people have COPD and 3 million people have died of COPD worldwide.Citation3 In addition, the WHO has predicted that COPD will become the third leading cause of death by 2030.Citation3 In a recent study, patients with COPD are at a higher risk of cardiovascular mortality.Citation4 COPD is characterized by dyspnea, poor health-related quality of life (HRQL), and insufficient cardiorespiratory endurance during exercise.Citation5 Even in patients with mild COPD, exercise performance is compromised, and exercise intolerance is also common.Citation5 Impaired exercise capacity and insufficient cardiorespiratory endurance are correlated with poor prognosis in patients with COPD.Citation5 Early recognition of patients with poor cardiorespiratory endurance may help physicians to provide early management to prevent cardiovascular complications.
Traditionally, spirometry is recommended by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) for the diagnosis of COPD.Citation6,Citation7 However, the parameters of spirometry, such as forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1) is a poor predictor of functional performance, cardiorespiratory endurance, and HRQL in patients with COPDCitation5,Citation8 Although, the 6 minutes walking test or HRQL questionnaires are good tools to evaluate functional performance of patients, these parameters cannot reflect the physiological response to exercise and we cannot define the causes of poor functional performance by the 6 minutes walking distance or HRQL scores. Therefore, it is necessary to develop new parameters to evaluate exercise performance and cardiorespiratory response in patients with COPD. The cardiopulmonary exercise test (CPET) is a good tool for evaluating exercise capacity, respiratory function, and circulatory function.Citation9 The physiological data from CPET can help physicians better understand the patient’s cardiorespiratory responses to exercise.Citation10
Work efficiency (WE) measures the relationship between changes in oxygen consumption (VO2) to workload (WR).Citation9 WE measures oxygen kinetics and is an index of overall oxygen consumption efficiency during exercise. However, studies about the role of WE in patients with COPD are quite limited. Since WE is an index of overall oxygen consumption efficiency, we hypothesized that patients with poor WE should have poor exercise capacity and HRQL. Therefore, this study aimed to comprehensively evaluate the relationship between WE and exercise capacity, HRQL, respiratory, and circulatory parameters in patients with COPD.
Patients and Methods
Patient Recruitment
There were 78 patients with COPD with exertional dyspnea (modified Medical Research Council, mMRC≧2) who were recruited from the outpatient clinic of the Pulmonology Department from August 2018 until June 2020.Citation11 According to WE obtained from CPET, patients were divided into two groups: group I had normal WE, and group II had poor WE.
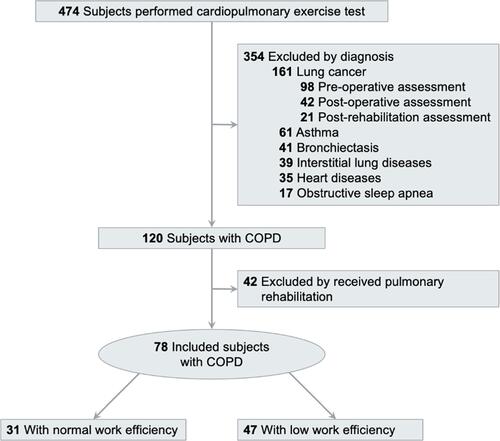
The inclusion criteria were as follows: (1) COPD diagnosed based on the GOLD criteria with FEV1/FVC <70%; (2) patients were stable without acute exacerbations in the last 3 months, and (3) patients could move independently and were willing to perform the exercise test. We excluded (1) patients receiving pulmonary rehabilitation in the past year; (2) patients who suffered from acute exacerbation in the last 3 months; (3) patients with severe musculoskeletal diseases that rendered them unable to perform exercise tests; (4) patients with a history of other lung diseases, such as asthma, lung cancer, pneumoconiosis, bronchiectasis, tuberculosis, and interstitial lung disease; (5) patients with a history of cardiovascular diseases such as congestive heart failure, myocardial infarction, primary pulmonary hypertension, pulmonary embolism, and (6) patients who were unwilling to participate exercise tests. The study flow chart is shown in .
The study was approved by the Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation Institutional Review Board (Protocol Number: 08-XD-035) in accordance with the Declaration of Helsinki. All patients signed informed consent forms.
Pulmonary Function Test
Patients performed pulmonary function tests using a spirometer (Medical Graphics Corporation; St Paul, MN, USA), in accordance with the standards of the American Thoracic Society.Citation12 Patients were requested to take a maximal inspiration and then to forcefully expel air as much as possible. The grade of airflow obstruction was assessed using FEV1% according to the GOLD guidelines.Citation11
Cardiopulmonary Exercise Test
All patients underwent the CPET with an incremental, symptom-limited protocol using a cycle ergometer (Lode Corival, the Netherlands). The expired air was analyzed breath by breath (Breeze suite 6.1, Medical Graphics Corporation; St Paul, MN, USA) to evaluate oxygen consumption (VO2), carbon dioxide output (VCO2), tidal volume (VT), and end tidal PCO2 (PETCO2). Oxygen saturation was measured using a pulse oximeter (SpO2). The respiratory rate (RR), electrocardiogram, heart rate (HR), and blood pressure (BP) were continuously monitored.
The patients underwent incremental CPET with an individualized ramp protocol, and the load was set up to finish between the 10th and 12th minutes of exertion.Citation13 Patients underwent a 2-minute warm-up phase (unloaded cycling), following which the WR was increased constantly in increments of 10, 15, or 20 watt/min, depending on the patient’s estimated subjective functional capacity.Citation13 During the exercise test, patients were requested to maintain a cycling frequency of approximately 60 revolutions per minute. Patients were requested to perform maximal exercise to evaluate their highest possible performance. The maximum effort was attained when patients showed signs of intense exertion such as sweating, unsteady biking, severe dyspnea, muscle fatigue (reluctance to continue exercising despite strong encouragement), a heart rate (HR) of >180 beats/min, or respiratory exchange rate (RER) >1.09 during exercise.Citation13 The maximum VO2 (VO2max) was calculated by the mean values of VO2 over the last 30 seconds of the exercise test.
WE, the relationship between VO2 and WR during exercise,Citation13 was defined as the VO2/WR slope during exercise. The slope of the VO2/WR relationship was determined using linear regression analysis. The normal range for WE is 8.6–10.1 mL/min/watt. A WE of <8.6 mL/min/watt is indicative of poor WE.Citation9 The VO2 at anaerobic threshold (AT) was identified by the VCO2 versus VO2 plot.Citation14 An AT of <40% of the predicted VO2max is indicative of low AT%.Citation9 The oxygen pulse was defined as VO2 divided by HR (O2P = VO2/HR) at maximal exercise. A O2P of <80% of the predicted value is indicative of low O2P%.Citation9
Respiratory Muscle Strength
The respiratory muscle strength, maximal inspiratory pressure (MIP), and maximal expiratory pressure (MEP) were measured using a pressure gauge (Respiratory Pressure Meter, Micro Medical Corp, England). MIP and MEP were measured five times, and the highest value was used as the final value.Citation15 For the MIP, patients were asked to exhale to the residual volume and then perform a rapid maximal inspiratory effort, sustained for 1–2 seconds. For MEP, patients were asked to inspire to the total lung capacity and then perform a rapid and maximal expiratory effort, sustained for 1–2 seconds.
HRQL
HRQL was assessed using a respiratory disease-specific questionnaire. The validated Chinese version of the St. George’s Respiratory Questionnaire (SGRQ), which had four components (total, symptom, activity, and impact), was used.Citation16 SGRQ scores ranged from 100 (worst) to 0 (best).Citation16
Dyspnea Score
The dyspnea score was evaluated using a Borg scale with 10-point scores; higher scores indicated more dyspnea.Citation17 During CPET, dyspnea scores were evaluated at rest and during exercise.
Statistical Analysis
All parameters are expressed as mean ± standard deviation (SD). An independent sample t-test was used to compare parameters between the two groups. We used the Chi-square test to compare sex, stages of COPD, and smoking status between group I and group II. Correlation analyses were performed to evaluate the correlation between WE, predicted FEV1%, predicted FVC%, VO2max, WR, O2P%, AT%, dyspnea score, and HRQL. The strength of the correlation was determined according to Evan’s classification.Citation18 All statistical analyses were performed using SPSS, version 24.0 (SPSS, Inc., Chicago, IL, USA).
Results
Baseline Characteristics
Of the 78 patients with COPD, 31 patients had normal WE (group I) and 47 had poor WE (group II). The clinical characteristics of the patients are shown in . The mean age was 67.6 ± 9.9 years and 71.5.9 ± 7.9 years in groups I and II, respectively (p > 0.05). The mean body mass index was 23.1 ± 3.2 and 22.6 ± 4.0 kg/m2 in groups I and II, respectively (p > 0.05). The mean FEV1% was 49.4 ± 14.2% and 47.8 ± 17.4% in groups I and II, respectively (p > 0.05). Ninety-four percent of patients in group I and 96% of patients in group II had moderate to very severe airflow obstruction (GOLD stages II to IV), and the severity of COPD was not significantly different between the two groups (p> 0.05). However, Group II had more current smokers and ex-smokers than group I (p < 0.05).
Table 1 Baseline Characteristics
Respiratory Parameters of Patients with Normal and Poor WE ()
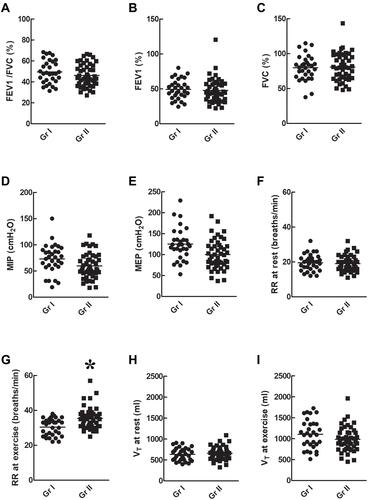
There was no significant difference in the respiratory parameters such as FEV1/FVC% (), FEV1% (), FVC% (), MIP (), and MEP () between groups I and II (p > 0.05). The RR at rest () was not significantly different between groups I and II (p > 0.05) but the RR () during exercise was higher in group II than in group I (p < 0.05). The VT at rest () or during exercise () was not significantly different between groups I and II (p > 0.05).
Figure 2 Respiratory parameters. There was no significant difference in respiratory parameters such as FEV1/FVC% (A), FEV1% (B), FVC% (C), MIP (D), MEP (E), RR at rest (F), and VT at rest (H) or during exercise (I) between group I and group II (p > 0.05). However, the RR during exercise (G) was higher in group II than in group I (p < 0.05). *p < 0.05 as comparison between groups I and II.
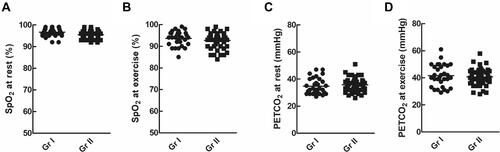
Circulatory Parameters of Patients with Normal and Poor WE ()
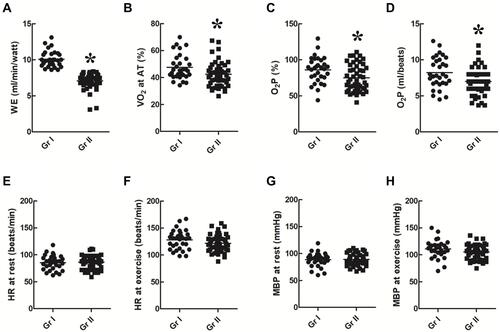
WE (), VO2max () or AT% (), and O2P% () were higher in group I than in group II (p < 0.05). There was no significant difference between HR at rest () or during exercise () and MBP at rest () or during exercise () between the two groups (p > 0.05).
Figure 3 Circulatory parameters. WE (A), VO2 at maximal exercise (B) or AT (C), and O2P (D) were higher in group I than in group II (p < 0.05). There was no significant difference in HR at rest (E) or during exercise (F) and in MBP at rest (G) or during exercise (H) between the two groups (p > 0.05). *p<0.05 as comparison between group I and II.
Gas Exchanges of Patients with Normal and Poor WE ()
There was no significant difference between SpO2 at rest () or during exercise () and PETCO2 at rest () or during exercise () between the two groups (p > 0.05)
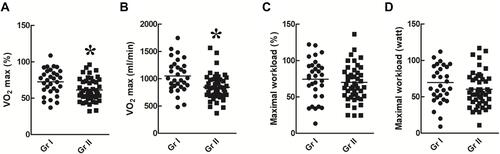
Exercise Capacity of Patients with Normal and Poor WE ()
VO2max% () and VO2max () were significantly higher in group I than in group II (p < 0.05). However, there was no significant difference in maximal WR% () and maximal WR () between the two groups (p > 0.05).
Figure 5 Exercise capacity. Maximal VO2% (A) and maximal VO2 (B) were higher in group I than in group II (p < 0.05). However, there was no significant difference in the maximal workload% (C) and maximal workload (D) between the two groups (p > 0.05). *p<0.05 as comparison between group I and II.
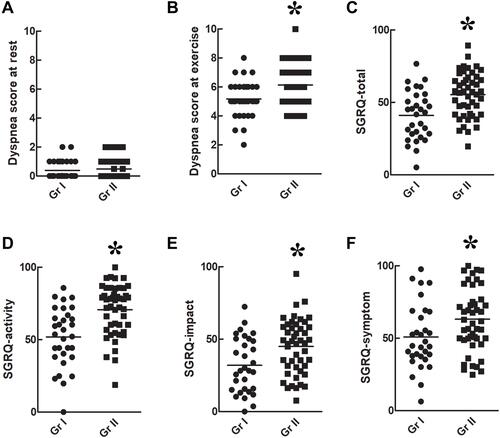
Dyspnea Score and HRQL ()
Dyspnea scores at rest () were similar between the two groups (p > 0.05), but dyspnea scores during maximal exercise () were significantly higher in group II than in group I (p < 0.05). SGRQ-total (), -activity (), -impact (), and -symptom () scores were significantly higher in group II than in group I (p < 0.05).
Figure 6 Dyspnea score and health-related quality of life. Dyspnea scores at rest (A) were similar between the two groups (p > 0.05), but dyspnea scores at maximal exercise (B) were significantly higher in group II than that in group I (p < 0.05). The SGRQ-total (C), -activity (D), -impact (E), and -symptom (F) scores were significantly poorer in group II than that in group I (p<0.05). *p<0.05 as comparison between group I and II.
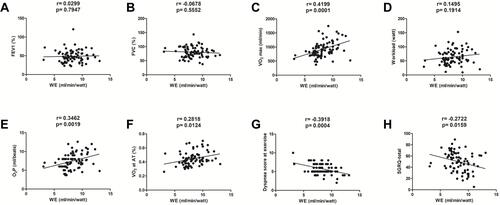
Correlation Between WE and Lung Function, Exercise Capacity, VO2 at the AT, O2P, Dyspnea Score, and HRQL ()
Comparison of important parameters such as lung function, exercise capacity, AT, HRQL and correlations between WE and these parameters are shown in . The patients of poor WE had lower exercise capacity, earlier anerobic threshold, poorer HRQL and more exertional dyspnea during exercise (all p<0.05). There was no significant correlation between WE and either FEV1% (, p> 0.05, no correlation) or FVC% (, p > 0.05, no correlation). A significant correlation was found between WE and VO2max (r = 0.4199, p = 0.0001, moderate correlation, ), but not between WE and WRmax (p > 0.05, no correlation, ). There were significant correlations between WE and O2P (r = 0.3462, p = 0.0019, weak correlation, ), VO2 at the AT (r = 0.2818, p = 0.0124, weak correlation, ), dyspnea score at exercise (r = 0.3918, p = 0.0004, weak correlation, ), and SGRQ-total score (r = 0.2722, p = 0.0159, weak correlation, ).
Table 2 Difference and Correlation Between WE and Variables
Figure 7 Correlation between WE and lung function, VO2 at maximal exercise and AT, O2P, dyspnea score, and HRQL. There was no significant correlation between WE and FEV1% (A) or FVC% (B) (both p > 0.05) between the two groups. A significant correlation was found between WE and VO2 (r = 0.4199, p = 0.001) (C), but not between WE and workload at maximal exercise (p > 0.05) (D). There were significant correlations between WE and O2P (r= 0.3462, p= 0.0019) (E), VO2 at the AT (r = 0.2818, p = 0.0124) (F), dyspnea score during exercise (r= 0.3918, p= 0.0004) (G), and SGRQ-total score (r = 0.2722, p = 0.0159) (H).
Discussion
There are some important findings in this study. Compared to patients with normal WE, those with poor WE had worsening HRQL, exercise intolerance, exertional dyspnea, and poor circulatory parameters. Correlation analysis revealed that WE was correlated with exercise capacity, dyspnea and HRQL. In addition, patients who were current smokers or ex-smokers had poor WE.
In patients, the efficiency of exercise depends on their respiratory (gas exchange), circulatory (oxygen delivery), musculoskeletal systems (extract oxygen to perform exercise), and metabolism by cellular mitochondria.Citation19 WE is a measurement from VO2 kinetics during exercise and is an index of overall oxygen consumption efficiency. The VO2 kinetics, the rate of oxygen consumption related to work rate, is considered as an indicator of cardiovascular efficiency and coupling O2 delivery to peripheral muscles. Uncoupling of this procedure results in the impaired ability to increase oxygen consumption. Lower WE therefore implies a poor exercise efficiency.Citation20
When the circulatory system and oxygen extraction match the demands of skeletal muscles during exercise, overall oxygen consumption efficiency is maintained. The increase in oxygen consumption is proportional to the incremental work during exercise.Citation13 Normally, the VO2/WR linearity corresponds to the increased watt, regardless of age and sex.Citation13,Citation20 However, when either oxygen delivery or oxygen extraction is impaired, the overall oxygen consumption during exercise is impaired, with presentation of a flattening or shallow downward shifting of VO2 at a given WR and WE is decreased.Citation19,Citation20 WE, therefore, reflects the capacity of aerobic metabolism during exercise.Citation20 The loss of the linear relationship between VO2 and WR indicates impaired oxygen consumption efficiency. Therefore, WE that indicates VO2 kinetics can help us assess oxygen consumption efficiency in patients with COPD.Citation20 WE can help physicians to assess the efficiency of cardiovascular oxygen delivery, and peripheral oxygen extraction.
When the metabolic demands begin to exceed oxygen delivery to the contracting muscles, anaerobic metabolism begins.Citation21 During an exercise test, the AT is the exercise level that reflects the metabolic condition of anaerobic glycolysis.Citation20,Citation21 Anaerobic glycolysis results in the production of lactic acid and metabolic acidosis-stimulating chemoreceptors, which lead to excessive ventilator responses.Citation20,Citation21 When exercise exceeds the AT, patients have high RR and minute ventilation. If the patient’s oxygen consumption efficiency is poor, early anaerobic metabolism occurs during exercise. In such patients, early anaerobic metabolism results in early lactic acidosis, which in turn leads to higher RR and ventilation during exercise. Higher RR and ventilation during exercise further results in more exertional dyspnea and exercise intolerance.Citation21 In our study, we found that patients with poor WE experienced earlier AT, higher RR, and more exertional dyspnea during exercise than patients with normal WE. Exertional dyspnea and exercise intolerance are associated with poor HRQL.Citation22 Our results further revealed that patients with poor WE had poorer HRQL, including SGRQ-symptom and SGRQ-activity scores.
Oxygen delivery is a major determinant of oxygen consumption efficiency. Oxygen delivery depends on a transport system, the cardiovascular system.Citation20 Inadequate oxygen delivery to the skeletal muscles leads to poor VO2 kinetics and anaerobic metabolism. Lower WE and early anaerobic metabolism occur in such conditions. Therefore, patients with cardiovascular dysfunction exhibit an attenuation of O2 transport and poor WE.Citation20 When oxygen delivery is impaired, VO2 fails to linearly increase with the increase in work rate, which in turn decreases WE.Citation20 O2P is often used as indicator of stroke volume.Citation14 In our study, WE was significantly correlated with O2P at maximal exercise.
Mitochondrial function is important for providing oxidative capacity in skeletal muscle during exercise.Citation23,Citation24 Therefore, mitochondrial function is critical for exercise efficiency.Citation23,Citation24 Many studies have shown that patients with COPD may have mitochondrial dysfunction, including a reduction in oxidative capacity, and enhanced reactive oxygen species (ROS) productionCitation23 Therefore, COPD is associated with chronic inflammation and oxidative stress.Citation23 It is important to assess mitochondrial function in COPD; however, muscle biopsies are needed for the determination of mitochondrial density, which is not always possible in the clinical setting. Patients with poor mitochondrial function with impaired oxidative capacity often present with poor WE.Citation24 Therefore, WE could be an indirect indicator of mitochondrial function. However, not all COPD patients suffer from mitochondrial dysfunction. The WE parameter obtained from CPET can help physicians identify patients who may have poor mitochondrial function.
Traditionally, WE is considered as a parameter of cardiovascular function that WE was considered to be reduced in cardiovascular disorders and normal in pulmonary diseases such as COPD.Citation25 However, in our study, we found a large number (47/78) of patients with COPD had poor WE. Mirdamadi et al also evaluated 37 patients with COPD by CPET and they also found that some patients with COPD had poor WE.Citation26 They showed a significant negative correlation between WE and the body-mass index, airflow obstruction, dyspnea, and exercise capacity (BODE) index, which is one parameter of COPD severity. Patients with more severe COPD had lower WE.Citation26 Another study assessing 58 patients attending CPET showed that WE was considered as one parameter of impaired peripheral oxygenation (IPO). COPD patients with IPO pattern showed poorer circulatory parameters and more dyspnea than those without IPO.Citation27
In our study, although there were significant correlations between WE and exercise capacity, exertional dyspnea score and HRQL, the correlation strength was only weak to moderate. The causes of exercise intolerance and poor HRQL in COPD are often multiple factors including impaired ventilatory efficiency, respiratory muscle dysfunction, peripheral skeletal muscle deconditioning, cardiovascular disorders, and anxiety.Citation28 As a result, it is rational that the correlation between WE and exercise capacity is weak to moderate. Therefore, when treating COPD patients with exercise intolerance and poor HRQL, these multiple factors should be taken into consideration and appropriate treatment must be provided. In this study, we further observed that poor efficiency of oxygen consumption is yet another factor associated with exercise intolerance and poor HRQL.
Clinical Implication
Exertional dyspnea, exercise intolerance, and physical inactivity are common in COPD and are strong predictors of reduced survival.Citation21 Aerobic fitness during exercise is important for patients with COPD. Overall oxygen consumption efficiency during exercise depends on oxygen delivery (cardiovascular system), oxygen extraction (skeletal muscle), and mitochondrial function.Citation13 Lower exercise efficiency causes patients to expend more energy for a given physical exertion and, therefore, hampers activities of daily living.Citation24 Clinically, echocardiography is often used to assess cardiac function. However, echocardiography at rest cannot assess the cardiovascular response during exercise. Mitochondrial function is important in COPD, but it is difficult to assess mitochondrial function clinically. CPET is a noninvasive exercise test that can objectively assess overall oxygen consumption efficiency by WE.Citation19 WE provides information about the circulatory function, tissue oxygen extraction and consumption during exercise.
Limitations of This Study
This study has some limitations. First, we suggested that WE plays a role in patients with COPD. Patients with poor WE had poor exercise capacity, HRQL and more exertional dyspnea. However, the prognostic value of WE for survival or acute exacerbation is unknown. Further studies are necessary to assess its prognostic value. Second, WE is a physiological parameter to assess overall oxygen consumption efficiency. It cannot specifically determine whether poor oxygen consumption efficiency is due to cardiovascular, muscular, or mitochondrial dysfunction. However, it is a good parameter to assess the overall oxygen consumption efficiency during exercise.Citation13 This is important to understanding exercise physiology in COPD. Third, there was no control group in the current study. WE is a parameter to assess the efficiency of oxygen consumption and is not influenced by age, sex or training status. We focused on the roles of WE in patients with COPD. We aimed to compare the exercise capacity, HRQL and other parameters in patients with COPD with normal or poor WE. We did not aim to compare WE between COPD patients and normal subjects. Therefore, there was no control group in this study.
Conclusions
WE is an index of overall efficiency of oxygen consumption during exercise and is associated with exercise capacity, exertional dyspnea, and HRQL. Patients with poor WE had exercise intolerance, poorer HRQL, higher respiratory rates, more exertional dyspnea and earlier anaerobic metabolism during exercise.
Acknowledgments
This study was supported by grants from the Taipei Tzu Chi Hospital and the Buddhist Tzu Chi Medical Foundation (TCRD-TPE-109-60 and TCMF-CP 109-02).
Disclosure
The authors have no financial or other potential conflicts of interest to disclose.
References
- Ko FWS, Sin DD. Twenty-five years of respirology: advances in COPD. Respirology. 2020;25(1):17–19. doi:10.1111/resp.13734
- Montagnani A, Mathieu G, Pomero F, et al. Hospitalization and mortality for acute exacerbation of chronic obstructive pulmonary disease (COPD): an Italian population-based study. Eur Rev Med Pharmacol Sci. 2020;24(12):6899–6907. doi:10.26355/eurrev_202006_21681
- World Health Organization. Chronic obstructive pulmonary disease (COPD). http://www.emro.who.int/health-topics/chronic-obstructive-pulmonary-disease-copd/index.html. Accessed January 26, 2021.
- Wang M, Lin EP, Huang LC, Li CY, Shyr Y, Lai CH. Mortality of cardiovascular events in patients with COPD and preceding hospitalization for acute exacerbation. Chest. 2020;158(3):973–985. doi:10.1016/j.chest.2020.02.046
- Punekar YS, Riley JH, Lloyd E, Driessen M, Singh SJ. Systematic review of the association between exercise tests and patient-reported outcomes in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;Volume 12(12):2487–2506. doi:10.2147/COPD.S100204
- López-Campos JL, Soler-Cataluña JJ, Miravitlles M. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2019 report: future challenges. Arch Bronconeumol. 2020;56(2):65–67. doi:10.1016/j.arbres.2019.06.001
- Vogelmeier CF, Criner GJ, Martínez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease. Informe 2017 de la iniciativa global para el diagnostico, tratamiento y prevencion de la enfermedad pulmonar obstructiva cronica: resumen ejecutivo de gold. Arch Bronconeumol. 2017;53(3):128–149. doi:10.1016/j.arbres.2017.02.001
- Johnson KM, Safari A, Tan WC, et al. Heterogeneity in the respiratory symptoms of patients with mild-to-moderate COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3983–3995. doi:10.2147/COPD.S184424
- Herdy AH, Ritt LE, Stein R, et al. Cardiopulmonary exercise test: background, applicability and interpretation. Arq Bras Cardiol. 2016;107(5):467–481. doi:10.5935/abc.20160171
- Hsieh MJ, Lan CC, Chen NH, et al. Effects of high-intensity exercise training in a pulmonary rehabilitation programme for patients with chronic obstructive pulmonary disease. Respirology. 2007;12(3):381–388. doi:10.1111/j.1440-1843.2007.01077.x
- Song JH, Lee CH, Um SJ, et al. Clinical impacts of the classification by 2017 gold guideline comparing previous ones on outcomes of COPD in real-world cohorts. Int J Chron Obstruct Pulmon Dis. 2017;(13):3473–3484.
- Culver BH, Graham BL, Coates AL, et al. Recommendations for a standardized pulmonary function report. An official American thoracic society technical statement. Am J Respir Crit Care Med. 2017;196(11):1463–1472. doi:10.1164/rccm.201710-1981ST
- Wasserman K, Hansen JE, Sue DY, et al. Principles of Exercise Testing and Interpretation. Fifth ed. Lippincott Williams & Wilkins; 2012:17.
- Wu CW, Hsieh PC, Yang MC, Tzeng IS, Wu YK, Lan CC. Impact of peak oxygen pulse on patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2019;14:2543–2551. doi:10.2147/COPD.S224735
- McConnell AK, Copestake AJ. Maximum static respiratory pressures in healthy elderly men and women: issues of reproducibility and interpretation. Respiration. 1999;66(3):251–258. doi:10.1159/000029386
- Wang KY, Chiang CH, Maa SH, Shau WY, Tarn YH. Psychometric assessment of the Chinese language version of the St. George’s respiratory questionnaire in Taiwanese patients with bronchial asthma. J Formos Med Assoc. 2001;100(7):455–460.
- Cheng ST, Wu YK, Yang MC, et al. Pulmonary rehabilitation improves heart rate variability at peak exercise, exercise capacity and health-related quality of life in chronic obstructive pulmonary disease. Heart Lung. 2014;43(3):249–255. doi:10.1016/j.hrtlng.2014.03.002
- Evans JD. Straightforward Statistics for the Behavioral Sciences. Pacific Grove, Calif: Brooks/Cole Publishing; 1996.
- Takken T, Mylius CF, Paap D, et al. Reference values for cardiopulmonary exercise testing in healthy subjects - an updated systematic review. Expert Rev Cardiovasc Ther. 2019;17(6):413–426. doi:10.1080/14779072.2019.1627874
- Guazzi M, Bandera F, Ozemek C, Systrom D, Arena R. Cardiopulmonary exercise testing? J Am Coll Cardiol. 2017;70(13):1618–1636. doi:10.1016/j.jacc.2017.08.012
- O’Donnell DE, Elbehairy AF, Faisal A, Webb KA, Neder JA, Mahler DA. Exertional dyspnoea in COPD: the clinical utility of cardiopulmonary exercise testing. Eur Respir Rev. 2016;25(141):333–347. doi:10.1183/16000617.0054-2016
- Bui KL, Nyberg A, Maltais F, Saey D. Functional tests in chronic obstructive pulmonary disease, part 1: clinical relevance and links to the international classification of functioning, disability, and health. Ann Am Thorac Soc. 2017;14(5):778–784. doi:10.1513/AnnalsATS.201609-733AS
- Nam HS, Izumchenko E, Dasgupta S, Hoque MO. Mitochondria in chronic obstructive pulmonary disease and lung cancer: where are we now? Biomark Med. 2017;11(6):475–489. doi:10.2217/bmm-2016-0373
- Broskey NT, Boss A, Fares EJ, et al. Exercise efficiency relates with mitochondrial content and function in older adults. Physiol Rep. 2015;3(6):12418. doi:10.14814/phy2.12418
- Balady GJ, Arena R, Sietsema K, et al. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American heart association. Circulation. 2010;122(2):191–225. doi:10.1161/CIR.0b013e3181e52e69
- Mirdamadi M, Rahimi B, Safavi E, Abtahi H, Peiman S. Correlation of cardiopulmonary exercise testing parameters with quality of life in stable COPD patients. J Thorac Dis. 2016;8(8):2138–2145. doi:10.21037/jtd.2016.07.07
- Chuang ML, Huang SF, Su CH. Cardiovascular and respiratory dysfunction in chronic obstructive pulmonary disease complicated by impaired peripheral oxygenation. Int J Chron Obstruct Pulmon Dis. 2015;10:329–337. doi:10.2147/COPD.S76209
- Anzueto A, Miravitlles M. Pathophysiology of dyspnea in COPD. Postgrad Med. 2017;129(3):366–374. doi:10.1080/00325481.2017.1301190