Abstract
Background
αB-crystallin (HspB5) is a chaperone whose role as a marker of innate immunity activation as well as its therapeutic potential have recently been investigated in several inflammatory diseases: multiple sclerosis, myocardial ischemia, and Guillain–Barré syndrome.
Aim
The aim of this study is to determine the role of αB-crystallin in chronic obstructive pulmonary disease (COPD) pathogenesis and inflammation.
Materials
Plasma levels of αB-crystallin were studied in 163 patients: 52 healthy non-COPD smokers; 20 COPD smokers in Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages I–II; 43 COPD smokers in GOLD stages III-IV. Forty-eight patients were diagnosed with acute inflammatory respiratory disease. The plasma levels of αB-crystallin antibodies were determined by an enzyme-linked immunosorbent assay (Calbiochem), and were confirmed with Western blotting. Tissue expression of the protein was compared in three different groups of patients: COPD smokers, COPD nonsmokers, and in patients with age-related emphysema.
Results
The mean level of anti-αB-crystallin antibodies in non-COPD smokers was 0.291nm. In COPD smokers it was 0.352 nm and, in patients with inflammatory lung diseases, 0.433 nm. There was a statistically significant difference between COPD smokers and healthy non-COPD smokers (P = 0.010). The same could be observed comparing the group of patients with acute inflammation and non-COPD healthy smokers (P = 0.007). There was no statistically significant difference between patients with mild/moderate inflammation and those with severe COPD. Tissue detection of the protein showed that it was significantly overexpressed in COPD smokers in comparison to COPD nonsmokers and was only slightly expressed in patients with age-related emphysema.
Conclusion
αB-crystallin is increased in patients with inflammatory lung diseases. Though unspecific, it could be used in a panel of markers discerning COPD smokers from healthy nonsmokers. As αB-crystallin is a regulator of innate immunity and a therapeutic anti-inflammatory agent, its exact role in COPD pathogenesis and therapy should be explored further.
Keywords:
Introduction
The molecular basis for tobacco smoke-induced airway diseases is poorly understood. To unravel thoroughly the cellular and molecular events or signaling pathways that may contribute to the pathogenesis of smoke-induced emphysema or chronic obstructive pulmonary disease (COPD), gene expression profiling – serial analysis of gene expression and microarray analysis as well as proteomics – has recently been applied. The gene expression profiles of lung tissues from control smokers (Global Initiative for Chronic Obstructive Lung Disease [GOLD-0]) and moderate (GOLD-2) COPD smokers identified numerous classes of genes, the expression of which is altered in COPD patients. These include genes encoding molecules for signal transduction, receptor function, growth factor, nuclear chromatin and DNA binding, adhesion and cytoskeleton, metabolism, matrix, cell cycle, and oxidative stress such as the HSP70 protein heme oxygenase (decycling) 1 (HO-1).Citation1 The data from proteomics also confirms a large number of proteins related to cigarette smoke induced endoplasmic reticulum stress, repair/injury proteins, heat-shock proteins, apoptosis, and cell cycle-responsible molecules.Citation2
COPD is obviously a disease of imbalance of proteins – oxidant/antioxidant,Citation3 protease/antiprotease,Citation4 apoptosis/ proliferation,Citation5 acetylases/deacetylasesCitation6 – that can no longer perform their proper function to keep the homeostasis in the new environmental settings of the oxidative stress.
Molecular chaperones provide the functional activity of proteins. They counteract the formation of aberrantly folded polypeptides and allow their correct refolding under stress recovery. Chaperones are responsible for protein folding and allow the functional state of cells to be maintained by preventing irreversible protein unfolding and aggregation. Their expression is one of the most evolutionarily conserved protective mechanisms in cells. They are stimulated under “stress” (thermal, metabolic, oxidative, etc) when the conditions of the cell environment are deleterious and alter the protein folding and their proper biological activity.Citation7,Citation8 Heat-shock proteins also participate in the protein triage and modulate the ubiquitin-proteasome pathway, promoting the degradation of irreversibly denatured proteins. They play critical roles as major cellular antistress and antidisease mechanisms.Citation9,Citation10 Defective chaperones are most probably an additional factor, accompanying the development and progression of senescence and age-associated diseases (neurodegenerative, cancer, atherosclerosis, COPD, etc) most of which are aggravated by stress.Citation11,Citation12
Recent studies shed light on the role of chaperones in COPD pathogenesis, describing them as one of the first molecules that are upregulated. Data are obtained with gene expression and proteomic studies in Hsp70, Hsp27, and Hsp32 (heme oxygenase).Citation1,Citation2 Elevated plasma levels of heat-shock proteins (Hsp70, Hsp27, Hsp32) have also been reported in COPD patients, presenting a new concept for the disease: a chaperonopathy.Citation13,Citation14
The aim of this study was to analyze the clinical significance of the small heat-shock protein αB-crystalline as a biomarker in COPD pathology, progression, and diagnosis. The relation of αB-crystalline plasma levels with already known pathological markers (matrix metallopeptidase-9 [MMP-9] and high-sensitivity C-reactive protein [hsCRP]) and its tissue expression in lung samples from COPD smokers and non-COPD smokers will also be discussed.
Materials and methods
The expression of αB-crystalline was examined in two different groups of patients: (1) the plasma levels were determined in a prospective study including 163 patients (COPD and non-COPD smokers) as well as patients with acute respiratory tract infection; (2) the tissue expression was determined on archival specimens of a retrospective group of patients.
Plasma levels of αB-crystalline, MMP-9, and hsCRP
Plasma levels of αB-crystalline were determined in a total of 163 patients. The study protocol was approved by the Ethics Committee of the Medical University in Sofia. All patients gave their informed consent before entering the study. Fifty-two healthy smoking volunteers, 48 smokers with acute inflammatory respiratory diseases, and 63 COPD smokers were recruited from the Clinic of Pulmonology in the period 2010–2011. Patients were randomly selected for the study. There were exclusion criteria only in the COPD group. Patients were excluded for a severe form of COPD with more than four exacerbations per year, an exacerbation or any cause of hospitalization that required antibiotic treatment or the systemic application of steroids within the last 6 weeks before entering the study, a history of respiratory tract infection within the last 4 weeks, or a history of cancer. All the patients were properly treated, referring to the GOLDCitation15 criteria and had combined inhalation therapy (long-acting β-agonists/glucocorticoids ± long-acting muscarinic antagonist). Patients’ characteristics are presented in .
Table 1 Patient characteristics for whom plasma MMP-9, hsCRP, and αB-crystallin were investigated
Pulmonary function tests were performed using a Profiler spirometer (MedGraphics, St Paul, MN). Measurements were made before and 20 minutes after inhalation of 400 μg salbutamol. COPD was defined according to GOLD (2008) criteria.Citation15
Blood samples were collected before any treatment and interventions. Plasma was acquired after centrifugation and aliquots were kept frozen at −20°C until testing.
Plasma levels of small heat-shock protein anti-αB-crystallin antibodies
Enzyme-linked immunosorbent assay (ELISA)
Anti-αB-crystallin antibodies in plasma were determined by ELISA using 96-well microtiter plates. As an antigen we used α-crystallin isolated by gel chromatography of porcine eye lens extract.Citation16 Polyacrylamide gel electrophoresis (PAGE; 12% running gel) was used in order to demonstrate the purity of the α-crystallin fraction. Polystyrene 96-well assay plates were coated with 10 mg/mL of antigen and incubated for 1 hour at room temperature (RT) and at 4°C overnight. Plates were washed three times with 0.05% Tween 20 phosphate-buffered saline (PBS). Nonspecific binding was reduced by incubating with 0.2% Tween 20 PBS for 2 hours at RT. Plates were washed again. Serum dilutions of 1:50 in PBS, 50 mL well added to each well and incubated for 2 hours at RT. After another washing, the plates were incubated for 1 hour at RT with rabbit peroxidase-conjugated anti-human IgG (gamma chain-specific; Sigma-Aldrich, St Louis, MO) (diluted 1:10,000) as a secondary antibody. The reaction was developed using ex tempore prepared solution of o-phenylenediamine in citrate buffer, pH 5.0 containing 0.015% HO and stopped 20 minutes later with 10% HSO. The optical density was measured at 492 nm on the ELISA reader. All samples were analyzed in duplicates. The inter- and intra-assay variability was <10%.
Western blotting
In 31 of the plasma samples Western blotting was also performed. Samples for Western blotting were subjected to sodium dodecyl sulfate/PAGE electrophoresis on a 12% polyacrylamide minigel. After electrophoresis, protein lanes were blotted onto a poly(vinylidene fluoride) (Millipore, Billerica, MA) membrane according to the manufacturer’s instructions for 50 minutes. The membrane was blocked with dry powder milk for 2 hours at RT. Incubation of filters with the primary antibody (diluted 1:25) was performed overnight at 4°C. After rinsing three times with PBS the secondary (anti-human IgG, conjugated to peroxidase; Sigma-Aldrich) antibody was added (diluted 1:500) for 1 hour at RT. A PBS rinse was again performed. The reaction was stopped with distilled water. Antibody binding to blotted antigens was revealed by incubation with diaminobezidin.
MMP-9
Plasma levels of MMP-9 were measured only in 26 COPD patients with GOLD III (GOLD, 2008) using RayBiotech (Norcross, GA) Human MMP-9 ELISA kit (Cat # ELH MMP9-001) according to the manufacturer’s instructions. In brief, plasma samples and standards were incubated in 96-well microtiter plates, precoated with human MMP-9. After a washing step, horseradish peroxidase (HRP)-conjugated antibody was added and plates were incubated for 24 hours. Plates were washed and TMB substrate was added. Color development was stopped and optical density determined at 450 nm. The amount of protein in each sample was calculated according to a standard curve of optical density values constructed for known levels of MMP-9. According to the manufacturer, the sensitivity of ELISA was determined to be 10 pg/mL.
hsCRP
Plasma levels of hsCRP were determined in 26 COPD patients with GOLD III (GOLD, 2008). The plasma levels were routinely analyzed in the Department of Laboratory Medicine at the Medical University of Sofia.
Immunohistochemical expression of small heat-shock protein αB-crystalline
We studied the tissue expression of the small heat-shock protein αB-crystalline in archival specimens of 28 COPD patients, 26 non-COPD smokers, and 14 patients with age-related emphysema. COPD was defined according to the 2008 GOLD criteria.
Archival specimen and medical data were retrospectively obtained by the Pathology Unit of the Medical University, Sofia. Appropriate tissue specimens were collected among the archive in the period 2006–2011. summarizes patient characteristics.
Table 2 Patients’ characteristics with investigated tissue samples
We studied 28 consecutive bronchial biopsies from patients with a history of cigarette smoking (mean 27.8 packs/ year consumption) affected by moderate-to-severe COPD.
Immunohistochemistry
The slides were deparaffinized in xylene and hydrated through graded ethanol. The endogenous peroxidase activity was blocked by 5 minutes of incubation in a 3% hydrogen peroxide–methanol buffer. Subsequently, the slides were incubated with 2.5% normal horse serum/PBS for 30 minutes at RT to reduce nonspecific background staining. A primary polyclonal anti-rabbit αB-crystalline antibody (Calbiochem Cat No: 238702; Novabiochem, Boston, MA) was applied for 24 hours; human (rabbit) (diluted at a 1:1000 in PBS) was applied for 24 hours at 2°C–4°C. This was followed by a series of three rinses of PBS. A subsequent incubation with secondary biotinylated anti-rabbit IgG antibody (diluted 1:400) was performed. After a series of PBS rinses, streptavidin– HRP Universal Elite ABC kit (Vectastain, Burlingame, CA) was applied for 1 hour at 37°C in the humidity chamber. Slides were again rinsed three times in PBS and visualized by a 5-minute incubation with liquid 303-diaminobezidin in buffered substrate. Finally, slides were counterstained with hematoxylin and eosin. In negative controls, normal horse serum was used and primary antibodies were omitted.
Evaluation of immunohistochemical staining
The evaluation of the slides was done independently by two investigators. They examined the slides by light microscopy (Nikon, Tokyo, Japan) (100 × 2.5) in a blinded manner with respect to the clinical data. In case of disagreement, both reached a consensus by jointly evaluating the slides, using a multihead microscope (DMLS; Leica Microsystems GmbH, Wetzlar, Germany). Immunostaining was classified according to its intensity as follows: 0 = no staining; 1 = weak staining; 2 = moderate staining; and 3 = strong staining.
The whole slide was analyzed. The number of cells with one and the same intensity of staining was presented as a percentage. Results are presented as the mean percentage of cells with one and the same intensity of staining ± standard deviation (SD) for the whole study group.
Statistical methods
SPSS Software (IBM, Armonk, NY) was used to analyze the results. Data are expressed as mean ± SD. Pair-wise comparisons between groups were performed using a t-test (when a normal distribution was found) and a Mann-Whitney test (in case of a lack of a normal distribution). Correlation coefficients were calculated using the Spearman rank method or Pearson correlation coefficient. A P-value < 0.05 was considered to be statistically significant.
Results
Plasma levels of anti-αB-crystalline antibodies between groups
The mean values of anti-αB-crystalline antibodies in COPD smokers and healthy smokers are presented in .
Table 3 Plasma levels of anti-αB-crystalline antibodies between groups
As αB-crystalline was not normally distributed between groups, data are presented as mean ± SD. In COPD patients, the plasma levels of αB-crystalline antibodies are 0.352 ± 0.12 nm; in healthy smokers, 0.291 ± 0.07 nm; and in smokers with inflammatory lung diseases, 0.433 ± 0.27 nm. A statistically significant difference was established between the COPD patients and the healthy smoking volunteers (P = 0.01) and between smokers with inflammatory lung diseases and the healthy smoking volunteers (P = 0.007). In comparison, there was not a significant difference between the COPD patients between groups. This result holds even after adjustment for age, pack years, and forced expiratory volume in 1 second (FEV1).
Applying correlation analysis no association could be established between age, pack years, or spirometric measurements (FEV1) and the plasma levels of anti-αB-crystalline antibodies.
Plasma levels of αB-crystalline, MMP-9, hsCRP, and clinical characteristics of COPD patients
In order to unravel the role of αB-crystalline in COPD pathology and progression we analyzed the correlation of the studied biomarker with the clinical parameters characterizing COPD. No statistically significant difference was found. The Pearson coefficient was insignificant when analyzing the correlation between age, pack years, FEV1, and the plasma levels of anti-αB-crystalline antibodies ().
Table 4 Plasma levels of anti-αB-crystalline antibodies, clinical parameters, and inflammatory markers of COPD smokers
In comparing the mean plasma levels of anti-αB-crystalline antibodies between the groups with mild COPD (GOLD I–II) and those of severe and very severe disease (GOLD III–IV), no statistically important difference was found.
Plasma levels of anti-αB-crystalline antibodies, MMP-9, and hsCRP
In 26 COPD patients (GOLD III), the well-accepted markers for inflammation and extracellular degradation were determined. The mean values for MMP-9 and hsCRP were 0.899 ± 0.30 pg/mL and 4.35 ± 3.11 mg/L, respectively.
No relation between these markers and anti-αB-crystalline antibodies could be demonstrated. Results for MMP-9 (P = 0.760) and hsCRP (P = 0.911) are shown in .
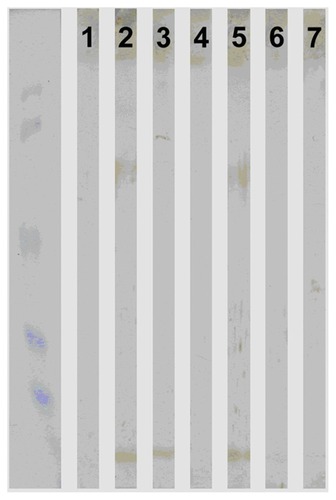
Western blotting
The specificity of the results of plasma anti-αB-crystalline antibodies was confirmed with Western blotting. The plasma samples of 31 patients – 20 with COPD and 11 healthy smokers were investigated in addition with Western blotting – are shown in . There was good coincidence between ELISA results and the Western blotting.
Tissue expression of αB-crystalline
αB-crystalline was detected in the alveolar pneumocytes. The bronchial epithelium was also stained, but only in the cytoplasm. Although only partially, there were a few areas where basal epithelial cells of the ciliated bronchial epithelium showed cytoplasmic staining and no nuclear staining. Macrophages infiltrating the samples were also positive for αB-crystalline. They had cytoplasmic staining, but the nuclear staining varied from intensive to faint. Apoptotic and necrotic cells had faint cytoplasmic and intensive nuclear staining. Intensive nuclear staining was also detected in cells undergoing mitosis ().
Figure 2 Tissue expression of αB-crystalline in lung tissues from non-COPD smokers, age-related emphysema, and COPD patients.
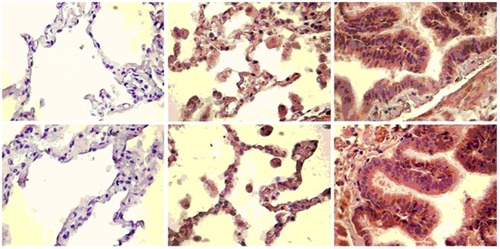
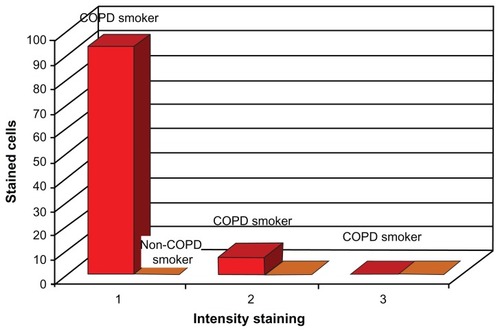
The pathologic assessment of bronchial biopsies from all COPD patients showed that the tissue samples of this study group contained mainly alveolar epithelium. In the COPD patients, immunopositivity for αB-crystalline was as follows: 92.86% of the cells in the samples had intensive staining, 7.14% had moderate staining, and none of the samples had cells that were faintly stained. Regarding the patients with age-related emphysema, intensive staining was observed in 35.7%, moderate staining in 14.2%, and weak staining in 50.1% of the cells. The non-COPD smokers showed no staining (see and ).
Table 5 Tissue expression of αB-crystalline in COPD-smokers, non-COPD smokers, and age-related emphysema
Figure 3 Schematic representation of the tissue expression of αB-crystalline from COPD-smokers, non-COPD smokers, and age-related emphysema.
Abbreviation: COPD, chronic obstructive pulmonary disease.
In analyzing the age, duration of the risk factor exposure, and the spirometric characteristics between groups, we did not find statistically significant differences that could potentially influence the expression of αB-crystalline.
Discussion
Oxidative stress does exist in the lungs and it is inevitable, keeping in mind its physiology. Maintaining its balance is a kind of self-protection mechanism for the organ. COPD is a disease of imbalance, with oxidant/antioxidant,Citation3 protease/ antiprotease,Citation4 apoptosis/proliferation,Citation5 acetylases/deacetylases,Citation6 and accelerated agingCitation17 consuming the physiologically relevant protective mechanisms. Despite the many possible etiologies triggering its development, the underlying pathophysiology however remains poorly understood. In most cases, COPD develops in smokers, which leads us to think that it is necessarily related to smoking. However, only 20% of smokers develop COPD. Moreover, the presence of nonsmokers with COPD is vaguely understood. Bouchecareilh and BalchCitation18 have presented nonsmokers’ COPD as a proteinopathy, a failure of the proteostasis, where disbalance in lung cells, even in the absence of exogenous noxa, triggers the cell stress response and sets a “danger” signal to the innate immunity.
Recently many studies have tried to determine COPD as a chaperonopathology, presenting the significance of different extracellular cell stress (heat-shock) proteins (hsp90, hsp70, hsp60, and hsp27) both as diagnostic markers and as mediators that activate the Toll-like receptors and provoke inflammation.Citation1,Citation2,Citation18 Cigarette smoke condensate is claimed to be a major stimulus for Hsp70 secretion, which binds to TLR4 and stimulates IL-8 and neutrophil recruitment.Citation13 It is also among the early upregulated transcripts in serial gene expression analysis. Proteomics define Hsp27 as among the upregulated proteins in lung lysates. Plasma levels of Hsp27 have been defined as COPD diagnostic markers and markers of disease progression.Citation2
Our previous work showed that elevated αB-crystalline (hspB5) can be found both in non-small-cell lung cancer patients and COPD patients.Citation19 In the present study, we confirmed the tissue expression of αB-crystalline in bronchial biopsies of COPD smokers. We compared it to non-COPD smokers. It could be observed that most of the COPD-smokers had intense staining in comparison to the non-COPD smokers who had no staining. Despite the fact that we do not have a reliable quantitative marker evaluating the oxidative stress, the two groups smoked a comparable number of pack years. So it is assumable that they were exposed to similar levels of oxidative stress. Thus, in COPD lungs αB-crystalline could reflect an oxidative imbalance and could be a mechanism of the chaperone system for lung protection. It could be possible that in the COPD smokers, the induction of higher levels of chaperones is required to keep the proteostasis, the proper folding, and functioning of the proteins under the same levels of oxidative stress.
As a cell stress protein, αB-crystalline is transiently induced as a result of intense oxidative metabolism in various organs such as the muscles, kidneys, and heart. In these organs, αB-crystalline is only upregulated when its cytoprotective properties are required.Citation11 It has antioxidant ability and increases cell resistance to oxidative injuries. It has been reportedCitation20 both in cell cultures and whole animals that αB-crystalline expression correlates to decreased levels of reactive oxygen species, nitric oxide, and of lipid peroxidation.Citation21,Citation22
Another function of αB-crystalline that could explain its participation in lung epithelial injury is its significance for the protection of the cytoskeleton.Citation23,Citation24 αB-crystalline is involved in the control of cytoskeletal organization during heat and oxidative stress. It maintains the polymerization-depolymerization processes of F-actin and thus is directly responsible for both cell integrity and intracellular contacts.Citation25,Citation26 αB-crystalline is a well-known stabilizer of the intermediate filaments and plays a major role in cytoskeletal architecture homeostasis.Citation27 It has been demonstrated in epithelial cells that signalling pathways, activated by disrupture of microfilaments, intermediate filaments, and microtubules, lead to the phosphorylation of crystalline, underlying the biological importance of this heat-shock protein in preserving the integral cell architecture.Citation28
In addition to the tissue expression, we have shown significant increases of αB-crystalline antibodies in plasma samples taken from the peripheral blood flow of patients suffering from COPD and acute respiratory infection as compared to healthy non-COPD smokers. This effect could probably be due to increased tissue devastation, reflecting the degree of oxidative stress and inflammation in both acute and chronic inflammatory settings. In patients with acute inflammation and oxidative stress (acute respiratory tract infection) αB-crystalline antibodies reach the highest values (0.433 nm). In persistent inflammation (COPD smokers and chronic oxidative injury) it is 0.352 nm. In non-COPD healthy smokers, it is the lowest (0.291 nm). We can speculate that the extracellular plasma levels reflect the degree of inflammation and oxidative stress. The higher levels of antibodies in patients with acute respiratory infection could be attributed to the fact that heat-shock proteins are the most evolutionary conserved proteins in pro- and eukaryotecs, and a concomitant bacterial infection could provoke the generation of αB-crystalline antibodies in acute respiratory tract infections.
On the other hand, HspB5 is actively secreted from normal cells via exosomes. The higher tissue expression may also correspond to an increased quantity of secreted HspB5, which is itself immunogenic.Citation29
As the age, pack years, and the degree of airway obstruction in the three groups in which plasma levels of αB-crystallin was measured are similar, it is highly probable that high plasma levels are the major factor determining the high plasma levels of αB-crystallin antibodies.
Analyzing the role of αB-crystalline in COPD progression we cannot find a significant difference between the early (GOLD I–II) and late (GOLD III–IV) stages. No correlation between αB-crystalline antibodies and the generally accepted markers of inflammation (hsCRP and MMP-9) could be established. However before making certain conclusions, we should bear in mind a major drawback of the study, which deters the precise clinical interpretation of αB-crystallin in COPD pathology, is that many more of our patients are in the severe COPD group. Another factor that could also be confounding is that all the patients have a strict control of their disease and receive supportive inhalation therapy (long-acting β-agonists/ glucocorticoids ± long-acting muscarinic antagonist).
In summary, αB-crystalline generally acts as an antiapoptotic mediator and can be seen as an endogenous immunosuppressive attempt to control excessive inflammation in COPD. Its plasma contents showed statistically significant potential to determine the occurrence of COPD among smokers. Further explorations are needed to determine optimal cut-off values and improve the proposed sensitivity and specificity of HspB5 in clinical settings.
Disclosure
The authors report no conflicts of interest in this work.
References
- NingWLiCJKaminskiNComprehensive gene expression profiles reveal novel pathways related to the pathogenesis of chronic obstructive pulmonary diseaseProc Natl Acad Sci U S A2004101148951490015469929
- HuROuyangQDaiATanSXiaoZTangCHeat shock protein 27 and cyclophilin A associate with the pathogenesis of COPDRespirology201116698399321585617
- NadeemARajHGChhabraSKIncreased oxidative stress and altered levels of antioxidants in chronic obstructive pulmonary diseaseInflammation2005291233216502343
- DemedtsIKBrusselleGGBrackeKRVermaelenKYPauwelsRAMatrix metalloproteinases in asthma and COPDCurr Opin Pharmacol20055325726315907912
- DemedtsIDemoorTBrackeKRJoosGFBrusselleGGRole of apoptosis in the pathogenesis of COPD and pulmonary emphysemaRespir Res200675316571143
- ItoKItoMElliottWMDecreased histone deacetylase activity in chronic obstructive pulmonary diseaseN Engl J Med2005352191967197615888697
- CraigEAThe heat shock responseCRC Crit Rev Biochem19851832392802412760
- EllisRJDiscovery of molecular chaperonesCell Stress Chaperones1996131551609222600
- ParcellierASchmittEGurbuxaniSHSP27 is a ubiquitin-binding protein involved in I-kappaBalpha proteasomal degradationMol Cell Biol200323165790580212897149
- LinDIBarbashOKumarKGPhosphorylation-dependent ubiquitination of cyclin D1 by the SCF(FBX4-alphaB crystallin) complexMol Cell200624335536617081987
- ArrigoAPSimonSGibertBHsp27 (HspB1) and alphaB-crystallin (HspB5) as therapeutic targetsFEBS Lett2007581193665367417467701
- CalderwoodSKKhalequeMASawyerDBCioccaDRHeat shock proteins in cancer: chaperones of tumorigenesisTrends Biochem Sci200631316417216483782
- DozENoulinNBoichotECigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependentJ Immunol200818021169117818178857
- HuROuyangQDaiATanSXiaoZTangCHeat shock protein 27 and cyclophilin A associate with the pathogenesis of COPDRespirology201116698399321585617
- GOLD criteria 2008 [homepage on the Internet]Global Initiative for Chronic Obstructive Lung Disease (GOLD) Available from: http://www.goldcopd.orgAccessed August 8, 2012
- TrifonovaNKalaydjievSStamenovaMTrifonovaRBreipohlWPorcine eye lens crystallins: antigenic similarity with human crystallins and tools for the detection of anti-crystallin antibodiesGraefes Arch Clin Exp Ophthalmol2002240977778112271377
- ItoKBarnesPJCOPD as a disease of accelerated lung agingChest2009135117318019136405
- BouchecareilhMBalchWEProteostasis: a new therapeutic paradigm for pulmonary diseaseProc Am Thorac Soc20118218919521543800
- ChernevaRPetrovaDGeorgievOChronic obstructive pulmonary disease – chaperonopathologyKian-ChungOChronic Obstructive Pulmonary Disease – Current Concepts and PracticeNew York, NYInTech201293113
- YanLJChristiansESLiuLXiaoXSohalRSBenjaminIJMouse heat shock transcription factor 1 deficiency alters cardiac redox homeostasis and increases mitochondrial oxidative damageEMBO J200221195164517212356732
- FirdausWJWyttenbachADiaz-LatoudCCurrieRWArrigoAPAnalysis of oxidative events induced by poyglutamin huntingtin exon 1 that are differentially restored by expression of heat shock proteins or treatment with an antioxidantFEBS J2006273133076309316817855
- PrévilleXGaestelMArrigoAPPhosphorylation is not essential for protection of L929 cells by Hsp25 against H2O2-mediated disruption actin cytoskeleton, a protection which appears related to the redox change mediated by Hsp25Cell Stress Chaperones1998331771879764758
- BenndorfRHayessKRyazantsevSWieskeMBehlkeJLutschGPhosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activityJ Biol Chem19942693220780207848051180
- MounierNArrigoAPActin cytoskeleton and small heat shock proteins: how do they interact?Cell Stress Chaperones20027216717612380684
- JogNRJalaVRWardRARaneMJHaribabuBMcLeishKRHeat shock protein 27 regulates neutrophil chemotaxis and exocytosis through two independent mechanismsJ Immunol200717842421242817277149
- SinghBNRaoKSRamakrishnaTRangarajNRaoChMAssociation of alphaB-crystallin, a small heat shock protein, with actin: role in modulating actin filament dynamics in vivoJ Mol Biol2007366375676717196975
- BennardiniFWrzosekAChiesiMAlpha-B crystalline in cardiac tissue. Association with actin and desmin filamentsCirc Res19927122882941628387
- LaunayNGoudeauBKatoKVicartPLilienbaumACell signaling pathways to alphaB-crystallin following stresses of the cytoskeletonExp Cell Res2006312183570358416979163
- RothbardJBKurnellasMPBrownellSTherapeutic effects of systemic administration of chaperone αB-crystallin associated with binding proinflammatory plasma proteinsJ Biol Chem2012287139708972122308023