Abstract
Purpose
In performing surgery for lung cancer, emphysema is a risk factor related to postoperative respiratory complications (PRC). However, few studies have addressed the risk of radiological emphysematous volume affecting PRC. The aim of this study was to investigate the relationship between emphysematous volume as measured on 3-dimensional computed tomography and PRC.
Patients and Methods
We reviewed 342 lung cancer patients undergoing lobectomy between 2013 and 2018. The percentage of low attenuation area (LAA%) was defined as the percentage of the lung area showing attenuation of −950 Hounsfield units or lower. Preoperative factors including age, sex, body mass index, smoking index, respiratory function, tumour histology, and LAA% were evaluated. PRC included pneumonia, atelectasis, prolonged air leakage, empyema, hypoxia, ischemic bronchitis, bronchopleural fistula, and exacerbation of interstitial pneumonia. Uni- and multivariable analyses were performed to investigate the relationship between independent clinical variables and postoperative adverse events.
Results
Median LAA% was 5.0% (range, 0–40%) and PRC was observed in 50 patients (14.6%). Patients who presented with PRC showed significantly high LAA% compared to those without complications (median: 8.1% vs 3.8%; p < 0.001). Based on univariable analysis, age, sex, smoking index, percentage of forced expiratory volume in 1 s (FEV1.0%), histology, and LAA% were significant predictors for PRC. Multivariable analysis revealed higher LAA% as a significant risk factor for PRC (odds ratio = 1.040; 95% confidence interval, 1.001–1.080; p = 0.046).
Conclusion
In addition to respiratory function with spirometry, LAA% can be used as a predictor of PRC.
Introduction
Despite marked advances in surgical procedures for non-small cell lung cancer (NSCLC), pulmonary resection still carries a risk of postoperative respiratory complications (PRC). Complication rates following lobectomy, which is a standard operative procedure for NSCLC, range from 6% to 34.2%.Citation1–Citation3 Preoperative risk assessment is generally performed based on detailed algorithms provided by three clinical guidelines (American College of Chest Physicians, European Respiratory Society and European Society of Thoracic Surgery).Citation4,Citation5 According to these guidelines, several spirometric parameters remain standard for preoperative risk assessment, including forced expiratory volume in 1 s (FEV1.0) and diffusing capacity of the lungs for carbon monoxide (DLCO). However, objectivity in pulmonary function tests remains lacking due to insufficient respiratory effort, particularly among elderly patients with chronic obstructive pulmonary disease (COPD).Citation6
Several recent studies have identified that quantitative computed tomography (CT) assessment using low attenuation area (LAA) correlates well with pulmonary emphysema.Citation7–Citation9 Previous report revealed that emphysema on CT was associated with significantly increased odds of lung cancer.Citation10,Citation11 Moreover, the distribution pattern of emphysema may affect both lung cancer risk and tumour location.Citation12,Citation13 Emphysema is a well-known risk factor for postoperative air leak, as the most frequent adverse event after lung resection.Citation14 Although increasing evidence seems to demonstrate a link between LAA and pulmonary emphysema, scant information has been published regarding the association between LAA and PRC.Citation15 Accordingly, combined assessment of the LAA and spirometric parameters has been hypothesized to contribute to the accurate prediction of PRC. The objective of the present study was to analyse the predictive value of LAA in terms of PRC among patients with NSCLC who underwent lobectomy.
Materials and Methods
Study Population
Patients who underwent pulmonary lobectomy for primary NSCLC at the University of Tsukuba Hospital between January 2013 and December 2018 were reviewed. Inclusion criteria were as follows: 1) pulmonary lobectomy for primary NSCLC without neoadjuvant chemotherapy or radiotherapy; 2) availability of high-resolution computed tomography for the whole lung with slice intervals ≤2 mm; and 3) spirometric measurements of lung function conducted before surgical intervention. The medical records of all patients were reviewed to determine age, sex, smoking index, respiratory function on spirometry, and histopathological factors. The study was conducted according to the principles of the Declaration of Helsinki. Ethical approval for the study was obtained from the institutional review board of the University of Tsukuba Hospital (approval number R01-258). All data in this retrospective study were fully anonymized and informed consent from the patients was obtained using the opt-out method with a disclosure document.
Pulmonary Function Tests
Spirometric variables were obtained within 2 months preoperatively and included forced vital capacity (FVC), FEV1.0, and DLCO. Percentage predicted values were calculated using the age, sex, and height of patients.
Operative and Perioperative Management
Under single-lung anaesthesia, lobectomy was basically performed by video-assisted thoracic surgery with four incisions without rib spreading. One specific chief surgeon (Y.S) was present for all lung lobectomies and critical portion of each surgical procedure was carefully supervised. All patients were extubated in the operating room. A 24-Fr chest tube with continuous channels was placed in the posterior pleural space via the apical space under continuous suction at −7 cmH2O. Rehabilitation was started on postoperative day (POD) 1. Chest X-ray and blood tests were routinely performed on POD 1–4, 7, and on POD 1, 4, and 7, respectively.
CT Examination and Quantitative Emphysema Measurement
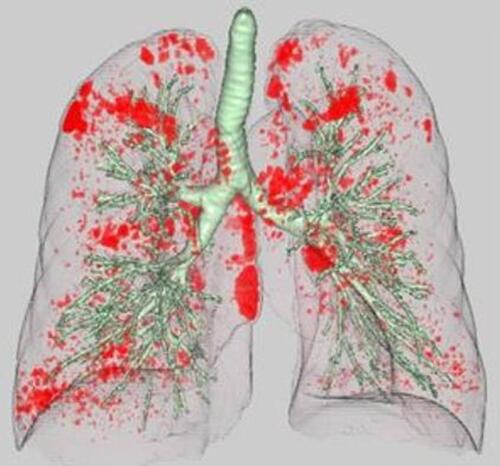
HRCT images were obtained from two multidetector-row CT scanners (Brilliance 64 and Brilliance iCT 256; PHILIPS Electronics, Eindhoven, the Netherlands). A standard contrast-enhanced scanning protocol was performed to evaluate from the lung apex to the diaphragm using the following parameters: 120 kilovoltage peak; 180–280 mAs; resolution, 512×512 pixels, and scanning duration, 0.5 s. Axial images were reconstructed with a slice thickness of 1 or 2 mm. Images were photographed using a window level of −500 to −700 HU with a window width of 1000–2000 HU (lung window setting) and a level of 30–60 HU with a window width of 350–600 HU (mediastinal window setting). All preoperative CT data were transferred to a computer workstation (SYNAPSE VINCENT version 3.0; FUJIFILM, Tokyo, Japan). Threshold limits of −600 to −1024 Hounsfield units (HU) were automatically applied to exclude soft tissue surrounding the lungs as well as large vessels within the lungs. The trachea, main bronchus, and lobar to segmental bronchus were automatically removed from the 3-dimensional model of the whole lung. Lung field area with attenuation values less than −950 HU were considered as LAA, as previously reported.Citation16 The ratio of the number of voxels with attenuation values lower than −950 HU among the total number of voxels for the whole lung was considered to indicate the percentage of the low attenuation area (LAA%) ().
Postoperative Respiratory Complication
In this study, PRC were selected from complications equal to or higher than grade 2 according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0.Citation17 PRC included 1) pneumonia (fever with infiltrative shadow on chest radiograph); 2) atelectasis (endobronchial sputum drainage required); 3) prolonged air leakage (air leakage persisting beyond 7 days postoperatively); 4) empyema (pleural effusion with positive bacteriological confirmation); 5) hypoxia (required home oxygen therapy); 6) ischemic bronchitis (defined on capillary hyperplasia, mucosal defect, and ulceration with bronchoscopic inspection); 7) bronchopleural fistula; and 8) exacerbation of interstitial pneumonia.
Statistical Analysis
According to past reports, radiological emphysema has been defined as being present in more than 10% of the lung.Citation18,Citation19 In this current study, patients were divided into two groups: a high LAA% group, representing patients with LAA% more than or equal to 10%, and a low LAA% group, representing patients with LAA% less than 10%. Patient characteristics and PRC were evaluated between groups. Data with a normal distribution are expressed as mean ± standard deviation (SD), and values for categorical variables are given as percentages. A χ2 test was used to compare categorical variables. Mann–Whitney U testing was used for non-parametric data. Preoperative factors including age, sex, body mass index, smoking index, respiratory function, tumour histology (adenocarcinoma or others), and LAA% were evaluated. Variables showing values of p < 0.05 in univariable analysis were placed into logistic regression multivariable analysis to identify predictors of postoperative respiratory complications. All statistical analyses were performed using SPSS® version 25.0 (IBM Corporation, Armonk, NY). Statistical analyses were considered significant for values of p < 0.05.
Results
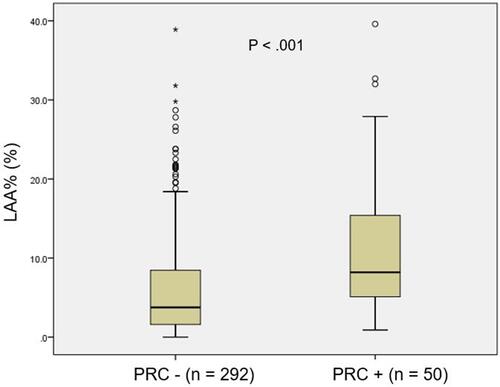
summarizes patient and tumour characteristics. The cohort in this study comprised 143 men (41.8%) and 199 women (58.2%). Mean diameter of primary tumours was 25.5 ± 14.0 mm (range, 8–90 mm). Adenocarcinoma constituted the most common pathological subtype (n = 272, 79.5%), followed by squamous cell carcinoma (n = 53, 15.5%). Mean and median LAA% were 7.0 ± 7.4% (range, 0–39.6%) and 5.0%, respectively. PRC were observed in 50 patients (14.6%). LAA% values for patients who presented with PRC were significantly higher compared to those without PRC (median, 8.1% vs 3.8%; p<0.001) ().
Table 1 Patient Characteristics (n = 343)
Figure 2 Box plot comparing the percentage of low attenuation area (LAA%) between patients with and without postoperative respiratory complications (PRC).
PRC in the high LAA% group (LAA% ≥10%) and low LAA% group (LAA% <10%) are shown in . These included pneumonia (n = 6), atelectasis (n = 8), prolonged air leakage (n = 13), empyema (n = 4), hypoxia (n = 5), ischemic bronchitis (n = 3), bronchopleural fistula (n = 1), acute exacerbation of interstitial lung disease (n = 4), and other adverse events (n = 6). The overall PRC rate was significantly greater in the high LAA group (25.0%) than in the low LAA group (11.5%, p<0.05). Of these, patients who developed postoperative pneumonia (5.0%) and hypoxia (5.0%) were significantly higher in high LAA% group (p=0.029, 0.012, respectively).
Table 2 Postoperative Respiratory Complications in High and Low LAA% Groups
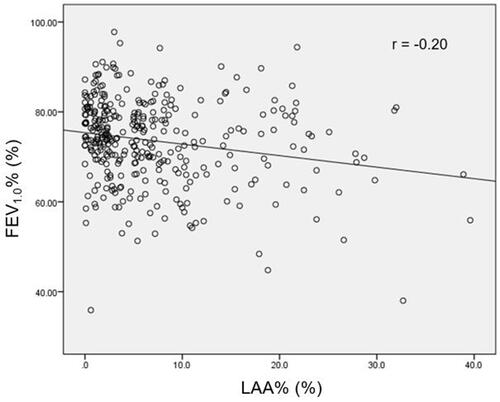
shows the correlation between LAA% and FEV1.0%. Only a weak negative correlation between these two variables was identified (r = −0.20). shows the results of univariable and multivariable analysis for predicting PRC. Based on the univariable analysis, male sex, age, smoking index, tumour histology, FEV1.0%, %DLCO, and LAA% were significant predictors for PRC. Conversely, no significant relationships were seen for BMI, whole tumour size, or %FVC. In order to reduce the impact of confounding variables, both smoking index and tumour histology were excluded from the subsequent multivariable analysis. Male sex (odds ratio [OR] 4.876, p < 0.001), %DLCO (OR 0.975, p = 0.002), and LAA% (OR 1.040, p = 0.046) showed significance predicting PRC after lobectomy.
Table 3 Uni- and Multivariable Analysis of Clinical Factors for Predicting PRC
Figure 3 Scatter plot showing the relationship between FEV1.0% and percentage of low attenuation area (LAA%). The r value indicates the Pearson correlation coefficient.
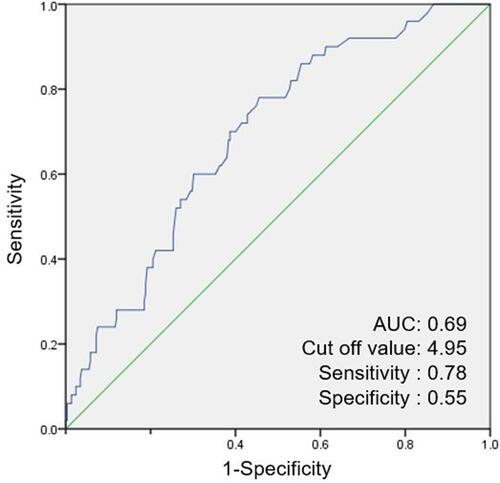
The ROC curve analysis revealed that the optimal cut-off values of LAA% for predicting PRC was 4.950 (). The area under the ROC curves of the LAA% was 0.693. These cut-off values yielded a sensitivity of 0.780 and a specificity of 0.545 for LAA%.
Discussion
The present study investigated associations between the ratio of emphysematous volume and PRC in patients who underwent lobectomy for primary lung cancer. We found that LAA% had significant impacts on prediction of PRC according to multivariable logistic regression analysis. In general, pulmonary emphysema is pathologically defined as an abnormal enlargement of the airspaces, accompanied by destruction of the alveolar walls.Citation20 These abnormal changes are observed on chest CT as the relative areas of lung occupied by attenuation values lower than −800 to −970 HU.Citation21–Citation23 In this study, LAA was defined as areas below −950 HU, because this value had been commonly used to define radiological emphysema and correlates well with pathological severity of emphysema.Citation24–Citation26 Since emphysematous lungs comprise fragile lung parenchyma combined with chronic bronchitis, patients with pulmonary emphysema could reasonably be expected to show higher complication rates after lung surgery. However, preoperative risk assessment has been practically performed only using functional evaluation by pulmonary function testing, cardiopulmonary exercise testing, and maximal exercise O2 consumption.Citation27 Radiological evaluation has not yet been established as an appropriate risk indicator, but whether structural emphysematous changes impact the tolerability of lung cancer surgery remains unclear.
Recent studies have addressed the capability of CT to accurately assess the severity of pulmonary emphysema.Citation28,Citation29 Such findings support the hypothesis that radiological evaluation can be applied for preoperative risk assessment as well as pulmonary function testing. Emphysematous changes on chest CT could be considered to correlate strongly with FEV1.0, because cigarette smoking is the common causative factor for both lower pulmonary function and emphysematous changes. However, conflicting results have suggested discrepancies between functional and radiological findings. Ueda et al showed that the extent of emphysema on chest CT did not correlate well with the percentage-predicted FEV1 (r = −0.375).Citation30 Kim et al also concluded that no strong correlation exists between severity of emphysema and FEV1.0% (r = −0.216), consistent with our results.Citation31 Our cohort included patients who showed severe pulmonary emphysematous changes on CT with normal pulmonary functions. One reason explaining the discrepancy is the heterogeneity in smoker responses to tobacco. Studies have reported that smoking-induced tissue damage varies among individuals depending on sex, genetic status, and type of exposure to tobacco smoke.Citation32–Citation34 Such reports indicate that some populations are more likely to develop radiological emphysema inconsistent with their pulmonary functions. Another possible explanation is that results from pulmonary function testing are commonly affected by several factors including patient effort, cognitive function, and the skill of the examiner. FEV1.0 obtained by spirometry does not necessarily reflect an objective result, especially in elderly patients. Therefore, in addition to respiratory function with spirometry, objective radiological evaluation seems warranted to achieve accurate preoperative risk assessment.
Quantitative CT analysis is widely accepted as an objective tool to evaluate the severity of pulmonary emphysema. In the present study, patients with high LAA%, defined using the 10% cut-off value, showed a higher PRC than patients with low LAA%. Several microenvironmental factors observed in patients with emphysema may explain the higher rates of inflammatory complications. Emphysematous changes pathologically involve abnormal, permanent enlargement of the tiniest airspaces including the alveoli and respiratory bronchioles, attributed to colonization with or infection by a potentially pathogenic organism.Citation35 Moreover, emphysema patients develop small airway stenosis due to the loss of elastic recoil of the lung, resulting in deficient mucociliary clearance.Citation36 Our study clearly demonstrated that PRC was linked with high LAA% because radiological emphysematous changes might represent disruptions of alveolar structures and surrounding microvessels. These results indicate that the degree of radiological lung parenchymal damage can be used as a predictor of PRC.
In our multivariable analysis, %DLCO was a significant factor for predicting PRC. This result was consistent with previous prospective studies from the Society of Thoracic Surgeons General Thoracic Surgery Database.Citation37,Citation38 FEV1.0%, which is an established prognostic factor for postoperative respiratory adverse events, was not shown to be a significant independent predictor in multivariable modelling. One possible reason to explain these results might be derived from the hypothesis that the presence and severity of emphysema were not directly linked to airflow limitation as measured on spirometry.Citation39,Citation40 According to previous studies, emphysema and airway wall thickening may already be present in most COPD patients, but some do not yet show airflow limitation.Citation41 Based on these hypotheses, declines in FEV1.0 cannot be evident unless the degree of emphysema reaches a particular threshold. Preoperative risk assessment with quantitative emphysema evaluation may thus allow more accurate prediction of PRC than that with pulmonary function testing alone.
Several potential limitations need to be considered for the present study. First, this investigation was a retrospective study conducted in a single institute and the number of patients with PRC was relatively small. Second, all the CT scans in the current study were performed with contrast and injection rate and time to scan might have been slightly different in each case. This indicates that image acquisition protocol with contrast agents could affect the HU value of the lung parenchyma. However, this was the single-center study and the contrast-enhanced CT scan was performed in the same protocol. Moreover, it is considered that contrast agents do not tend to accumulate on the emphysematous lung and the results would not be different regardless of whether contrast agent was used. Therefore, we consider the effect of contrast-enhanced CT could be neglectable Third, we measured emphysema severity for global lungs, because precise lobulation in three-dimensional software was sometimes challenging in patients with incomplete lobulation of the lungs. Further evaluation of the difference in LAA distribution between upper and lower lobes using fissure integrity scores may be ideal.
Conclusion
LAA% showed a significant correlation with the incidence of PRC after lobectomy. Our findings may have important clinical implications, in that combining radiological assessment of emphysema with functional evaluation by spirometry may facilitate accurate prediction of PRC after lobectomy for NSCLC.
Acknowledgments
The poster’s abstract of the present study was published in “Poster Abstracts” in American Journal of Respiratory and Critical Care Medicine 2020;201:A4730: https://doi.org/10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A4730.
Disclosure
The authors report no conflicts of interest in this work.
References
- Berry MF, Hanna J, Tong BC, et al. Risk factors for morbidity after lobectomy for lung cancer in elderly patients. Ann Thorac Surg. 2009;88(4):1093–1099. doi:10.1016/j.athoracsur.2009.06.012
- Ziarnik E, Grogan EL. Postlobectomy early complications. Thorac Surg Clin. 2015;25:355–364. doi:10.1016/j.thorsurg.2015.04.003
- Sandri A, Papagiannopoulos K, Milton R, et al. Major morbidity after video-assisted thoracic surgery lung resections: a comparison between the European Society of Thoracic Surgeons definition and the Thoracic Morbidity and Mortality System. J Thorac Dis. 2015;7:1174–1180.
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e166S–90S.
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemoradiotherapy). Eur Respir J. 2009;34:17–41. doi:10.1183/09031936.00184308
- Yoon HY, Kim TH, Seo JB, et al. Effects of emphysema on physiological and prognostic characteristics of lung function in idiopathic pulmonary fibrosis. Respirology. 2019;24:55–62. doi:10.1111/resp.13387
- Yamamoto T, Kadoya N, Sato Y, et al. Prognostic value of radiation pneumonitis after stereotactic body radiotherapy: effect of pulmonary emphysema quantitated using CT images. Clin Lung Cancer. 2018;19:e85–e90. doi:10.1016/j.cllc.2017.05.022
- Murakami J, Ueda K, Tanaka T, et al. Grading of emphysema is indispensable for predicting prolonged air leak after lung lobectomy. Ann Thorac Surg. 2018;105:1031–1037. doi:10.1016/j.athoracsur.2017.11.053
- Kim YS, Kim EY, Ahn HK, et al. Prognostic significance of CT-emphysema score in patients with advanced squamous cell lung cancer. J Thorac Dis. 2016;8:1966–1973. doi:10.21037/jtd.2016.06.70
- Smith BM, Pinto L, Ezer N, et al. Emphysema detected on computed tomography and risk of lung cancer: a systematic review and meta-analysis. Lung Cancer. 2012;77(1):58–63. doi:10.1016/j.lungcan.2012.02.019
- Nishio M, Kubo T, Togashi K. Estimation of lung cancer risk using homology-based emphysema quantification in patients with lung nodules. PLoS One. 2019;14:e0210720. doi:10.1371/journal.pone.0210720
- Gonzalez J, Henschke CI, Yankelevitz DF, et al. Emphysema phenotypes and lung cancer risk. PLoS One. 2019;14(7):e0219187. doi:10.1371/journal.pone.0219187
- Lim J, Shin KM, Lee K, et al. Relationship between emphysema severity and the location of lung cancer in patients with chronic obstructive lung disease. AJR Am J Roentgenol. 2015;205:540–545. doi:10.2214/AJR.14.13992
- Gilbert S, Maghera S, Seely AJ, et al. Identifying patients at higher risk of prolonged air leak after lung resection. Ann Thorac Surg. 2016;102:1674–1679. doi:10.1016/j.athoracsur.2016.05.035
- Sato S, Nakamura M, Shimizu Y, et al. The impact of emphysema on surgical outcomes of early-stage lung cancer: a retrospective study. BMC Pulm Med. 2019;19:73. doi:10.1186/s12890-019-0839-1
- Park KJ, Bergin CJ, Clausen JL. Quantitation of emphysema with three-dimensional CT densitometry: comparison with two-dimensional analysis, visual emphysema scores, and pulmonary function test results. Radiology. 1999;211:541–547. doi:10.1148/radiology.211.2.r99ma52541
- National Cancer Institute, National Institutes of Health, US Department of Health and Human Services. Common Terminology criteria for adverse events (CTCAE), Version 5.0. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed August 23, 2021.
- Wilson DO, Weissfeld JL, Balkan A, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178:738–744. doi:10.1164/rccm.200803-435OC
- Gullon JA, Suarez I, Medina A, et al. Role of emphysema and airway obstruction in prognosis of lung cancer. Lung Cancer. 2011;71(2):182–185. doi:10.1016/j.lungcan.2010.05.018
- Kemp SV, Polkey MI, Shah PL. The epidemiology, etiology, clinical features, and natural history of emphysema. Thorac Surg Clin. 2009;19:149–158. doi:10.1016/j.thorsurg.2009.03.003
- Occhipinti M, Paoletti M, Bartholmai BJ, et al. Spirometric assessment of emphysema presence and severity as measured by quantitative CT and CT-based radiomics in COPD. Respir Res. 2019;20:101. doi:10.1186/s12931-019-1049-3
- Dournes G, Laurent F, Coste F, et al. Computed tomographic measurement of airway remodeling and emphysema in advanced chronic obstructive pulmonary disease. Correlation with pulmonary hypertension. Am J Respir Crit Care Med. 2015;191:63–70. doi:10.1164/rccm.201408-1423OC
- Matsuoka S, Yamashiro T, Washko GR, et al. Quantitative CT assessment of chronic obstructive pulmonary disease. Radiographics. 2010;30(1):55–66. doi:10.1148/rg.301095110
- Müller NL, Staples CA, Miller RR, et al. “Density mask.” An objective method to quantitate emphysema using computed tomography. Chest. 1988;94:782–787. doi:10.1378/chest.94.4.782
- Gevenois PA, De Vuyst P, de Maertelaer V, et al. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996;154:187–192. doi:10.1164/ajrccm.154.1.8680679
- Madani A, Zanen J, de Maertelaer V, et al. Pulmonary emphysema: objective quantification at multi-detector row CT- -comparison with macroscopic and microscopic morphometry. Radiology. 2006;238:1036–1043. doi:10.1148/radiol.2382042196
- Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax. 2010;65:iii1–27. doi:10.1136/thx.2010.145938
- Haruna A, Muro S, Nakano Y, et al. CT scan findings of emphysema predict mortality in COPD. Chest. 2010;138:635–640. doi:10.1378/chest.09-2836
- Diaz AA, Bartholmai B, San José Estépar R, et al. Relationship of emphysema and airway disease assessed by CT to exercise capacity in COPD. Respir Med. 2010;104:1145–1151. doi:10.1016/j.rmed.2010.02.023
- Ueda K, Jinbo M, Li TS, et al. Computed tomography-diagnosed emphysema, not airway obstruction, is associated with the prognostic outcome of early-stage lung cancer. Clin Cancer Res. 2006;12:6730–6736. doi:10.1158/1078-0432.CCR-06-1196
- Kim YS, Kim EY, Ahn HK, et al. Prognostic significance of CT-emphysema score in patients with advanced squamous cell lung cancer. J Thorac Dis. 2016;8:1966–1973.
- Dransfield MT, Washko GR, Foreman MG, et al. Gender differences in the severity of CT emphysema in COPD. Chest. 2007;132(2):464–470. doi:10.1378/chest.07-0863
- Manichaikul A, Hoffman EA, Smolonska J, et al. Genome-wide study of percent emphysema on computed tomography in the general population. The multi-ethnic study of atherosclerosis lung/SNP health association resource study. Am J Respir Crit Care Med. 2014;189:408–418. doi:10.1164/rccm.201306-1061OC
- Sharafkhaneh A, Hanania NA, Kim V. Pathogenesis of emphysema: from the bench to the bedside. Proc Am Thorac Soc. 2008;5:475–477. doi:10.1513/pats.200708-126ET
- Eom JS, Song WJ, Yoo H, et al. Chronic obstructive pulmonary disease severity is associated with severe pneumonia. Ann Thorac Med. 2015;10:105–111. doi:10.4103/1817-1737.151441
- Bustamante-Marin XM, Ostrowski LE. Cilia and mucociliary clearance. Cold Spring Harb Perspect Biol. 2017;9:a028241. doi:10.1101/cshperspect.a028241
- Agostini P, Cieslik H, Rathinam S, et al. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax. 2010;65:815–818. doi:10.1136/thx.2009.123083
- Ferguson MK, Gaissert HA, Grab JD, et al. Pulmonary complications after lung resection in the absence of chronic obstructive pulmonary disease: the predictive role of diffusing capacity. J Thorac Cardiovasc Surg. 2009;138:1297–1302. doi:10.1016/j.jtcvs.2009.05.045
- Lutchmedial SM, Creed WG, Moore AJ, et al. How common is airflow limitation in patients with emphysema on CT scan of the chest? Chest. 2015;148(1):176–184. doi:10.1378/chest.14-1556
- Makita H, Nasuhara Y, Nagai K, et al. Characterisation of phenotypes based on severity of emphysema in chronic obstructive pulmonary disease. Thorax. 2007;62:932–937. doi:10.1136/thx.2006.072777
- Mohamed Hoesein FAA, de Jong PA, Lammers JJ, et al. Airway wall thickness associated with forced expiratory volume in 1 second decline and development of airflow limitation. Eur Respir J. 2015;45:644–651. doi:10.1183/09031936.00020714