Abstract
Up to 50% of patients with chronic obstructive pulmonary disease (COPD) in stable state may carry potentially pathogenic microorganisms (PPMs) in their airways. The presence of PPMs has been associated with increased symptoms, increased risk and severity of exacerbations, a faster decline in lung function and impairment in quality of life. Although some clinical trials have demonstrated a reduction in exacerbations in patients chronically treated with systemic antibiotics, particularly macrolides, the selection of patients was based on the previous frequency of exacerbations and not on the presence of PPMs in their airways. Therefore, unlike in bronchiectasis, there is a lack of evidence-based recommendations for assessment and treatment of the presence of PPMs in either single or repeated isolations in COPD. In this article, we propose that chronic bronchial infection (CBI) in COPD be defined as the isolation of the same PPM in at least three sputum samples separated by more than one month; we review the impact of CBI on the natural course of COPD and suggest a course of action in patients with a single isolation of a PPM or suspected CBI. Antibiotic treatment in stable COPD should be recommended based on four main criteria: a) the presence of comorbid bronchiectasis, b) the demonstration of a single or multiple isolation of the same PPM, c) the clinical impact of CBI on the patients, and d) the type of PPM, either Pseudomonas aeruginosa or non-pseudomonal PPM. These recommendations are derived from evidence generated in patients with bronchiectasis and, until new evidence specifically obtained in COPD is available, they may help in the management of these challenging patients with COPD. Existing evidence suggests that inhaled therapy is insufficient to manage patients with moderate-to-severe COPD, frequent exacerbations, and CBI. New studies must be conducted in this particularly demanding population.
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of morbidity and mortality worldwide, and its prevalence is expected to continue to grow.Citation1,Citation2 Tobacco smoke and the inhalation of other toxic gases or fumes are its main known causes, and these external causes mainly act by generating and sustaining bronchial and parenchymal inflammation leading to the development of the characteristic lesions of chronic bronchitis and emphysema.Citation3 These two clinical phenotypes are responsible for the main clinical manifestations of the disease, the symptoms of cough and sputum production, and the progressive exertional dyspnea and airflow limitation.Citation3
The natural course of COPD is punctuated by exacerbations, which are episodes of an acute increase of respiratory symptoms (ie increased cough, sputum production and/or dyspnea) as a consequence of bacterial or viral infection in the majority of cases.Citation4–Citation6 These exacerbations may have different degrees of severity, from mild and self-limiting episodes to severe symptoms that may lead to hospital admission or even death.Citation6–Citation8 Exacerbations may be frequent in some patients with COPD, the so-called frequent exacerbators, and have been associated with more rapid disease progression.Citation9,Citation10
Nowadays, treatment of COPD is basically symptomatic with the use of long-acting bronchodilators that improve airflow limitation and reduce air trapping, thereby relieving dyspnea and improving quality of life.Citation11–Citation13 Despite the inflammation present in patients with COPD, no specific anti-inflammatory treatment is available for COPD. Inhaled corticosteroids (ICS) are effective in reducing the risk of exacerbation and improving symptoms in patients with frequent exacerbations and an eosinophilic inflammatory profile, but have very limited efficacy (if any) in infrequent exacerbators without eosinophilic inflammation.Citation11–Citation15 Interestingly, COPD patients with frequent exacerbations without an eosinophilic profile usually have potentially pathogenic microorganisms (PPM) in their airwaysCitation16 and the use of ICS in these patients may increase the risk of isolation of Pseudomonas aeruginosa (PA),Citation17,Citation18 bacterial exacerbations or pneumonia.Citation19,Citation20 In these cases, oral anti-inflammatory treatment with roflumilast or macrolides can be effective in preventing exacerbations, but the use of these drugs is limited by the frequent appearance of side effects and the possible development of bacterial resistance.Citation21
Despite the frequency and importance of the presence of PPM in respiratory secretions of COPD patients in stable state and their relationship with the risk of exacerbations,Citation22 little attention has been given to the assessment and treatment of bacterial infection in stable COPD in clinical practice guidelines. In this article we address the importance of the isolation of PPM in respiratory samples in stable COPD and suggest new lines of research to prevent exacerbations and improve outcomes in patients with COPD.
Bacterial Isolation in Sputum of Stable COPD. Why is It Important?
Bronchial inflammation caused by tobacco smoke or other toxic inhalations leads to impairment of the antimicrobial defenses of the airways, reducing secretory IgA, a defective bacterial phagocytosis by macrophages, increasing mucus secretion and impairing mucociliary clearance, among other mechanisms, that explain the adhesion of PPMs to bronchial epithelial cells and their proliferation in the airways of patients with COPD.Citation23 This alteration of bronchial defenses may appear early in the course of the disease, as reflected by changes in the composition of the normal pulmonary microbiota already observed in smokers with normal lung function, but more markedly in those with established COPD.Citation24
As a consequence of the persistence and increased bacterial proliferation in the airways, the isolation of MPPs, basically Haemophilus influenzae, Moraxella catarrhalis, Streptococcus pneumoniae and PA, in stable COPD patients is not uncommon, since they may appear in up to 30–50% of severe cases.Citation22,Citation25 This isolation of PPMs in sputum in stable COPD patients has usually been defined as bronchial colonization, similarly to the isolation of bacteria in the skin or the gut. However, several studies have demonstrated that the isolation of PPMs even without the association of increased respiratory symptoms is not innocuous but is associated with enhanced bronchial and systemic inflammation,Citation26–Citation29 an increased frequency and severity of exacerbations,Citation30,Citation31 accelerated decline in lung function,Citation32 the possible development of bronchiectasis,Citation33 impaired quality of life,Citation34 an increase in cardiovascular events,Citation35 and even with increased mortality in the case of the isolation of PA.Citation36 Therefore, the presence of PPMs in the lower airways of patients with COPD does not fulfill the criteria of colonization, which does not harm the host, but instead it constitutes a type of bronchial infection.Citation37–Citation39
The Concept of Acute and Chronic Bronchial Infection in COPD
The natural history of COPD is punctuated by episodes of an acute increase of respiratory symptoms, which in most cases are caused by infection.Citation5,Citation6,Citation22 These episodes may be caused by the acquisition of a new bacterial strain or by an increase in the bacterial load in the airwaysCitation40,Citation41 and are considered as acute bronchial infection which requires directed antibiotic treatment.Citation4,Citation6,Citation42
In contrast, the persistence of PPMs in the lower airways in patients with stable COPD, not associated with acute symptoms of exacerbation, fulfills the characteristics of a chronic, low intensity infection and has been named chronic bronchial infection (CBI).Citation37,Citation39 However, despite its multiple deleterious effects, there is no universally accepted approach to its definition and treatment as yet.
A recent expert consensus proposed a definition of CBI in stable COPD as 3 or more positive sputum cultures by the same PPM over 1 year, separated by at least 1 month.Citation39 This definition derives from the evidence obtained from bronchiectasis studies, which suggest that the clinical consequences of bronchial infection may differ in cases of a single or repeated isolation of the same PPM.Citation43,Citation44 This is very likely to also be the case in COPD; a recent study has shown that CBI was associated with an increased risk of pneumonia, especially in patients under ICS treatment and with less than 100 blood eosinophils/µL, an effect that was not observed in cases of a single isolation.Citation19 In another study, CBI was associated with an almost 4-fold increased risk of cardiovascular events (especially coronary events) compared with patients without PPM isolation or only one isolation during follow-up.Citation35 Moreover, the number of PPM isolations was independently associated with the development or progression of bronchiectasis in COPD.Citation33 Taken together, these studies suggest that CBI in COPD has a special role in the pathogenesis and progression of COPD, beyond that of a single PPM isolation.Citation22,Citation25,Citation37,Citation38
The isolation of PA in sputum in COPD deserves special attention. Old studies have shown that PA has a role in exacerbations, particularly in more severe COPD patients,Citation45 and new evidence suggest that PA may be associated with increased mortality in COPD;Citation46,Citation47 all these studies support a role of PA in the progression of the disease. Murphy et alCitation48 observed that acquisition of PA was frequently followed by the development of an exacerbation and that approximately one third of PA strains demonstrated persistence, irrespective of the development of an immunological response mediated by antibodies. The clinical impact of the persistence of (or CBI by) PA has only recently been described; one study observed that CBI by PA was associated with increased mortality (hazard ratio 3.06 [1.8–5.2], p = 0.001),Citation46 but a single isolation of PA was not,Citation46,Citation49 and that the risk factors for a first PA isolation and PA persistence were different.Citation46
All these results suggest that PA may have increased virulence compared with other PPMs, even when it is isolated only once. This would be particularly true in patients with COPD and associated bronchiectasis, because the single isolation of PA in bronchiectasis has demonstrated to have an impact on the evolution of the disease.Citation50 It is also likely that a single isolation of H. influenzae may have an impact on the pathogenesis of COPD, since it is associated with increased local and systemic inflammation,Citation28,Citation29,Citation51 but evidence about the long-term clinical impact of a single isolation of H. influenzae is lacking.
Should We Treat Chronic Bronchial Infection in COPD?
Despite the evidence about the pathogenic effect of the presence of bacteria in the lower airways in stable COPD, there is a lack of evidence-based recommendations for the diagnosis and treatment of CBI in COPD and, therefore, its management remains controversial.Citation11,Citation39 It is of note that a significant number of COPD patients with frequent exacerbations and CBI may have bronchiectasis when investigated by computed tomography (CT) scans.Citation52,Citation53 In these cases, it is sensible to follow the recommendations of the bronchiectasis guidelines, which establish clear recommendations about the treatment of CBI,Citation43,Citation44 but no formal recommendations have been established for COPD patients with CBI without bronchiectasis. Nevertheless, some course of action should be recommended for these particularly challenging and severe COPD patients and based on different recent evidence, it should be possible to reach a consensus on treatment of CBI in COPD while awaiting the results of randomized clinical trials.Citation39
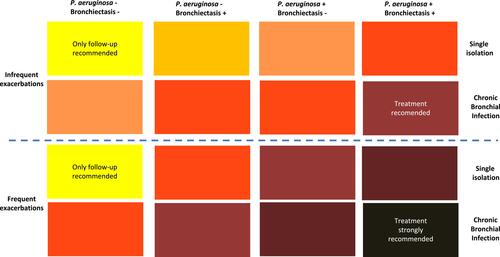
The recommendations for the treatment of CBI should be based on four factors: a) the clinical impact; b) the type of PPM, either PA or non-PA associated CBI; c) the presence of bronchiectasis, and d) whether it is a single isolation or CBI.
A) Clinical impact of CBI: The impact of CBI on COPD has already been extensively described above, but unfortunately, it is only feasible to measure and identify the history and severity of exacerbations in clinical practice. Although CBI may impact other outcomes, such as a decline in lung function or impairment of quality of life, these are not usually quantified and require prolonged follow-up, which make them of little use for demonstrating the clinical impact of CBI in routine clinical practice.
B) PA versus non-PA CBI: Data from bronchiectasis patients and some epidemiological data on COPD suggest that the isolation of PA may be associated with a worse prognosis than the isolation of other PPMs,Citation50,Citation54 although it is possible that H. influenzae may have a similar impact on the course of COPD.Citation28,Citation29,Citation38
C) Presence of bronchiectasis: The demonstration of bronchiectasis probably indicates a longer evolution of the CBI in COPD, since some studies showed that bronchiectasis in COPD may develop because of repeated exacerbations and the presence of CBI.Citation33,Citation53 There are guidelines about the treatment of CBI in patients with bronchiectasis.Citation43,Citation44
D) Single or multiple isolations of the same PPM: Finally, the potential effect on the natural history of COPD of a single isolation of a PPM may be significantly lower than that associated with the CBI. Only the single isolation of PA, but not of other PPMs, in COPD has been associated with worse long-term outcomes.Citation46
By combining these four factors, a range of priorities for the treatment of CBI in COPD can be established. The strongest priority for treatment would be the presence of a CBI by PA in a patient with frequent exacerbations and bronchiectasis, while the other end of the spectrum; that is, the lowest priority, would be a patient without bronchiectasis, with infrequent exacerbations and a single isolation of a non-PA PPM ().
Figure 1 Strength of the recommendation of antimicrobial treatment of CBI in COPD.
How to Treat Chronic Bronchial Infection in COPD?
There are two main strategies for the treatment of CBI: improve host defenses or eradicate infecting PPMs. A series of candidate treatments for CBI is presented in . Unfortunately, there are no licensed therapies dedicated to improving host defenses in COPD for the prevention of CBI. Anti-influenza and anti-pneumococcal vaccines are indicated in patients with COPD to prevent influenza, invasive pneumococcal disease and pneumococcal pneumonia, but they have demonstrated a very limited effect in the prevention of exacerbations and it remains to be demonstrated whether they can prevent CBI.Citation11,Citation13 On the other hand, oral whole-cell killed nontypeable H. influenzae vaccine might provide some protection against CBI by this pathogen.Citation55 Other strategies include techniques to improve mucus clearance and the investigation of possible immunoglobulin (Ig) deficiencies that have been associated with increased risk of severe exacerbations and death and might be treated with Ig replacement.Citation56 In addition, general strategies of management of COPD may help to reduce the risk of CBI, such as the use of long-acting bronchodilators due to their effect in facilitating an adequate bronchomotor tone and the reduction of mucus hypersecretion with anticholinergics.Citation57 Similarly, pulmonary rehabilitation has demonstrated to facilitate the mucociliary function and reduce the frequency of exacerbations ().Citation4
Table 1 Candidate Treatment Strategies for Chronic Bronchial Infection in COPD
Regarding antimicrobial treatment, there are few studies conducted with systemic antibiotics and these have been inconclusive because their limited efficacy in the prevention of exacerbations must be balanced with the possible side effects related to the prolonged use of systemic antibiotics and the possible selection of resistant strains.Citation58 A randomized, double-blind, placebo-controlled trial with moxifloxacin 400 mg daily for 5 days in 40 patients with stable COPD with a previous isolation of PPM in sputum showed a significant difference in eradication (75% versus 30%; p<0.01), but the frequency of acquisition of a new PPM was high and similar in both treatment groups. Consequently, the prevalence of a positive sputum culture for PPM at 8 weeks was also similar between treatment arms.Citation59 No difference was found in the number of patients with exacerbations during the 5-month follow-up; however, only the acquisition of a new PPM during follow-up showed a statistically significant relationship with the occurrence of an exacerbation.Citation59 Regarding chronic administration of antimicrobials, a sub-analysis of the PULSE study, which analyzed the efficacy and safety of 5 days of moxifloxacin every other month for one year versus placebo, observed a significant reduction of exacerbations of 45% (p=0.006) with moxifloxacin in patients who presented with dark sputum at baseline.Citation60 This is relevant because dark sputum is very closely related to the presence of CBI even in patients with stable COPD.Citation61 Unfortunately, the majority of studies selected patients based on the frequency of exacerbations, and not on the presence of CBI.
Macrolides, which have antibacterial, immunomodulatory and anti-inflammatory activity, have been shown to reduce the number of exacerbations in patients with COPD.Citation62 This effect was more pronounced in patients with three or more exacerbations in the previous year and irrespective of the presence of bronchiectasis.Citation63 However, again these studies were not carried out in groups of patients with CBI, and the potential side effects and the development of resistance to these antimicrobials may limit their use in routine clinical practice.Citation21
Inhaled antibiotics have been successfully used in patients with bronchiectasis associated or not with cystic fibrosis. These antibiotics can reach high local concentrations without an excess of systemic side effects; however, their efficacy is not yet proven in COPD patients with CBI. A recent large observational study suggested their efficacy in reducing the frequency of exacerbations in severe patients with COPD, but they were not selected for the presence of CBI, and the type of antibiotic and treatment regimens were not standardized.Citation64
A final consideration is that effective antimicrobial treatment of bacterial exacerbations may be the first strategy to prevent CBI. The persistence of PPMs, even at low bacterial loads, after the treatment of an exacerbation may be the first step to the development of a CBI.Citation41,Citation65,Citation66 The lack of eradication has been associated with increased inflammation and a shorter period until the next exacerbation;Citation66,Citation67 therefore, antibiotic treatment of exacerbations must aim at bacterial eradication in addition to clinical improvement.
Conclusions and Future Research
Despite the advances in research about infection in COPD, several unanswered questions about CBI still remain (). Chronic bronchial infection is frequent in patients with moderate to severe COPD; up to 50% of patients may carry PPMs in their bronchial tree during stable state.Citation37,Citation68 The persistence of PPMs in the lungs of patients with COPD may be defined as a CBI due to its impact on symptoms, exacerbations, and the natural history of the disease.Citation38 Due to its deleterious effects, CBI should be considered a treatable trait of COPD.Citation13,Citation69,Citation70 Patients with moderate to severe COPD and frequent exacerbations should be investigated for the presence of CBI and bronchiectasis with serial sputum samples and high-resolution CT scan. Until more evidence is available, CBI in COPD should be treated taking into consideration the presence of bronchiectasis, the type of PPM isolated, the clinical impact and the presence of a single or multiple isolates of the same PPM. Patterns of treatment should follow the evidence generated in bronchiectasis with either eradication with systemic antibiotics or long-term treatment with macrolides or inhaled antibiotics.
Box 1 Ten Unanswered Questions in Relation to Chronic Bronchial Infection in COPD
Treatment of severe COPD should go beyond the usual inhalers and, in particular in frequent exacerbators, the infective component of the disease should be investigated and treated. However, future studies must clarify several aspects of the diagnosis, impact and treatment of CBI. First, an international consensus should be established to define the different microbiological issues arising in COPD patients: the differentiation between bronchial colonization (if it really exists) and CBI; the definition and consequences of an initial, intermittent, or CBI; and the definition of the eradication of a given PPM from the airways, either spontaneously or after treatment. Second, the relevance, frequency and processing methods of sputum sampling in patients with stable COPD should be established. Microbiological monitoring of sputum is essential for these purposes, and it should be carried out at each clinical visit in COPD patients with frequent exacerbations able to expectorate.Citation39 Third, the relevance of using molecular methods such as polymerase chain reaction or sequencing to complement the culture of respiratory samples should be determined.Citation71 Fourth, the importance of performing a CT scan for the diagnosis of associated bronchiectasis due to the clinical, prognostic and therapeutic repercussions of the combination of the two diseases must be demonstrated.Citation52,Citation72 Fifth, trials aimed at the study of the safety and efficacy of treatments for CBI in patients with COPD and frequent exacerbations must be designed.
Furthermore, some key aspects remain to be clarified, with at least three of these being of special importance, and thus appropriate topics for future studies. 1. Is COPD with CBI a differentiated clinical phenotype of COPD? Although scientific evidence is still lacking in this regard, according to the definition of the clinical phenotype of COPD described by Han et al,Citation73 this is very likely the case. These patients present a different clinical, prognostic and therapeutic profile, especially if they have bronchiectasis. It is possible that there is an “infectious” phenotype of COPDCitation37 in patients genetically predisposed to bronchial infection and exposed to certain environmental factors that should be treated early to avoid the disease progressing towards an irreversible alteration of the lung parenchyma and airways; 2. Should patients with COPD and CBI be treated with ICS? There is no clear answer to this question, although it is likely that the most sensible decision might be to minimize their use and dosage, especially bearing in mind the frequent presence of bronchiectasis in these patients (a disease in which ICS are not indicatedCitation74) and the marked and well-known immunosuppressant properties of this treatment.Citation75–Citation77 Future studies are needed to investigate the impact of ICS treatment on patients with COPD and CBI, as well as interaction with blood eosinophil concentrations.Citation19,Citation78,Citation79 As an example, a recent study showed that COPD patients with CBI treated with ICS had a higher risk of future pneumonia only if they had less than 100 blood eosinophils/µL.Citation19
Conclusions
There is evidence of the impact of the presence of PPMs in bronchial secretions of patients with COPD. Nonetheless, there is very limited information about the management strategy for patients with COPD and CBI. It is clear that the usual inhaled therapy is insufficient to manage patients with moderate-to-severe COPD, frequent exacerbations, and CBI. Therefore, it is necessary to conduct clinical trials in patients with COPD and CBI to shed light on their prognosis and on the role of different treatments such as ICS, macrolides, or different systemic and inhaled antibiotics. In the latter respect, an analysis of the efficacy of the different inhaled antibiotics currently on the market would be very relevant.
Abbreviations
CBI, Chronic bronchial infection; COPD, Chronic obstructive pulmonary disease; CT, Computed tomography; ICS, Inhaled corticosteroid; Ig, Immunoglobulin; IgA, Immunoglobulin A; PA, Pseudomonas aeruginosa; PPM, Potentially pathogenic microorganism.
Disclosure
Miguel Angel Martinez-Garcia has received fees from Chiesi, GlaxoSmithKline, Menarini, Rovi, Bial, Zambon, Vitalaire, TEVA, Grifols and Novartis, consulting fees from Grifols, Zambon and TEVA, and research grants from TEVA, Zambon and Vitalaire. Marc Miravitlles has received speaker fees from AstraZeneca, Atrina Therapeutics, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Menarini, Rovi, Bial, Sandoz, Zambon, CSL Behring, Grifols and Novartis, consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, Kamada, CSL Behring, Laboratorios Esteve, Ferrer, Johnson & Johnson, Mereo Biopharma, Palobiofarma SL, Verona Pharma, TEVA, Spin Therapeutics, pH Pharma, Novartis, Sanofi, ONO Pharma, Takeda, and Grifols and research grants from Grifols. The authors report no other conflicts of interest in this work.
Additional information
Funding
References
- Soriano JB, Kendrick PJ, Paulson KR; GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585–596. doi:10.1016/S2213-2600(20)30105-3
- Soriano JB, Alfageme I, Miravitlles M, et al. Prevalence and determinants of COPD in Spain: EPISCAN II. Arch Bronconeumol. 2021;57(1):61–69. doi:10.1016/j.arbr.2020.07.017
- Agustí A, Hogg JC, Drazen JM. Update on the pathogenesis of Chronic obstructive pulmonary disease. N Engl J Med. 2019;381:1248–1256. doi:10.1056/NEJMra1900475
- Wedzicha JA, Miravitlles M, Hurst JR, et al. Management of COPD exacerbations: an European Respiratory Society/American Thoracic Society (ERS/ATS) guideline. Eur Respir J. 2017;49:1600791. doi:10.1183/13993003.00791-2016
- Anzueto A, Miravitlles M. Chronic obstructive pulmonary disease exacerbations: a need for action. Am J Med. 2018;131:15–22. doi:10.1016/j.amjmed.2018.05.003
- Soler-Cataluña JJ, Piñera P, Trigueros JA, et al. Spanish COPD guidelines (GesEPOC) 2021 update diagnosis and treatment of COPD exacerbation syndrome. Arch Bronconeumol. 2022;58:159–170. doi:10.1016/j.arbres.2021.05.011
- Halpin DMG, Miravitlles M, Metzdorf N, Celli B. Impact and prevention of severe exacerbations of COPD: a review of the evidence. Int J Chron Obstruct Pulmon Dis. 2017;12:2891–2908. doi:10.2147/COPD.S139470
- Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi:10.1136/thx.2005.040527
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa0909883
- Soler-Cataluña JJ, Miralles C. Exacerbation Syndrome in COPD: a paradigm shift. Arch Bronconeumol. 2021;57(4):246–248. doi:10.1016/j.arbr.2020.07.021
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2021. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf. Accessed December 16, 2021.
- Montes de Oca M, López Varela MV, Acuña A, et al. Incorporating new evidence on inhaled medications in COPD. The Latin American Chest Association (ALAT) 2019. Arch Bronconeumol. 2020;56(2):106–113. doi:10.1016/j.arbr.2019.09.002
- Miravitlles M, Calle M, Molina J, et al. Spanish COPD Guidelines (GesEPOC) 2021: updated Pharmacological treatment of stable COPD. Arch Bronconeumol. 2022;58:69–81. doi:10.1016/j.arbres.2021.03.005
- López-Campos JL, Carrasco-Hernández L, Román Rodríguez L, Quintana-Gallego E, Carmona Bernal C, Alcázar Navarrete B. The clinical implications of triple therapy in fixed-dose combination in COPD: from the trial to the patient. Arch Bronconeumol. 2020;56(4):242–248. doi:10.1016/j.arbres.2019.11.011
- Ruano-Raviña A, Fernández-Villar A, López-Campos JL. Coping with low mortality and exacerbation rate differences between COPD triple therapy studies, and a proposal for upcoming studies. Arch Bronconeumol. 2020;56(5):336–338. doi:10.1016/j.arbr.2019.11.009
- Kolsum U, Donaldson GC, Singh R, et al. Blood and sputum eosinophils in COPD; relationship with bacterial load. Respir Res. 2017;18(1):88. doi:10.1186/s12931-017-0570-5
- Shafiek H, Verdú J, Iglesias A, et al. Inhaled corticosteroid dose is associated with Pseudomonas aeruginosa infection in severe COPD. BMJ Open Respir Res. 2021;8(1):e001067. doi:10.1136/bmjresp-2021-001067
- Eklöf J, Ingebrigtsen TS, Sørensen R, et al. Use of inhaled corticosteroids and risk of acquiring Pseudomonas aeruginosa in patients with chronic obstructive pulmonary disease. Thorax. 2021:thoraxjnl-2021-217160. doi: 10.1136/thoraxjnl-2021-217160
- Martinez-Garcia MA, Faner R, Oscullo G, et al. Inhaled steroids, circulating eosinophils, chronic airway infection and pneumonia risk in chronic obstructive pulmonary disease: a network analysis. Am J Respir Crit Care Med. 2020;201(9):1078–1085. doi:10.1164/rccm.201908-1550OC
- Miravitlles M, Auladell-Rispau A, Monteagudo M, et al. Systematic review on long-term adverse effects of inhaled corticosteroids in the treatment of COPD. Eur Respir Rev. 2021;30(160):210075. doi:10.1183/16000617.0075-2021
- Wedzicha J, Calverley P, Albert R, et al. Prevention of COPD exacerbations: an European Respiratory Society/American Thoracic Society (ERS/ATS) guideline. Eur Respir J. 2017;50:1602265. doi:10.1183/13993003.02265-2016
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–2365. doi:10.1056/NEJMra0800353
- Tanno A, Fujino N, Yamada M, et al. Decreased expression of a phagocytic receptor Siglec-1 on alveolar macrophages in chronic obstructive pulmonary disease. Respir Res. 2020;21(1):30. doi:10.1186/s12931-020-1297-2
- Millares L, Pascual S, Montón C, et al. Relationship between the respiratory microbiome and the severity of airflow limitation, history of exacerbations and circulating eosinophils in COPD patients. BMC Pulm Med. 2019;19(1):112. doi:10.1186/s12890-019-0867-x
- Miravitlles M, Anzueto A. Chronic respiratory infection in patients with chronic obstructive pulmonary disease: what is the role of antibiotics? Int J Mol Sci. 2017;18:E1344. doi:10.3390/ijms18071344
- Sethi S, Maloney J, Grove L, Wrona C, Berenson CS. Airway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(9):991–998. doi:10.1164/rccm.200509-1525OC
- Zhang M, Li Q, Zhang XY, Ding X, Zhu D, Zhou X. Relevance of lower airway bacterial colonization, airway inflammation, and pulmonary function in the stable stage of chronic obstructive pulmonary disease. Eur J Clin Microbiol Infect Dis. 2010;29:1487–1493. doi:10.1007/s10096-010-1027-7
- Tufvesson E, Bjermer L, Ekberg M. Patients with chronic obstructive pulmonary disease and chronically colonized with Haemophilus influenzae during stable disease phase have increased airway inflammation. Int J Chron Obstruct Pulmon Dis. 2015;10:881–889. doi:10.2147/COPD.S78748
- Finney LJ, Ritchie A, Pollard E, Johnston SL, Mallia P. Lower airway colonization and inflammatory response in COPD: a focus on Haemophilus influenzae. Int J Chron Obstruct Pulmon Dis. 2014;9:1119–1132. doi:10.2147/COPD.S54477
- Patel IS, Seemungal TA, Wilks M, Lloyd-Owen SJ, Donaldson GC, Wedzicha JA. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax. 2002;57:759–764. doi:10.1136/thorax.57.9.759
- Leung JM, Tiew PY, Aogain M, et al. The role of acute and chronic respiratory colonization and infections in the pathogenesis of COPD. Respirology. 2017;22:634–650. doi:10.1111/resp.13032
- Wilkinson TM, Patel IS, Wilks M, Donaldson GC, Wedzicha JA. Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:1090–1095. doi:10.1164/rccm.200210-1179OC
- Martinez-Garcia MA, de la Rosa-carrillo D, Soler-Cataluna JJ, et al. Bronchial infection and temporal evolution of bronchiectasis in patients with chronic obstructive pulmonary disease. Clin Infect Dis. 2021;72(3):403–410. doi:10.1093/cid/ciaa069
- Banerjee D, Khair OA, Honeybourne D. Impact of sputum bacteria on airway inflammation and health status in clinical stable COPD. Eur Respir J. 2004;23:685–691. doi:10.1183/09031936.04.00056804
- Martinez-Garcia MÁ, Faner R, Oscullo G, et al. Chronic bronchial infection and incident cardiovascular events in chronic obstructive pulmonary disease patients: a long-term observational study. Respirology. 2021;26(8):776–785. doi:10.1111/resp.14086
- Eklof J, Sorensen R, Ingebrigtsen TS, et al. Pseudomonas aeruginosa and risk of death and exacerbations in patients with chronic obstructive pulmonary disease: an observational cohort study of 22 053 patients. Clin Microbiol Infect. 2020;26:227–234. doi:10.1016/j.cmi.2019.06.011
- Matkovic Z, Miravitlles M. Chronic bronchial infection in COPD. Is there an infective phenotype? Respir Med. 2013;107:10–22. doi:10.1016/j.rmed.2012.10.024
- López-Campos JL, Miravitlles M, De la Rosa Carrillo D, Cantón R, Soler-Cataluña JJ, Martinez-Garcia MA. Current challenges in chronic bronchial infection in patients with chronic obstructive pulmonary disease. J Clin Med. 2020;9:E1639. doi:10.3390/jcm9061639
- De la Rosa Carrillo D, López-Campos JL, Alcázar Navarrete B, et al. Consensus document on the diagnosis and treatment of chronic bronchial infection in chronic obstructive pulmonary disease. Arch Bronconeumol. 2020;56(10):651–664. doi:10.1016/j.arbres.2020.04.023
- Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347(7):465–471. doi:10.1056/NEJMoa012561
- Miravitlles M. Exacerbations of chronic obstructive pulmonary disease: when are bacteria important? Eur Respir J Suppl. 2002;36:9s–19s. doi:10.1183/09031936.02.00400302
- Llor C, Moragas A, Hernández S, Bayona C, Miravitlles M. Efficacy of antibiotic therapy for acute exacerbations of mild to moderate COPD. Am J Respir Crit Care Med. 2012;186:716–723. doi:10.1164/rccm.201206-0996OC
- Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):1700629. doi:10.1183/13993003.00629-2017
- Martinez-Garcia MA, Maiz L, Olveira C, et al. Spanish guidelines on the evaluation and diagnosis of bronchiectasis in adults. Arch Bronconeumol. 2018;54(2):79–87. doi:10.1016/j.arbr.2017.07.013
- Miravitlles M, Espinosa C, Fernández-Laso E, Martos JA, Maldonado JA, Gallego M; Study Group of Bacterial Infection in COPD. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Chest. 1999;116:40–46. doi:10.1378/chest.116.1.40
- Martínez-García MÁ, Faner R, Oscullo G, et al. Risk factors and relation with mortality of a new acquisition and persistence of Pseudomonas aeruginosa in COPD patients. COPD. 2021;18(3):333–340. doi:10.1080/15412555.2021.1884214
- Martinez-García MA, Rigau D, Barrecheguren M, et al. Long-term risk of mortality associated with isolation of Pseudomonas aeruginosa in COPD: a prognostic systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2022;17:371–382. doi:10.2147/COPD.S346294
- Murphy TF, Brauer AL, Eschberger K, et al. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(8):853–860. doi:10.1164/rccm.200709-1413OC
- Boutou AK, Raste Y, Reid J, Alshafi K, Polkey MI, Hopkinson NS. Does a single Pseudomonas aeruginosa isolation predict COPD mortality? Eur Respir J. 2014;44(3):794–797. doi:10.1183/09031936.00023414
- Araújo D, Shteinberg M, Aliberti S, et al. The independent contribution of Pseudomonas aeruginosa infection to long-term clinical outcomes in bronchiectasis. Eur Respir J. 2018;51:1701953. doi:10.1183/13993003.01953-2017
- Wang Z, Maschera B, Lea S, et al. Airway host-microbiome interactions in chronic obstructive pulmonary disease. Respir Res. 2019;20(1):113. doi:10.1186/s12931-019-1085-z
- Polverino E, Dimakou K, Hurst J, et al. The overlap between bronchiectasis and chronic airways diseases: state of the art and future directions. Eur Respir J. 2018;52:pii: 1800328. doi:10.1183/13993003.00328-2018
- Martínez-García MA, Miravitlles M. Bronchiectasis in COPD patients. More than a comorbidity? Int J Chron Obstruct Pulmon Dis. 2017;12:1401–1411. doi:10.2147/COPD.S132961
- Chen CL, Huang Y, Yuan JJ, et al. The roles of bacteria and viruses in bronchiectasis exacerbation: a Prospective Study. Arch Bronconeumol. 2020;56(10):621–629. doi:10.1016/j.arbr.2019.12.014
- Clancy RL, Crip Ps AW. An oral whole-cell killed nontypeable Haemophilus influenzae immunotherapeutic for the prevention of acute exacerbations of chronic airway disease. Int J Chron Obstruct Pulmon Dis. 2019;25(14):2423–2431. doi:10.2147/COPD.S217317
- Cowan J, Mulpuru S, Abdallah SJ, et al. A randomized double-blind placebo-control feasibility trial of immunoglobulin treatment for prevention of recurrent acute exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2021;3(16):3275–3284. doi:10.2147/COPD.S338849
- Anzueto A, Miravitlles M. Considerations for the correct diagnosis of chronic obstructive pulmonary disease and its management with bronchodilators. Chest. 2018;154:242–248. doi:10.1016/j.chest.2018.02.023
- Miravitlles M, Anzueto A. Antibiotic prophylaxis in COPD: why, when, and for whom? Pulm Pharmacol Ther. 2015;32:119–123. doi:10.1016/j.pupt.2014.05.002
- Miravitlles M, Marín A, Monsó E, et al. Efficacy of moxifloxacin in the treatment of bronchial colonization in COPD. Eur Respir J. 2009;34:1066–1071. doi:10.1183/09031936.00195608
- Sethi S, Jones PW, Theron MS, et al. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res. 2010;11:10. doi:10.1186/1465-9921-11-10
- Miravitlles M, Marin A, Monsó E, et al. Colour of sputum is a marker of bacterial colonization in COPD. Respir Res. 2010;11:58. doi:10.1186/1465-9921-11-58
- Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689–698. doi:10.1056/NEJMoa1104623
- Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (Columbus): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2:361–368. doi:10.1016/S2213-2600(14)70019-0
- De la Rosa Carrillo D, Martínez-García MÁ, Barreiro E, et al. Effectiveness and safety of inhaled antibiotics in patients with chronic obstructive pulmonary disease. A Multicentre Observational Study. Arch Bronconeumol. 2022;58:11–21. doi:10.1016/j.arbres.2021.03.009
- Chen K, Pleasants KA, Pleasants RA, et al. A systematic review and meta-analysis of sputum purulence to predict bacterial infection in COPD exacerbations. COPD. 2020;17(3):311–317. doi:10.1080/15412555.2020.1766433
- Sethi S, Anzueto A, Miravitlles M, et al. Determinants of bacteriological outcomes in exacerbations of chronic obstructive pulmonary disease. Infection. 2016;44:65–76. doi:10.1007/s15010-015-0833-3
- White AJ, Gompertz S, Bayley DL, et al. Resolution of bronchial inflammation is related to bacterial eradication following treatment of exacerbations of chronic bronchitis. Thorax. 2003;58(8):680–685. doi:10.1136/thorax.58.8.680
- Armitage MN, Spittle DA, Turner AM. A systematic review and meta-analysis of the prevalence and impact of pulmonary bacterial colonisation in stable state Chronic Obstructive Pulmonary Disease (COPD). Biomedicines. 2021;10(1):81. doi:10.3390/biomedicines10010081
- Cardoso J, Ferreira AJ, Guimarães M, Oliveira AS, Simão P, Sucena M. Treatable Traits in COPD - A Proposed Approach. Int J Chron Obstruct Pulmon Dis. 2021;18(16):3167–3182. doi:10.2147/COPD.S330817
- Pérez de Llano L, Miravitlles M, Golpe R, et al. A proposed approach to Chronic Airway Disease (CAD) using therapeutic goals and treatable traits: a look to the future. Int J Chron Obstruct Pulmon Dis. 2020;15:2091–2100. doi:10.2147/COPD.S263430
- Monso E. Look at the wood and not at the tree: the microbiome in chronic obstructive lung disease and cystic fibrosis. Arch Bronconeumol. 2020;56:5–6.
- Traversi L, Miravitlles M, Martinez-Garcia MA, et al. ROSE: radiology, obstruction, symptoms and exposure - A Delphi consensus definition of the association of COPD and bronchiectasis by the EMBARC Airways Working Group. ERJ Open Res. 2021;7(4):00399–2021. doi:10.1183/23120541.00399-2021
- Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182:560–598. doi:10.1164/rccm.200912-1843CC
- Henkle E, Curtis JR, Chen L, et al. Comparative risks of chronic inhaled corticosteroids and macrolides for bronchiectasis. Eur Respir J. 2019;54(1):1801896. doi:10.1183/13993003.01896-2018
- Singanayagam A, Glanville N, Girkin JL, et al. Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations. Nat Commun. 2018;9(1):2229.
- Singanayagam A, Glanville N, Cuthbertson L, et al. Inhaled corticosteroid suppression of cathelicidin drives dysbiosis and bacterial infection in chronic obstructive pulmonary disease. Sci Transl Med. 2019;11:507. doi:10.1126/scitranslmed.aav3879
- Contoli M, Pauletti A, Rossi MR, et al. Long-term effects of inhaled corticosteroids on sputum bacterial and viral loads in COPD. Eur Respir J. 2017;50(4):1700451. doi:10.1183/13993003.00451-2017
- Miravitlles M, Monteagudo M, Solntseva I, Alcázar B. Blood eosinophil counts and their variability and risk of exacerbations in COPD: a Population-Based Study. Arch Bronconeumol. 2021;57(1):13–20. doi:10.1016/j.arbr.2019.12.021
- Golpe R, Dacal D, Sanjuán-López P, Martín-Robles I, Pérez-de-llano LA. Plasma eosinophil count and patient-centered events in chronic obstructive pulmonary disease in real-life clinical practice. Arch Bronconeumol. 2020;56(2):129–130. doi:10.1016/j.arbr.2019.09.001
