Abstract
In its 2021 strategy report, the Global Initiative for Chronic Obstructive Lung Disease states: “we no longer refer to asthma-COPD overlap (ACO), instead we emphasize that asthma and COPD are different disorders, although they may […] coexist in an individual patient. If a concurrent diagnosis of asthma is suspected, pharmacotherapy should primarily follow asthma guidelines, but pharmacological and non-pharmacological approaches may also be needed for their COPD.” What does this mean for the treating physician? In this review, we explore the implications of this new guidance on treating patients with chronic obstructive pulmonary disease, arguing for a personalized approach to treatment.
ACO: No Longer Fit for Purpose?
The coexistence of asthma and COPD in the same patient is not a new concept, with references in the literature dating back to the 1760s.Citation1 First coined by Gibson et al in 2009,Citation2 asthma–COPD overlap (ACO) has gathered much global interest in the past decade, resulting in >350 publications on the subject within the past 5 years alone (PubMed search string as of 09 March 2021: “asthma-COPD overlap”). So why, despite this increased knowledge and interest in ACO,Citation3 has Global Initiative for Chronic Obstructive Lung Disease (GOLD) chosen to revise its position?Citation4 The answer to this may lie in the lack of a unified definition for ACO. In 2015, a Global Initiative for Asthma (GINA)/GOLD joint publication was developed by the Science Committees of both GINA and GOLD based on a review of available literature.Citation5 It defined ACO syndrome as characterized by persistent airflow limitation, with several features usually associated with asthma and several features usually associated with COPD. However, it also acknowledged that a specific definition was not possible due to the lack of evidence. Some consensus documents, such as those from SpainCitation6,Citation7 and the Czech Republic,Citation8 describe ACO using diagnostic criteria, though the latest update to the Spanish document does not include ACO.Citation9 Similarly, ACO was addressed in the 2017 update of the Canadian Thoracic Society position statementCitation10 on the treatment of COPD, but was not included in the 2019 update due to a lack of evidence.Citation11 Since the 2015 GINA/GOLD consensus document was published,Citation5 there have been some attempts to work towards a consensus definition.Citation12 However, research into ACO has been limited, with no randomized controlled trials conducted and no clear consensus on how to define or diagnose the condition.
Some physicians and researchers still consider ACO to be a specific syndrome,Citation13 and use of the term may have been helpful in some situations for primary care providers to ensure that characteristics of asthma in patients with COPD are identified and addressed.Citation14 However, the inclusion of heterogeneous populations of patients under the same term and the absence of clinical trial data to guide treatment may make treatment decisions challenging.Citation13 Many physicians and researchers see ACO as a theoretical construct with no clear biologic grounds and an imprecise definition that encompasses both long-standing asthmatics who smoke and develop chronic airflow obstruction, and patients with COPD who have blood eosinophilia or greater reversibility after a bronchodilator test.Citation7 Where lack of clarity in the definition of a disease exists, so too does a lack of confidence in its diagnosis and treatment. Some consensus documents contain precise classification criteria for ACO which can be used to assess prevalence in specific countries.Citation6,Citation8 However, the lack of an internationally accepted definition means that accurate comparisons of the disease epidemiology cannot be made between countries. Our own systematic review of seven studies, which included a total of >36,700 patients across 20 countries, revealed a relatively low prevalence of 1.8–15.9%.Citation15–Citation21 However, interpreting these studies is complicated by a lack of consistency in terms of patient selection (diagnostic criteria), definitions of pulmonary function impairment, and concomitant medication received by patients.Citation22 In short, the complexities surrounding the definition and diagnosis of ACO may be the driving force behind the recent change in the GOLD 2021 strategy report. The consequence of all this for primary care physicians is potential confusion and a lack of clarity on how to treat patients with COPD who show features of asthma. In the following section, we consider how best to treat this complex patient population.
Differential Diagnosis and Tailored Treatment
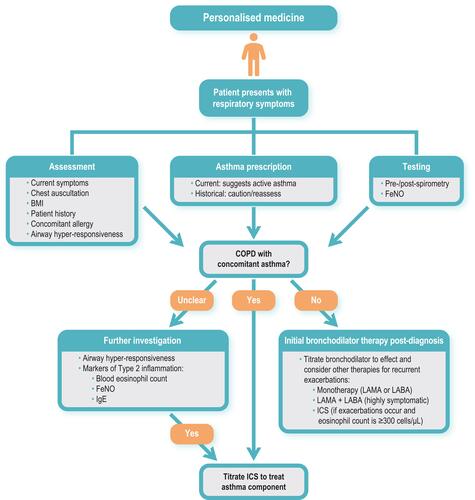
In no longer referring to the term “ACO” and increasing the emphasis on a treatment approach tailored to COPD and/or asthma,Citation4 GOLD puts the focus on personalized medicine – a concept gaining traction across many disease areas. Personalized medicine targets the individual needs of patients on the basis of the genetic, biomarker, phenotypic or psychosocial characteristics that distinguish them.Citation23 In essence, the new GOLD 2021 strategy report endorses a personalized approach, acknowledging that there are cases where COPD and asthma can coexist, and in these cases, “pharmacotherapy should primarily follow asthma guidelines”Citation4 (ie, use of inhaled corticosteroids [ICS]). However, in a new patient presenting with respiratory symptoms, it is important not to simply assume COPD with concomitant asthma without careful consideration of the presenting features.Citation4 The features of COPD and asthma are summarized in .
Table 1 Summary of Characteristics of COPD and Asthma
In order to make a confident diagnosis of concomitant asthma, we must consider the patient with COPD and ask how asthma was diagnosed. Misdiagnosis between COPD and other respiratory diseases – especially asthma – is common and may lead to inadequate treatment.Citation24 For the treatment of asthma, the most effective medication currently available remains low- or high-dose ICS,Citation25 whereas for COPD, the cornerstone of treatment is inhaled long-acting bronchodilators (alone or in combination), with ICS reserved as an add-on therapy for patients who are highly symptomatic, have a severe exacerbation history and high eosinophil count.Citation4 But what about patients with a history of asthma (perhaps diagnosed in childhood) who are not currently receiving any treatment – not even short-acting bronchodilators for symptomatic relief? Is it appropriate to treat these patients with ICS? Confusing a history of asthma with concomitant (and clinically relevant) asthma on presentation might lead physicians to favor a treatment choice that covers both diagnoses, even if their asthma was a feature early in life but is no longer clinically relevant.Citation26 Because primary care physicians often lack resources to help differentiate COPD from asthma such as spirometry, FeNO, full pulmonary function tests and computed tomography scans, they may rely on the historical diagnosis of asthma to guide their treatment choices, choosing to cover all bases by prescribing bronchodilators together with ICS. A current prescription for asthma medication or a history of asthma exacerbations in the years preceding consultation may therefore be the best confirmatory signals for asthma; if these are not available, re-assessment of bronchodilator responsiveness and eosinophil count should be carried out (). Ultimately, using diagnostic markers that have a high specificity gives a high degree of confidence in “ruling in” or identifying the presence of a particular trait,Citation27 such as asthma.
Figure 1 Assessments to guide treatment choice for patients with COPD presenting with asthma symptoms.
According to GINA, one of the diagnostic criteria for asthma is a post-bronchodilator increase in forced expiratory volume in 1 second (FEV1) of >12% and 200 mL from baseline (indicating reversible airflow limitation), with an increase of >15% and 400 mL providing greater confidence in the diagnosis.Citation25 In line with this, the GINA/GOLD publicationCitation5 and other consensus documentsCitation7 define a post-bronchodilator increase in FEV1 of 400 mL from baseline (ie, marked reversibility) as compatible with a diagnosis of ACO, but unlikely to be indicative of COPD, suggesting that this threshold might be useful for differential diagnosis. However, reversibility may not be the most reliable diagnostic tool, as large-scale studies have shown that bronchodilator responsiveness is at least as common in COPD as in asthma. Thus, measures of reversibility alone may be of limited value for differentiating between patients with asthma and COPD, and those with both.Citation28
So, what is the harm in treating a patient with COPD and suspected (but unconfirmed) or historical (and no longer clinically relevant) asthma with an ICS? It has been suggested that the main reason for diagnosing concomitant asthma in patients with COPD is to identify those who are likely to have a better response to ICS.Citation7 This is the correct motive, as the effectiveness of ICS (normally prescribed in combination with a long-acting β2-agonist [LABA]) in preventing exacerbations is well established.Citation29 But how should these patients be identified? Rather than relying on a historical diagnosis of asthma or bronchodilator responsiveness (as discussed), blood eosinophilia may be a more useful guide. In the MAJORICA study, for example, 27.4% of 603 patients with COPD fulfilled the 2015 GINA/GOLD definition of ACO.Citation30 These patients were more frequently treated with ICS and had a better prognosis relative to patients with COPD alone in terms of healthcare utilization (emergency department visits and all-cause hospitalizations). Assessment of the heterogeneity of these patients classified under the ACO umbrella showed that the prognosis of patients with eosinophilic COPD (COPD-Eo) differed from that of patients with a previous diagnosis of asthma.Citation30 Thus, from a practical point of view, distinguishing patients with COPD-Eo from those with a historical diagnosis of asthma may help to determine which patients would benefit most from ICS (though referral to a specialist center may be required to ascertain this). In abandoning the term ACO (in line with GOLD), it may be more useful then to consider T helper type 2 (Th2) in the blood as a surrogate marker (ie, treatable trait) of airway eosinophilia.Citation30 Increased levels of Th2 are associated with increased severity of COPD and “asthma-like” features (including a favorable corticosteroid response), suggesting that Th2 inflammation is important in a COPD subset that cannot be identified by a clinical history of asthma alone.Citation31 Additionally, serum levels of immunoglobulin E antibody and fractional exhaled nitric oxide are useful biomarkers in identifying the Th2 asthma phenotype ().Citation32,Citation33
Prescription of ICS for patients who do not have clinical features suggesting steroid responsiveness is associated with certain risks. Singh et al have estimated that the relative risk of severe pneumonia in patients with COPD was significantly higher when treated with ICS (1.57 [95% confidence interval 1.41–1.75]).Citation34 Pneumonia is a well-documented side effect of ICS,Citation35 but there are others, including tuberculosis,Citation36 osteoporosis with potential airflow obstruction,Citation37,Citation38 diabetes,Citation39 adverse metabolic effects,Citation40 glaucoma,Citation41 cataractsCitation42 and disruption of the lung microbiome,Citation43 and mycobacteria-associated bronchiectasis,Citation44 Therefore, the clinician must evaluate the risk–benefit ratio in each patient before the prescription of an ICS, taking the time to make a careful differential diagnosis to identify those patients with asthmatic features who are most likely to benefit ().Citation7
The similarities shared by COPD and asthma have the potential to confound diagnosis, but it is important to recognize that there are differentiating features – from etiology, symptoms, type of airway inflammation, inflammatory cells and mediators, consequences of inflammation, response to therapy, and disease course.Citation45 GOLD 2021 still recognizes that asthma and COPD may coexist in a patient; however, estimates of ACO prevalence vary (as previously discussed) and some evidence suggests that asthma is over-diagnosed in patients with COPD.Citation18 The following basic principle therefore holds true: if there are features of COPD, then the recommendations of GOLD should be followed, namely treat first with bronchodilators based on the GOLD ABCD classification (monotherapy or dual therapy), reserving treatment with ICS for patients in group D only, ie, those who are highly symptomatic (modified Medical Research Council grade 2 or COPD Assessment Test score ≥10) and have a history of two or more exacerbations per year (or one or more exacerbation leading to hospitalization).Citation4 Furthermore, use of ICS (in the form of LABA/ICS or LAMA/LABA/ICS) in these patients should only be considered if they have an eosinophil count higher than 300 eosinophils/µL (), with the benefits of use in patients with levels between 100 and 300 less clear, and <100 possibly causing harm.Citation46 However, the potential instability of eosinophil counts over time must be considered, as evidence from a large, population-based study suggests that the stability of blood eosinophil counts is significantly lower in patients with COPD compared with control subjects (85% stability after 6 months, declining to 65% at 2 years and progressively thereafter), with age and sex having a significant impact. Thus, more frequent re-assessment of eosinophilia in patients with COPD may be advisable.Citation47 In asthma, tiotropium can be added to LABA/ICS to help improve lung function and prevent exacerbations,Citation25 and single-device triple therapies (combining other long-acting muscarinic antagonists with LABA/ICS) are now available in Europe and the USA.Citation48,Citation49 For both COPD and asthma, non-pharmacologic management (vaccination, smoking cessation, trigger management, exercise, diet, inhaler technique and adherence to therapy) must also be considered as part of the holistic management of their disease.Citation4,Citation25
For a patient with COPD, a diagnosis of concomitant asthma must be thoroughly considered and based on an individualized assessment – in order to weigh up the benefits and risks of treating the individual with ICS. The use of ICS should also be considered in patients with eosinophilic COPD, taking into account the number of exacerbations and their triggers. For all other patients with COPD, disease management should follow guideline recommendations for the use of bronchodilator therapy.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Acknowledgments
Medical writing assistance was provided by Olive Denneny, MSc, of MediTech Media (UK) and was funded by Boehringer Ingelheim.
Disclosure
Dr. Roman-Rodriguez reports grants and personal fees from AstraZeneca and GSK, and personal fees from Boehringer-Ingelheim, Chiesi, Menarini, Mundipharma, Novartis, Pfizer, Teva, Trudell, and Bial, outside the submitted work. Dr. Kaplan reports personal fees and non-financial support from AstraZeneca and Boehringer Ingelheim, and personal fees from Covis, CSL Behring, GSK, Merck Frost, Teva, Trudel, Sanofi and Novartis, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- Gee S. The Lumleian lectures on bronchitis, pulmonary emphysema, and asthma: delivered before the Royal College of Physicians of London. Br Med J. 1899;1(1994):645–649. doi:10.1136/bmj.1.1994.645
- Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64(8):728–735. doi:10.1136/thx.2008.108027
- Miravitlles M. Asthma-COPD overlap (ACO) PRO-CON debate. ACO: call me by my name. COPD. 2020;17(5):471–473. doi:10.1080/15412555.2020.1817883
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2021 report) 2020; 2020. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf. Accessed February 2, 2021.
- Global Initiative for Asthma, Global Initiative for Chronic Obstructive Lung Disease. Diagnosis of diseases of chronic airflow limitation: asthma COPD and asthma - COPD overlap syndrome (ACOS); 2015. Available from: https://goldcopd.org/wp-content/uploads/2016/04/GOLD_ACOS_2015.pdf. Accessed December 4, 2020.
- Soler-Cataluna JJ, Cosio B, Izquierdo JL, et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch Bronconeumol. 2012;48(9):331–337. doi:10.1016/j.arbr.2012.06.017
- Miravitlles M, Alvarez-Gutierrez FJ, Calle M, et al. Algorithm for identification of asthma-COPD overlap: consensus between the Spanish COPD and asthma guidelines. Eur Respir J. 2017;49(5):1700068. doi:10.1183/13993003.00068-2017
- Koblizek V, Chlumsky J, Zindr V, et al. Chronic Obstructive Pulmonary Disease: official diagnosis and treatment guidelines of the Czech Pneumological and Phthisiological Society; a novel phenotypic approach to COPD with patient-oriented care. Biomed Papers Med Faculty Palacky Univ Olomouc. 2013;157(2).
- Miravitlles M, Calle M, Molina J, et al. Spanish COPD guidelines (GesEPOC) 2021: updated pharmacological treatment of stable COPD. Arch Bronconeumol. 2021. doi:10.1016/j.arbr.2021.03.014
- Bourbeau J, Bhutani M, Hernandez P, et al. CTS position statement: pharmacotherapy in patients with COPD—an update. Can J Respir Crit Care Sleep Med. 2017;1(4):222–241. doi:10.1080/24745332.2017.1395588
- Bourbeau J, Bhutani M, Hernandez P, et al. Canadian Thoracic Society Clinical Practice Guideline on pharmacotherapy in patients with COPD – 2019 update of evidence. Can J Respir Crit Care Sleep Med. 2019;3(4):210–232. doi:10.1080/24745332.2019.1668652
- Sin DD, Miravitlles M, Mannino DM, et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J. 2016;48(3):664–673. doi:10.1183/13993003.00436-2016
- Rogliani P, Ora J, Puxeddu E, et al. Airflow obstruction: is it asthma or is it COPD? Int J Chron Obstruct Pulmon Dis. 2016;11:3007–3013. doi:10.2147/COPD.S54927
- Moore C. Implementing an asthma and COPD overlap protocol in general practice. Nurs Times. 2020;116(4):31–34.
- Cosentino J, Zhao H, Hardin M, et al. Analysis of asthma-chronic obstructive pulmonary disease overlap syndrome defined on the basis of bronchodilator response and degree of emphysema. Ann Am Thorac Soc. 2016;13(9):1483–1489. doi:10.1513/AnnalsATS.201511-761OC
- Menezes AMB, Montes de Oca M, Perez-Padilla R, et al. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest. 2014;145(2):297–304. doi:10.1378/chest.13-0622
- Che-Morales JL, Valle-Pina J, Carrillo-Chan J. [Asthma-chronic obstructive pulmonary disease overlap syndrome: clinical-functional profile]. Rev Med Inst Mex Seguro Soc. 2019;57(5):284–290. Swedish.
- Nissen F, Morales DR, Mullerova H, et al. Concomitant diagnosis of asthma and COPD: a quantitative study in UK primary care. Br J Gen Pract. 2018;68(676):e775–e82. doi:10.3399/bjgp18X699389
- Barrecheguren M, Roman-Rodriguez M, Miravitlles M. Is a previous diagnosis of asthma a reliable criterion for asthma-COPD overlap syndrome in a patient with COPD? Int J Chron Obstruct Pulmon Dis. 2015;10:1745–1752. doi:10.2147/COPD.S87025
- Baron AJ, Flokstra-de Blok BMJ, van Heijst E, et al. Prevalence of asthma characteristics in COPD patients in a Dutch well-established asthma/COPD service for primary care. Int J Chron Obstruct Pulmon Dis. 2020;15:1601–1611. doi:10.2147/COPD.S247819
- Koblizek V, Milenkovic B, Barczyk A, et al. Phenotypes of COPD patients with a smoking history in Central and Eastern Europe: the POPE Study. Eur Respir J. 2017;49(5):pii:1601446. doi:10.1183/13993003.01446-2016
- Barczyk A, Maskey-Warzechowska M, Gorska K, et al. Asthma-COPD overlap-a discordance between patient populations defined by different diagnostic criteria. J Allergy Clin Immunol Pract. 2019;7(7):2326–36e5. doi:10.1016/j.jaip.2019.04.022
- Jameson JL, Longo DL. Precision medicine–personalized, problematic, and promising. N Engl J Med. 2015;372(23):2229–2234. doi:10.1056/NEJMsb1503104
- Miravitlles M, Andreu I, Romero Y, et al. Difficulties in differential diagnosis of COPD and asthma in primary care. Br J Gen Pract. 2012;62(595):e68–75. doi:10.3399/bjgp12X625111
- Global Initiative for Asthma. Global strategy for asthma management and prevention (2020 report); 2020. Available from: https://ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-final-_wms.pdf. Accessed May 13, 2020.
- Cataldo D, Derom E, Liistro G, et al. Overuse of inhaled corticosteroids in COPD: five questions for withdrawal in daily practice. Int J Chron Obstruct Pulmon Dis. 2018;13:2089–2099. doi:10.2147/COPD.S164259
- McDonald VM, Fingleton J, Agusti A, et al. Treatable traits: a new paradigm for 21st century management of chronic airway diseases: treatable traits down under international workshop report. Eur Respir J. 2019;53(5):1802058. doi:10.1183/13993003.02058-2018
- Janson C, Malinovschi A, Amaral AFS, et al. Bronchodilator reversibility in asthma and COPD: findings from three large population studies. Eur Respir J. 2019;54(3):1900561. doi:10.1183/13993003.00561-2019
- Tashkin DP, Strange C. Inhaled corticosteroids for chronic obstructive pulmonary disease: what is their role in therapy? Int J Chron Obstruct Pulmon Dis. 2018;13:2587–2601. doi:10.2147/COPD.S172240
- Toledo-Pons N, van Boven JFM, Roman-Rodriguez M, et al. ACO: time to move from the description of different phenotypes to the treatable traits. PLoS One. 2019;14(1):e0210915. doi:10.1371/journal.pone.0210915
- Christenson SA, Steiling K, van den Berge M, et al. Asthma-COPD overlap. Clinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191(7):758–766. doi:10.1164/rccm.201408-1458OC
- de Abreu FC, da Silva Júnior JLR, Rabahi MF. The fraction exhaled nitric oxide as a biomarker of asthma control. Biomark Insights. 2019;14:1177271919826550. doi:10.1177/1177271919826550
- Kuruvilla ME, Lee FE-H, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56(2):219–233. doi:10.1007/s12016-018-8712-1
- Singh S, Loke YK. Risk of pneumonia associated with long-term use of inhaled corticosteroids in chronic obstructive pulmonary disease: a critical review and update. Curr Opin Pulm Med. 2010;16(2):118–122. doi:10.1097/MCP.0b013e328334c085
- Leung JM, Sin DD. Inhaled corticosteroids in COPD: the final verdict is. Eur Respir J. 2018;52(6):1801940. doi:10.1183/13993003.01940-2018
- Brassard P, Suissa S, Kezouh A, et al. Inhaled corticosteroids and risk of tuberculosis in patients with respiratory diseases. Am J Respir Crit Care Med. 2011;183(5):675–678. doi:10.1164/rccm.201007-1099OC
- Buehring B, Viswanathan R, Binkley N, et al. Glucocorticoid-induced osteoporosis: an update on effects and management. J Allergy Clin Immunol. 2013;132(5):1019–1030. doi:10.1016/j.jaci.2013.08.040
- Sin DD, Man JP, Man SF. The risk of osteoporosis in Caucasian men and women with obstructive airways disease. Am J Med. 2003;114(1):10–14. doi:10.1016/s0002-9343(02)01297-4
- Price DB, Russell R, Mares R, et al. Metabolic effects associated with ICS in patients with COPD and comorbid type 2 diabetes: a historical matched cohort study. PLoS One. 2016;11(9):e0162903. doi:10.1371/journal.pone.0162903
- Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med. 2010;123(11):1001–1006. doi:10.1016/j.amjmed.2010.06.019
- Shroff S, Thomas RK, D’Souza G, et al. The effect of inhaled steroids on the intraocular pressure. Digit J Ophthalmol. 2018;24(3):6–9. doi:10.5693/djo.01.2018.04.001
- Weatherall M, Clay J, James K, et al. Dose-response relationship of inhaled corticosteroids and cataracts: a systematic review and meta-analysis. Respirology. 2009;14(7):983–990. doi:10.1111/j.1440-1843.2009.01589.x
- Singanayagam A, Glanville N, Cuthbertson L, et al. Inhaled corticosteroid suppression of cathelicidin drives dysbiosis and bacterial infection in chronic obstructive pulmonary disease. Sci Transl Med. 2019;11(507):eaav3879. doi:10.1126/scitranslmed.aav3879
- Brode SK, Campitelli MA, Kwong JC, et al. The risk of mycobacterial infections associated with inhaled corticosteroid use. Eur Respir J. 2017;50(3):1700037. doi:10.1183/13993003.00037-2017
- Cukic V, Lovre V, Dragisic D, et al. Asthma and chronic obstructive pulmonary disease (COPD) - differences and similarities. Mater Sociomed. 2012;24(2):100–105. doi:10.5455/msm.2012.24.100-105
- Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018;52(6):1801219. doi:10.1183/13993003.01219-2018
- Oshagbemi OA, Burden AM, Braeken DCW, et al. Stability of blood eosinophils in patients with chronic obstructive pulmonary disease and in control subjects, and the impact of sex, age, smoking, and baseline counts. Am J Respir Crit Care Med. 2017;195(10):1402–1404. doi:10.1164/rccm.201701-0009LE
- Novartis Pharmaceuticals UK Ltd. Enerzair breezhaler; 2020. Available from: https://www.medicines.org.uk/emc/product/11886/smpc#gref. Accessed January 20, 2021.
- GlaxoSmithKline. FDA approves Trelegy Ellipta as the first once-daily single inhaler triple therapy for the treatment of both asthma and COPD in the US 2020; 2020. Available from: https://www.gsk.com/en-gb/media/press-releases/fda-approves-trelegy-ellipta-as-the-first-once-daily-single-inhaler-triple-therapy-for-the-treatment-of-both-asthma-and-copd-in-the-us/. Accessed January 20, 2021.
