Abstract
Purpose
To identify clinical features and outcomes associated with pulmonary heart disease among patients with chronic obstructive pulmonary disease exacerbation (COPD), which may help reduce economic burden accrued over hospital stay and shorten length of stay (LOS).
Patients and Methods
Totally, 4386 patients with acute exacerbation of COPD (AECOPD) classified into pulmonary heart disease (PHD) group and non-pulmonary heart disease group, were included from the ACURE registry, a prospective multicenter patient registry study. Clinical features and outcomes were compared between groups.
Results
PHD patients had a more severe profile, including having higher scores of COPD assessment test and modified British Medical Research Council, worse lung function, more patients hospitalized more than once in the past year due to acute exacerbation of COPD, and more comorbidities. Furthermore, drug cost was higher and length of stay was longer in AECOPD patients with PHD.
Conclusion
AECOPD patients with PHD had a more severe profile and worse clinical outcomes, including higher drug cost and longer LOS. PHD is an independent risk factor of drug cost and LOS. Complicated with PHD in COPD/AECOPD patients with PHD means heavier disease burden and worse prognosis. It merits further study to focus on PHD management in COPD/AECOPD patients.
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide and imposes substantial economic and social burden.Citation1 Based on the Burden of Obstructive Lung Diseases (BOLD) program and other large-scale epidemiological studies, it is estimated that the number of COPD cases was 384 million in 2010, with a global prevalence of 11.7% (95% confidence interval (CI) 8.4–15.0%).Citation2 With the increasing prevalence of smoking in developing countries, and aging populations in high-income countries, the prevalence of COPD is supposed to rise over the next 40 years and by 2060 there may be over 5.4 million deaths annually from COPD and related conditions annually.Citation3 The overall prevalence of spirometry-defined COPD was 8.6% (95% CI 7.5–9.9) among the general Chinese population aged 20 years or older, accounting for 99.9 (95% CI 76.3–135.7) million people with COPD in China.Citation4 COPD was the third leading cause of death and accounted for more than 0.9 million deaths in 2013.Citation5
Cardiac dysfunction frequently occurs in patients with COPD. Pulmonary hypertension and pulmonary heart disease (PHD) are the typical classic cardiovascular comorbidities of COPD, which are characterized by pulmonary vascular remodeling and right ventricular hypertrophy, eventually right-side cardiac failure. The cardiovascular dysfunction is related to the risk of exacerbation, independent of previous history of exacerbations.Citation6 Several investigations have demonstrated that PHD is associated with a worsening prognosis, including increased risk of aggravation, decreased exercise ability, and reduced survival rate.Citation6–Citation8
There are few studies on PHD among patients with acute exacerbation of COPD (AECOPD), and there is no prospective multicenter study on the clinical characteristics and outcomes of AECOPD patients with PHD. Therefore, the purpose of this study was aimed to demonstrate clinical characteristics and outcomes of acute exacerbations in AECOPD patients with PHD.
Materials and Methods
Study Design and Participants
In the acute exacerbation of chronic obstructive pulmonary disease registry study (ACURE), we enrolled participants with acute exacerbation of COPD (AECOPD), with the purpose of investigating the demographic characteristics, clinical features, diagnoses, treatments, prognoses, and economic costs among AECOPD patients (ClinicalTrials.gov identifier: NCT02657525).
We planned to recruit 7600 COPD patients hospitalized for exacerbation in real-world settings from diverse areas of China with a 3-year follow-up. We started to enroll patients from September 1st, 2017, and follow-up is expected to end in December 2022. In Phase I of this study, data collection ended on February 25th, 2020. All participants in the study met the enrollment criteria and signed an informed consent form before enrollment. The eligibility criteria have been described elsewhere.Citation9
Protocol of ACURE has been approved by the ethics committee of China–Japan Friendship Hospital (approval number: 2015-88). The ACURE was conducted in accordance with the Declaration of Helsinki.
Procedures and Measurements
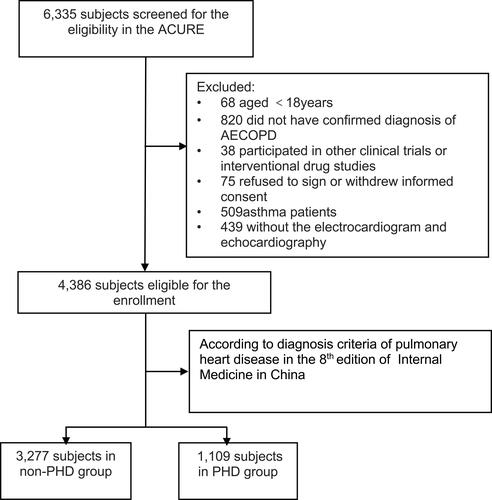
Initially, 6335 patients were screened in the ACURE study. Eligible criteria of this study were as follows: (1) 18 years or older; (2) patients had a confirmed diagnosis of AECOPD according to the GOLD 2017 report; (3) patients underwent echocardiography or electrocardiogram (ECG). Patients were excluded if they had a clinical diagnosis of asthma, acute left heart failure, rheumatic valvular heart disease, congenital heart disease, primary cardiomyopathy, or chronic constrictive pericarditis. After exclusion, 4386 patients from 163 centers were included for analysis. According to diagnosis criteria of pulmonary heart disease in the 8th edition of Internal Medicine in China, patients were divided into PHD group and non-PHD group. If patient’s ECG or echocardiography revealed one of the following features, he/she was classified into PHD group: (1) ECG revealed a pulmonary P wave; (2) ECG revealed right ventricular hypertrophy; (3) echocardiography revealed pulmonary hypertension; (4) echocardiography revealed right ventricular dilatation and right ventricular hypertrophy.Citation10 Finally, there were 1109 patients with PHD and 3277 without PHD. Details are shown in .
Figure 1 Flow chart of obtaining the study population.
Upon admission, each participant was required to complete a survey to collect their demographics, smoking history, medical history, and management of disease during a stable period. Demographics included age, sex, and body mass index (calculated from the height and weight measurements). Regarding smoking history, participants were characterized as current-smoker, former-smoker, and never-smoker. Medical history, including comorbidity, hospitalization and emergency room visits due to AECOPD in the past year, pulmonary function test (PFT), was compiled for each subject. The comorbidity was extracted from patient’s self-report, results of examination and discharge diagnosis. We assessed patient’s symptoms for the past 4 weeks before admission, due to scores of COPD assessment test (CAT) and the modified British Medical Research Council (mMRC).Citation11,Citation12 Physical examinations were conducted on admission, including pulse, systolic pressure, diastolic pressure, and respiratory rate. During the period of hospitalization, treatment and auxiliary examination results, including laboratory, PFT, ECG, and echocardiography, were recorded if available. The results of PFT were almost extracted from patients’ previous results. If the patient was lack of PFT results, he/she would undergo a PFT in follow-up after discharge. Details of the data collection have been described in previously published paper.Citation13 According to the 2017 Global Initiative for Chronic Obstructive Lung Disease (GOLD), the severity of airflow limitation was classified into four grades: GOLD 1 (forced expiratory volume in the first second, FEV1% predicted ≥ 80), GOLD 2 (50 ≤ FEV1% predicted < 80), GOLD 3 (30 ≤ FEV1% predicted < 50), and GOLD 4 (FEV1% predicted < 30). ECG and echocardiography were performed during the hospitalization. PFT, ECG, and echocardiography were performed by professional technicians and doctors. The central office underwent quality control in the report of lung function test by spot check. Details are shown in and .
Table 1 The Demographics, Smoking Status and Comorbidity of Study Population
Table 2 The Clinical Features of AECOPD Patients
At discharge, discharge diagnoses and clinical outcomes were recorded, including death, intensive care unit admission, hospitalization cost, and length of stay (LOS). AECOPD was diagnosed based on the diagnostic criteria of the 2017 GOLD guidelines. The hospitalization cost included drug cost, diagnostic-related fees (laboratory, inspection, et al), hospital stay, and other treatment-related fees. LOS was calculated as days by subtracting the admission date from the discharge date or death date at the index admission, which was the initial hospital stay. For patients readmitted frequently during the study period, their initial hospital stays were included for analyses. If patients were discharged and readmitted on the same day, the readmissions were recorded as the same hospitalization as the initial one.
Statistical Analysis
Statistical analysis was performed using SAS (V9.4 SAS Institute, Cary, NC, USA). For continuous variables, mean and standard deviation were used for data with normal distribution, while median and interquartile range (IQR) were described for non-normal data. Depending on the type of data distribution, t-test or Wilcoxon test was used for group comparisons, respectively. For categorical variables, frequencies and percentages were determined. Pearson’s chi-squared test or Fisher’s exact test was used for group comparisons. We used generalized linear model to assess the association of various factors with total cost, drug cost and LOS of AECOPD by relative risks (RRs) and 95% CIs. All statistical analyses were all two-sided tests with a significance level of 0.05.
Results
A total of 4386 AECOPD patients were included into the final analysis, with a large proportion of men (79.53%) and a mean age of 69.60 ± 9.24 years. In AECOPD patients, the number of patients with PHD was 1109 (25.28%), and the number of patients without PHD was 3277 (74.72%). The mean age of non-PHD group was 69.77 ± 9.33, and that of PHD group was 69.11 ± 8.99 (P = 0.41). Patients with PHD had more former smokers but fewer current smokers. Patients with PHD had more pulmonary and cardiovascular comorbidities, such as bronchiectasis (18.76% vs 15.87%, P = 0.026), pulmonary fibrosis (16.41% vs 12.42%, P <0.01), chronic heart failure (11.90% vs 4.94%, P < 0.01) and hypertension (39.95% vs 36.53%, P = 0.042). The details are shown in
The PHD group had lower FEV1/FVC, FVC, FEV1, and FEV1 /predicted after bronchodilator inhalation. Moreover, the proportion of GOLD 4 in the PHD group was 34.30% (330/1109), greater than that in the non-PHD group 28.04% (748/3277). Regarding hospitalization for more than once due to acute exacerbation in the past year, the PHD group had a higher proportion (58.88% vs 55.17%, P = 0.033). The symptoms of PHD group were more severe according to CAT (21.14 ± 7.12 vs 19.86 ± 6.95, P < 0.01) and mMRC scores (3.00 [2.00,4.00] vs 3.00 [2.00,3.00], P < 0.01). PHD patients’ pulse rate (90.44 ± 16.49 vs 88.75 ± 16.14, P < 0.01) was higher. In the analysis of arterial blood gas analysis, PaO2 (71.89 ± 24.83 vs 78.26 ± 26.71, P <0.01) was lower, and PaCO2 (48.37 ± 13.78 vs 45.28 ± 12.67, P < 0.01) was higher in the PHD group. The details are shown in
Regarding the clinical outcomes of interest, the median (IQR) of total hospitalization cost ($) was higher in PHD patients (1463.44 [1092.17, 2046.27] vs 1407.49 [1019.07, 1984.91], P < 0.01). The drug cost was also higher in PHD group (558.55 [386.06, 868.34] vs 542.35 [356.96, 804.73], P = 0.010). A gap of total cost between two groups was 55.95$, which was 16.2$ of drug cost. However, there was no difference in exam costs between two groups. In addition, the PHD group had longer LOS (10 [7,13] vs 9 [7,12], P < 0.01). There was no difference in all-cause readmission (2.71% vs 2.75%, P = 0.942) or COPD-related readmission (2.07% vs 2.75%, P = 0.952) between PHD group and non-PHD group in 30 ± 2 days after discharge. The analysis of death during hospitalization and 30 ± 2 days after discharge revealed no difference in two groups (0.63% vs 0.24%, P = 0.072). Details are shown in .
Table 3 The Clinical Outcomes of Study Population
In the multivariable regression model, older age, quicker pulse, higher CAT, thromboembolism, cancer were all associated with total cost of AECODP, and furthermore, older age, quicker pulse, higher CAT, thromboembolism, cancer, coronary heart disease and pulmonary heart disease were all associated with drug cost. And older age, quicker pulse, higher CAT, hospitalization due to AECOPD in past year, thromboembolism, cancer, coronary heart disease and pulmonary heart disease were all associated with length of stay. The results suggest that having a comorbidity of PHD was an independent risk factor on increasing in drug cost and LOS. However, PHD comorbidity had no effect on total cost. Details were shown in and .
Table 4 Multivariate Adjusted Relative Risk for Total Cost and Drug Cost in Study Population
Table 5 Multivariate Adjusted Relative Risk for Length of Stay in Study Population
Discussion
This study is a multicenter real-world prospective observational study to describe features of PHD among patients with AECOPD in China. The prevalence rate of PHD in patients with AECOPD was 25.28% in this study. The term “cor pulmonale/pulmonary heart disease” was used to define right ventricular dilation or right ventricular hypertrophy due to COPD. There is an increased mean pulmonary arterial blood pressure in pulmonary hypertension (PH) that causes an increased afterload for the right ventricle (RV) of the heart leading to right heart hypertrophy or RV dilatation and finally failure. COPD is the major cause of chronic respiratory insufficiency and PHD, and it is estimated to account for 80–90% of the cases.Citation14 The prevalence of PH in COPD patients varies considerably, ranging from 20% to 91%.Citation15 The incidence of severe PH in patients with GOLD4 was reported to be 3–5% (mPAP > 35 to 40mmHg).Citation16 Because the presence of PH and PHD clearly increases mortality, the occurrence of PH and PHD in COPD patients is of important prognostic relevance.Citation17,Citation18 To date, the exact prevalence of pulmonary hypertension and PHD in COPD is unknown because it is not feasible to perform right heart catheterization on a large-scale patient population.Citation6,Citation19 Prevalence and mortality of PHD in AECOPD were seldom reported in China and other countries during the past decades. PHD has not gained sufficient attention of health-care providers in clinical practice. In this study, only 176 (15.87%) AECOPD patients with PHD had been diagnosed with PHD, which means the majority of PHD diagnoses (933/1109, 84.13%) were missed. However, among patients without PHD, 216 (6.59%) non-PHD patients were misdiagnosed as PHD.
In our analysis, AECOPD patients with PHD had more severe clinical features and worse outcomes. There was obvious statistical significance in smoking status between two groups. PHD group had more former smokers and less current smokers than non-PHD group. Growing evidence supports the injurious effect of cigarette smoke on pulmonary endothelial cells and the roles of endothelial cell injury in development of pulmonary hypertension.Citation20 Wright et al reveal that PH is probably a result of the direct effect of tobacco smoke on aberrant vascular remodeling and aberrant vascular physiology.Citation21,Citation22 Tobacco smoking is also associated with higher frequency of cardiovascular comorbidities.Citation23 Smoking cessation is the single most cost-effective way to reduce the risk of development of COPD and worsening of the disease the patients with COPD.Citation1
The analysis of comorbidities revealed that COPD patients with PHD had more cardiopulmonary comorbidities, such as bronchiectasis, pulmonary fibrosis, chronic heart failure, and hypertension. COPD-overlap syndromes, including bronchiectasis-COPD and fibrosis-COPD, are risk factors of chronic hypoxia, which is the cause of PH. The severity of disease in PH-associated COPD significantly increases in individuals who also have pulmonary fibrosis or emphysema.Citation17 And Medrek et al also revealed that PH-COPD carried a much-effective diagnosis of various cardiac comorbidities.Citation7 Piccari et al had the same result that COPD patients with pulmonary vascular dysfunction had higher prevalence of systemic arterial hypertension.Citation24 Notably, this comorbidity-rich subtype had more frequent hospitalizations, with a worse prognosis and an increased risk of mortality in COPD patients.Citation25,Citation26
Similar to prior researches, our findings showed lower FEV1/FVC, FEV1 (L), FVC (L) and FEV1%predicted in patients with PHD.Citation27,Citation28 According to PFT, most patients with PHD were classified as GOLD 4. The risk of exacerbations is significantly higher in patients with GOLD 3 (severe) and GOLD 4 (very severe).Citation29,Citation30 PHD patients had lower PaO2 and higher PaCO2 in acute exacerbation, indicating that airflow obstruction and ventilatory dysfunction of COPD patients with PHD were severe than those without PHD.Citation31 Specifically, the pulse in PHD group was higher than that in non-PHD group. Patients with PHD had a more obvious compensatory performance. Long-term oxygen therapy (LTOT) is the major effective treatment of PHD.Citation32
In the analysis of symptoms to assess the severity of AECOPD patients, CAT and mMRC scores were higher in patients with PHD. In the past year, there was a larger proportion of patients in PHD group who were hospitalized more than once. Prior findings showed that the AECOPD hospitalization in the past year could increase the risk of the hospitalization for acute exacerbation in COPD patients in the following year.Citation33,Citation34 To sum up, AECOPD patients with PHD had a worse prognosis.
Concerning the outcomes of AECOPD patients with PHD, our study demonstrated that PHD patients had higher drug cost and longer LOS. The cost of hospitalization in the PHD group was $1463.44 in the PHD group, which was $1407.49 in the non-PHD group. The gap in the cost between the two groups was $55.95. Drug costs in non-PHD and PHD groups were $542.35 vs $558.55, which were significantly different. The results of the multivariable regression also suggested that PHD is an independent risk factor for medication cost. Second, the results showed that the LOS of the patients with and without PHD was 10 [7, 13] and 9 [7, 12], respectively. The multivariable regression analysis also suggested that PHD is an independent risk factor for LOS.
Finally, we also analyzed readmission and death within 30 ± 2 days after discharge. There were 30 (2.71%) patients readmitted in 30±2 days after discharge in PHD group, and among them 23 (23/30, 76.67%) patients were readmitted for COPD in PHD group. In non-PHD group, there were 90 (2.75%) readmitted in 30 ± 2 days after discharge, and 67 (67/90, 74.44%) readmitted for COPD in non-PHD. There were no difference in all-cause or COPD-related readmission within 30 ± 2 days after discharge in two group. To date, the conclusions of the risk and cause of readmission are inconsistent. Jiang et al revealed that having medical comorbidities, including osteoporosis, depression, pulmonary circulation disorders, and ischemic heart disease, were associated with increased probability of COPD-related 30-day readmission.Citation35,Citation36 More resources may be needed to generate a measurable effect on readmission rates. And there was also no difference in the death from admission to 30 ± 2 days after discharge. It is worth mentioning that only 15 patients died in this period, including 8 patients in non-PHD group (0.24%), 7 patients in PHD group (0.63%) and the P value was 0.072. Since the follow-up period was only 30 ± 2 days, the number of cases was relatively small. There may be some differences in readmission and death in the follow-up for a long time.
COPD exacerbations account for the greatest proportion of the total COPD burden on the healthcare system.Citation1 Considering of the prior studies and our research, patients with PHD had a higher risk of hospitalization due to exacerbation, and PHD is an independent risk factor for medication cost and LOS, that were obvious higher in PHD patients. Consequently, the disease burden of AECOPD patients with PHD was greater than that of patients without PHD.
Therefore, ways to reduce this burden should be explored. First, the overall prevalence of spirometry-defined COPD was 99.9 (95% CI 76.3–135.7) million people with COPD among the general Chinese population aged 20 years or older in 2015.Citation4 The prevalence rate of PHD in patients with AECOPD was 25.28% in our multicenter research. There would be a considerable number of COPD patients complicated with PHD. Our study found that only a small number of PHD patients were diagnosed timely, which suggests that it is necessary and urgent to perform large-scale population screening for PHD in COPD/AECOPD patients. Medrek et al suggest clinical characterization of COPD patients at risk who are progressing toward PH will aid therapeutic development at earlier stages of progressively fatal PH-COPD.Citation7 Early diagnosis could prompt treatment strategy in early stage of PHD. Second, PHD treatment is supposed to maintain lung function and keep oxygen saturation level at above 90%.Citation29 The management of COPD, especially slowing down the deterioration of lung function, is important in the intervention of PHD. We observed that clinical management of COPD during the stable period was unsatisfactory in China in another analysis of ACURE database.Citation13 Therefore, it needs to strengthen management of COPD in the stable stage needs to be popularized and strengthened, which could then delay the decline of lung function and reduce acute exacerbation of COPD. Third, PHD treatment is mainly to maintain lung function and oxygen therapy, which has remained almost unchanged for the past 3 decades.Citation31 There is no remarkable progress in the treatment of PHD in past decades. Studies on other treatments, such as diuretics, digoxin, anticoagulants, angiotensin converting enzyme inhibitors (ACEIs), and beta-blockers et al, yielded inconsistent findings.Citation37–Citation41 In general, the treatment and management of PHD require further studies.
There were several limitations in our study. First, the diagnosis of PHD was based on the echocardiography or ECG, not the right ventricular floating catheter examination. Although the right ventricular floating catheter examination is the gold standard for pulmonary hypertension and PHD, but it is not feasible to perform in large-scale population screening due to its invasiveness nature. In many studies, echocardiography and ECG also have high sensitivity and specificity in the diagnosis of PHD, and they are easily used and economical in large-scale surveys.Citation42−Citation43 Second, the patients enrolled in this study were all at the acute exacerbation stage of COPD, and we did not obtain the echocardiographic and ECG findings of patients at the stable stage before admission for AECOPD. Data collection was ended on 25th February 2020 in our study. The ACURE is an ongoing observational registry study. During the follow-up, evaluation of patients in stable stage would be collected, including symptoms, signs, frequency of exacerbation and comparison of the echocardiography, ECG, PFT, and blood gas analysis. Additionally, the details concerning treatment of PHD were not collected in our study, except the oxygen therapy; hence, we could not analyze the treatment of PHD profoundly. We need more further studies on management of PHD in COPD/AECOPD patients.
To the best of our knowledge, our study is the first multicenter, real-world, and large-scale prospective registry study to screen PHD among AECOPD patients by use of echocardiography and ECG. Our study demonstrated clinical outcomes of AECOPD patients with PHD, including higher drug cost and longer LOS. As the diagnosis of PHD in COPD is not necessarily associated with conventional measures of COPD severity, such as airflow limitations, we found an underestimation of PHD in patients with AECOPD. This indicates a potential need for management of COPD patients with PHD screening programs. Further studies focusing on PHD management are warranted.
Conclusion
AECOPD patients with PHD had a more severe profile, such as worse lung function, severe symptoms and hypoxemia, higher risk of hospitalization, and worse clinical outcomes, including higher drug cost and longer LOS. PHD is an independent risk factor of drug cost and LOS. COPD/AECOPD patients with PHD predict more disease burden and worse prognosis. Considering the underestimation of PHD, it is necessary and useful to focus on the diagnosis and treatment of PHD in COPD/AECOPD patients.
Acknowledgments
The authors thank all participants, staff and investigators involved in the ACURE from all participating sites for their efforts in providing and collecting the data used in this study.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2021 report. Available from: https://goldcopd.org/2021-gold-reports/. Accessed October 8, 2021.
- Global and regional estimates of COPD prevalence: systematic review and meta-analysis - PubMed [Internet]; [ cited June 16, 2021]. Available from: https://pubmed.ncbi.nlm.nih.gov/26755942/. Accessed October 8, 2021.
- World Health Organization. Projections of mortality and causes of death, 2016 and 2060. Available from: http://www.who.int/healthinfo/global_burden_disease/projections/en/. Accessed October, 2020.
- Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi:10.1016/S0140-6736(18)30841-9
- Zhou M, Wang H, Zhu J, et al. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387(10015):251–272. doi:10.1016/S0140-6736(15)00551-6
- Washko GR, Nardelli P, Ash SY, et al. Arterial vascular pruning, right ventricular size, and clinical outcomes in Chronic Obstructive Pulmonary Disease. A longitudinal observational study. Am J Respir Crit Care Med. 2019;200(4):454–461. doi:10.1164/rccm.201811-2063OC
- Medrek SK, Sharafkhaneh A, Spiegelman AM, et al. Admission for COPD exacerbation is associated with the clinical diagnosis of pulmonary hypertension: results from a retrospective longitudinal study of a Veteran population. COPD. 2017;14(5):484–489. doi:10.1080/15412555.2017.1336209
- Roversi S, Fabbri LM, Sin DD, et al. Chronic Obstructive Pulmonary Disease and cardiac diseases. an urgent need for integrated care. Am J Respir Crit Care Med. 2016;194(11):1319–1336. doi:10.1164/rccm.201604-0690SO
- Pei Z, Sun Y, Wang S, et al. Estimating mortality among inpatients with acute exacerbation of chronic obstructive pulmonary disease using registry data. Npj Prim Care Respir Med. 2020;30(1):28. doi:10.1038/s41533-020-0186-y
- Ge J, Xu Y. Internal Medicine. 8th ed. China: People’s Medical Publishing House; March, 2013:113.
- Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.00102509
- Cheng S-L, Lin C-H, Wang -C-C, et al. Comparison between COPD Assessment Test (CAT) and modified Medical Research Council (mMRC) dyspnea scores for evaluation of clinical symptoms, comorbidities and medical resources utilization in COPD patients. J Formos Med Assoc. 2019;118(1):429–435. doi:10.1016/j.jfma.2018.06.018
- Liang C, Mao X, Niu H, et al. Characteristics, management and in-hospital clinical outcomes among inpatients with acute exacerbation of Chronic Obstructive Pulmonary Disease in China: results from the phase I data of ACURE study. Int J Chron Obstruct Pulmon Dis. 2021;16:451–465. doi:10.2147/COPD.S281957
- Weitzenblum E. Chronic cor pulmonale. Heart. 2003;89(2):225–230. doi:10.1136/heart.89.2.225
- Shujaat A, Minkin R, Eden E. Pulmonary hypertension and chronic cor pulmonale in COPD. Int J Chron Obstruct Pulmon Dis. 2007;2:273–282.
- Opitz I, Ulrich S. Pulmonary hypertension in chronic obstructive pulmonary disease and emphysema patients: prevalence, therapeutic options and pulmonary circulatory effects of lung volume reduction surgery. J Thorac Dis. 2018;10(S23):S2763–74. doi:10.21037/jtd.2018.07.63
- Cuttica MJ, Kalhan R, Shlobin OA, et al. Categorization and impact of pulmonary hypertension in patients with advanced COPD. Respir Med. 2010;104(12):1877–1882. doi:10.1016/j.rmed.2010.05.009
- Naeije R. Pulmonary hypertension and right heart failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2(1):20–22. doi:10.1513/pats.200407-037MS
- NICE Guideline Updates Team (UK). Managing Pulmonary Hypertension and Cor Pulmonale: Chronic Obstructive Pulmonary Disease in Over 16s: Diagnosis and Management: Evidence Review a [Internet]. London: National Institute for Health and Care Excellence (UK); 2018 [ cited June 17, 2021]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK560180/. Accessed October 8, 2021.
- Lu Q, Gottlieb E, Rounds S. Effects of cigarette smoke on pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2018;314(5):L743–56. doi:10.1152/ajplung.00373.2017
- Wright J, Levy R, Churg A. Pulmonary hypertension in chronic obstructive pulmonary disease: current theories of pathogenesis and their implications for treatment. Thorax. 2005;60(7):605–609. doi:10.1136/thx.2005.042994
- Wright JL, Zhou S, Churg A. Pulmonary hypertension and vascular oxidative damage in cigarette smoke exposed eNOS(-/-) mice and human smokers. Inhal Toxicol. 2012;24(11):732–740. doi:10.3109/08958378.2012.715698
- Soumagne T, Guillien A, Roche N, et al. In patients with mild-to-moderate COPD, tobacco smoking, and not COPD, is associated with a higher risk of cardiovascular comorbidity. Int J Chron Obstruct Pulmon Dis. 2020;15:1545–1555. doi:10.2147/COPD.S253417
- Piccari L, Del Pozo R, Blanco I, et al. Association between systemic and pulmonary vascular dysfunction in COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:2037–2047. doi:10.2147/COPD.S257679
- Martínez-García MA, de la Rosa Carrillo D, Soler-Cataluña JJ, et al. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(8):823–831. doi:10.1164/rccm.201208-1518OC
- Putcha N, Drummond MB, Wise RA, et al. Comorbidities and Chronic Obstructive Pulmonary Disease: prevalence, influence on outcomes, and management. Semin Respir Crit Care Med. 2015;36(04):575–591. doi:10.1055/s-0035-1556063
- Kaushal M, Shah PS, Shah AD, et al. Chronic obstructive pulmonary disease and cardiac comorbidities: a cross-sectional study. Lung India. 2016;33(4):404–409. doi:10.4103/0970-2113.184874
- Buklioska-Ilievska D, Minov J, Kochovska-Kamchevska N, et al. Cardiovascular comorbidity in patients with Chronic Obstructive Pulmonary Disease: echocardiography changes and their relation to the level of airflow limitation. Open Access Maced J Med Sci. 2019;7(21):3568–3573. doi:10.3889/oamjms.2019.848
- Soriano JB, Lamprecht B, Ramírez AS, et al. Mortality prediction in chronic obstructive pulmonary disease comparing the GOLD 2007 and 2011 staging systems: a pooled analysis of individual patient data. Lancet Respir Med. 2015;3(6):443–450. doi:10.1016/S2213-2600(15)00157-5
- Blanco I, Tura-Ceide O, Peinado VI, et al. Updated perspectives on pulmonary hypertension in COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:1315–1324. doi:10.2147/COPD.S211841
- Sakao S. Chronic obstructive pulmonary disease and the early stage of cor pulmonale: a perspective in treatment with pulmonary arterial hypertension-approved drugs. Respir Investig. 2019;57(4):325–329. doi:10.1016/j.resinv.2019.03.013
- Branson RD. Oxygen therapy in COPD. Respir Care. 2018;63(6):734–748. doi:10.4187/respcare.06312
- Jennings JH, Thavarajah K, Mendez MP, et al. Predischarge bundle for patients with acute exacerbations of COPD to reduce readmissions and ED visits: a randomized controlled trial. Chest. 2015;147(5):1227–1234. doi:10.1378/chest.14-1123
- Hunter LC, Lee RJ, Butcher I, et al. Patient characteristics associated with risk of first hospital admission and readmission for acute exacerbation of chronic obstructive pulmonary disease (COPD) following primary care COPD diagnosis: a cohort study using linked electronic patient records. BMJ Open. 2016;6(1):e009121. doi:10.1136/bmjopen-2015-009121
- Jiang X, Xiao H, Segal R, Mobley WC, Park H. Trends in readmission rates, hospital charges, and mortality for patients with Chronic Obstructive Pulmonary Disease (COPD) in Florida from 2009 to 2014. Clin Ther. 2018;40(4):613–626.e1. doi:10.1016/j.clinthera.2018.03.006
- Alqahtani JS, Aquilina J, Bafadhel M, et al. Research priorities for exacerbations of COPD. Lancet Respir Med. 2021;9(8):824–826. doi:10.1016/S2213-2600(21)00227-7
- Rowan SC, Keane MP, Gaine S, et al. Hypoxic pulmonary hypertension in chronic lung diseases: novel vasoconstrictor pathways. Lancet Respir Med. 2016;4(3):225–236. doi:10.1016/S2213-2600(15)00517-2
- Alajaji W, Baydoun A, Al-Kindi SG, et al. Digoxin therapy for cor pulmonale: a systematic review. Int J Cardiol. 2016;223:320–324. doi:10.1016/j.ijcard.2016.08.018
- Sperrin M, Webb DJ, Patel P, et al. Chronic obstructive pulmonary disease exacerbation episodes derived from electronic health record data validated using clinical trial data. Pharmacoepidemiol Drug Saf. 2019;28(10):1369–1376. doi:10.1002/pds.4883
- Qiu J, Guo Y, Xu X, et al. Ginkgo leaf extract and dipyridamole injection for chronic cor pulmonale: a PRISMA-compliant meta-analysis of randomized controlled trials. Biosci Rep. 2020;40(3):BSR20200099. doi:10.1042/BSR20200099
- Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25):D109–D116. doi:10.1016/j.jacc.2013.10.036
- Mandoli GE, Sciaccaluga C, Bandera F, et al. Cor pulmonale: the role of traditional and advanced echocardiography in the acute and chronic settings. Heart Fail Rev. 2021;26(2):263–275. doi:10.1007/s10741-020-10014-4
- Hilde JM, Skjørten I, Grøtta OJ, et al. Right ventricular dysfunction and remodeling in chronic obstructive pulmonary disease without pulmonary hypertension. J Am Coll Cardiol. 2013;62(12):1103–1111. doi:10.1016/j.jacc.2013.04.091
