Abstract
Bronchodilators are central in the symptomatic management of chronic obstructive pulmonary disease (COPD). Long-acting muscarinic antagonists (LAMAs) and long-acting β2-agonists (LABAs) are the main classes of long-acting bronchodilators. To date, tiotropium is the only once-daily LAMA available for the treatment of COPD. Glycopyrronium is a novel LAMA, currently in development for COPD. Phase II studies have shown that glycopyrronium 50 μg once daily provides clinically significant 24-hour bronchodilation with a rapid onset of action, which is faster than that of tiotropium, and a favorable safety and tolerability profile. The Phase III GLycopyrronium bromide in COPD airWays (GLOW) program has now confirmed the long-term efficacy and tolerability of glycopyrronium 50 μg once daily. The three studies included in this program have further shown that the effect of glycopyrronium versus placebo is similar to that of tiotropium in reducing dyspnea and the risk of exacerbations, as well as improving lung function, exercise tolerance, and health status in patients with COPD. The safety profile of glycopyrronium is also similar to that of tiotropium in terms of overall incidence of adverse events and muscarinic side effects. Glycopyrronium could be an alternative choice to tiotropium, and like tiotropium, has the potential to be used as a monotherapy or combination therapy. Phase II studies have shown that a fixed-dose combination of glycopyrronium and the 24-hour LABA indacaterol, produces rapid and sustained bronchodilation compared with indacaterol monotherapy in patients with COPD. Phase III studies are currently ongoing to assess the long-term efficacy and safety of this combination.
Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable lung disease characterized by progressive airflow limitation.Citation1 It is a leading cause of mortality and morbidity worldwide, and represents a significant social and economic burden.Citation1 Characteristic symptoms of COPD, such as progressive dyspnea, cough, and sputum production, have a considerable impact on patient quality of life. Patients with COPD indicate the morning as the time when symptoms are more severe. In particular, shortness of breath is more frequent in the morning and severely restricts essential morning activities.Citation2–Citation4 Patients with severe to very severe airflow limitation have a greater range of symptoms and a higher burden of progressive disease compared with patients with more moderate disease.Citation1,Citation5
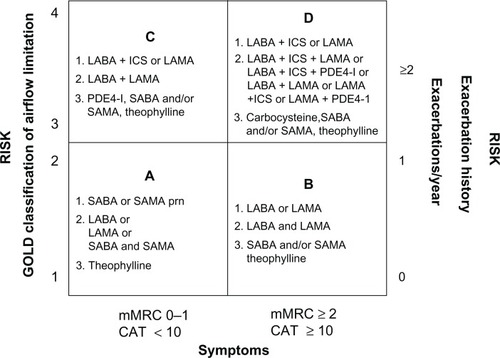
According to the Global Initiative for Obstructive Lung Disease (GOLD) 2011 strategy, the aim of pharmacologic management of COPD remains the relief of symptoms (especially dyspnea), reduction in the frequency and severity of exacerbations, and improvement of health status and exercise tolerance.Citation1 In addition, this new strategy recommends that the management of stable COPD should no longer be based solely on spirometric categories, but should include the impact of the disease on patients (determined by symptom burden and activity limitation) and risk of future events (especially exacerbations).Citation1 This revised recommendation reflects the approach used in multidimensional grading systems, such as the BODE index which includes the body mass index (B), degree of airflow obstruction (O), measured by the forced expiratory volume in one second (FEV1), dyspnea (D), and exercise capacity (E) as predictors of COPD-related hospitalization and mortality.Citation6
Bronchodilators are the cornerstone of pharmacologic therapy for COPD.Citation1 Long-acting inhaled bronchodilators are more convenient and more effective at sustaining symptomatic relief than short-acting bronchodilators.Citation1,Citation7,Citation8 Furthermore, longer-acting inhaled therapies improve patient compliance, as less frequent dosing may improve adherence.Citation9–Citation11 The GOLD 2011 strategy recommends long-acting bronchodilator therapy as a first choice in groups B, C, and D (low-risk more symptoms, high-risk fewer symptoms, and high-risk more symptoms, respectively) and a second choice (after rescue medication use) in group A (low risk less symptoms, ).Citation1
Figure 1 GOLD 2011 pharmacologic management of COPD based on combined assessment of airflow limitation, symptoms and exacerbations.
Adapted from Global Initiative for Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. GOLD; 2011.Citation1
Abbreviations: GOLD, Global Initiative for Obstructive Lung Disease; COPD, chronic obstructive pulmonary disease; LABA, long-acting β2-agonist; ICS, inhaled corticosteroid; LAMA, long-acting muscarinic antagonist; PDE4-inh, phosphodiesterase-4inhibitor; SABA, short-acting β2-agonist; SAMA, short-acting muscarinic antagonist; prn, pro re nata (as needed); mMRC, modified Medical Research Council; CAT, COPD Assessment Test.
Two classes of long-acting bronchodilators are available: long-acting β2-agonists (LABAs) and long-acting muscarinic antagonists (LAMAs). Currently, the only once-daily LAMA approved for maintenance therapy in COPD is tiotropium (Spiriva®, Boehringer Ingelheim Pharmaceuticals Inc, Ridgefield, CT); however, the once-daily Seebri® Breezhaler® (glycopyrronium, in development as NVA237, Novartis Pharma AG, Basel, Switzerland) was filed for approval in the European UnionCitation12 and in JapanCitation13 in 2011. Aclidinium bromide, a novel, twice-daily LAMA has recently been approved in the European Union and the US for the treatment of COPD.Citation14,Citation15 This review will focus on the novel LAMA glycopyrronium, and aims to provide pharmacokinetic and clinical evidence (focusing on data from Phase III studies) showing that this treatment will be a useful future addition to current pharmacologic strategies for COPD. Furthermore, clinical evidence will be considered in line with GOLD 2011 recommendations.
Pharmacology and pharmacokinetics
Glycopyrronium has a quaternary ammonium structure.Citation16 This minimizes its oral bioavailability, which should reduce systemic effects from any swallowed portion of the dose.Citation17
Mechanism and onset of action
Bronchoconstriction is caused by the action of acetylcholine (ACh) on muscarinic receptors. Muscarinic type-1 (M1) receptors on postganglionic nerves bind ACh released from preganglionic nerves, stimulating ACh release from postganglionic terminals. This binds to M3 receptors in the airways smooth muscle, stimulating bronchoconstriction ().Citation16
Figure 2 Glycopyrronium induces bronchodilation by inhibiting parasympathetically mediated bronchoconstriction.
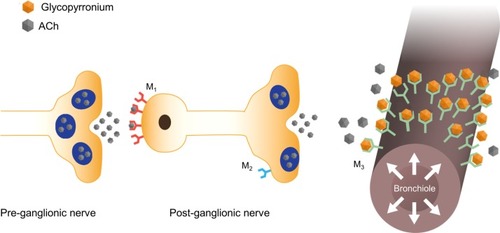
As with other LAMAs, the bronchodilator effect of glycopyrronium results from blockade of M1 and M3 receptors. A third type of receptor, the presynaptic inhibitory M2 receptor, is present not only in many different types of smooth muscle, where its function is not yet known,Citation18 but also on postganglionic nerves. In addition, these receptors are found in the heart and modulate pacemaker activity, atrioventricular conduction, and contractile force.Citation17,Citation19 Therefore, all LAMAs carry the risk of cardiovascular effects, such as tachycardia and arrhythmia. In preclinical studies, glycopyrronium showed a high degree of selectivity for M3 over M2 receptors and, compared with tiotropium, showed fewer systemic cardiovascular events.Citation16,Citation20,Citation21
As the binding of acetylcholine to muscarinic receptors triggers an increase in intracellular calcium, the onset of action of muscarinic antagonists can be measured using a cell-based in vitro calcium assay.Citation22 Studies using this assay have shown that glycopyrronium has a rapid onset of action, with a half-life (t1/2) of 6.1 ± 2.1 minutes for inhibition of methacholine-induced calcium release, which is approximately five times faster than that of tiotropium (t1/2 of inhibition 29.4 ± 4.2 minutes).Citation23
Drug delivery via Breezhaler® device
Glycopyrronium is delivered by the Breezhaler® device, a low-resistance, single-dose dry-powder inhaler suitable for use by patients of all ages with COPD and a wide range of disease severity.Citation24,Citation25 Studies with the Breezhaler® device have shown that it delivers a consistent dose of drug, irrespective of disease severity and patient age, with no reported device failures.Citation24,Citation25 The Breezhaler® device has a lower internal resistance than the Handihaler® device used to deliver tiotropium (specific airflow resistances of 2.2 and 5.1 × 10−2 kPa½ L−1 minute, respectively).Citation24 The internal resistance of the inhaler determines the effort patients have to make to achieve adequate inspiratory flows for effective and reproducible dose delivery.Citation26,Citation27 Low-resistance devices require less effort by the patients to generate adequate inspiratory flows than high-resistance devices, and are therefore more suitable for older patients and for those with severe COPD who may experience difficulties in generating these flows.
Clinical efficacy
A 50 μg dose glycopyrronium (50 μg refers to the quantity of the glycopyrronium moiety present in the capsule, which corresponds to a delivered dose of 44 μg) once daily in the morning provided significant bronchodilation over the course of the day, and was well tolerated in patients with moderate-to-severe COPD, as demonstrated in Phase II clinical studies.Citation28–Citation31 The Phase III GLycopyrronium bromide in COPD airWays (GLOW) program further evaluated the efficacy and safety of glycopyrronium 50 μg once daily in patients with moderate-to-severe COPD.Citation32–Citation34 GLOW1 evaluated the efficacy, safety, and tolerability of glycopyrronium 50 g once daily compared with placebo over 26 weeks in 822 patients.Citation32 GLOW2 evaluated the efficacy and safety of glycopyrronium 50 μg once daily compared with placebo and open-label tiotropium 18 μg once daily (as an active comparator) over 52 weeks in 1066 patients.Citation33 In both studies, outcomes measured included FEV1, dyspnea measured on the transition dyspnea index (TDI), health-related quality of life as assessed by the St George’s Respiratory Questionnaire (SGRQ), time to first moderate or severe COPD exacerbation, and mean daily rescue medication use over study duration. In the GLOW2 study, tiotropium was evaluated versus placebo as well as glycopyrronium for all endpoints, but the study was not powered to show the statistical superiority of glycopyrronium over tiotropium.Citation33 GLOW3 assessed the effects of glycopyrronium 50 μg once daily versus placebo on exercise tolerance.Citation34 Exercise tolerance was measured as exercise endurance time during a submaximal exercise tolerance test. Other key outcomes included spirometrically measured inspiratory capacity (IC) at isotime (the last matching time point at which the patient had a test result for both treatment periods) and exertional dyspnea (Borg CR10 scale). In all the studies in the GLOW program, safety was assessed by recording of treatment-emergent adverse events, monitoring of vital signs, and laboratory analyses.
Lung function
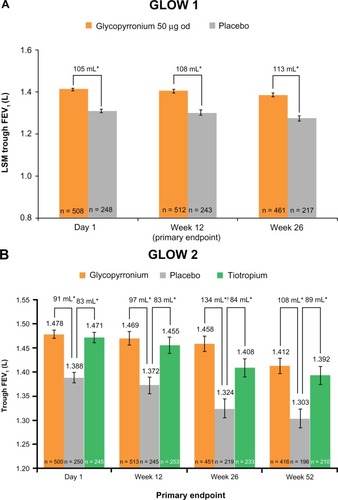
Trough FEV1 at Week 12 (primary endpoint) was significantly higher in patients receiving glycopyrronium compared with patients receiving placebo in both GLOW1 and GLOW2 (), with least squares means (LSM) treatment differences versus placebo of 108 mL and 97 mL (P < 0.001 for both).Citation32,Citation33 In the GLOW2 study, the efficacy of glycopyrronium versus placebo was similar to that of tiotropium (83 mL versus placebo; P < 0.001, ).Citation33 This improved bronchodilation was sustained until the end of the study in both GLOW1 and GLOW2 ().Citation32,Citation33
Figure 3 Glycopyrronium steady-state improvement in trough FEV1 achieved on day 1 and sustained until (A) week 26 (GLOW1) and (B) week 52 (GLOW2). Adapted from D’Urzo A, Ferguson GT, van Noord JA, et al. Efficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trial. Respir Res. 2011;12:156Citation32 and Kerwin E, Hébert J, Korenblat P, et al. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with moderate-to-severe COPD over 52 weeks: The GLOW2 study. Eur Respir J. July 26, 2012.Citation33
Abbreviations: FEV1, forced expiratory volume in one second; GLOW, GLycopyrronium bromide in COPD airWays; LSM, least squares means; SE, standard error; od, once daily.
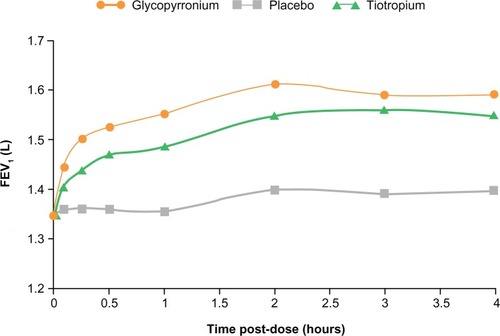
In GLOW1, glycopyrronium provided rapid bronchodilation, with a difference in mean FEV1 of 93 mL at 5 minutes and 144 mL at 15 minutes versus placebo, following the first dose on Day 1 (P < 0.001). On day 1 in the GLOW2 study, the mean FEV1 treatment difference for glycopyrronium versus placebo was 87 mL at 5 minutes and 143 mL at 15 minutes, while the difference for tiotropium versus placebo was 45 mL at 5 minutes and 78 mL at 15 minutes (all P < 0.001; ).Citation33 Essentially, glycopyrronium provided rapid bronchodilation following the first dose on Day 1, with significantly higher FEV1 from 5 minutes to 4 hours post-dose compared with placebo (P < 0.001) and tiotropium (P < 0.01; ).Citation33
Figure 4 FEV1 at each time point up to 4 hours post-dose on day 1in GLOW2. Reprinted from Kerwin E, Hébert J, Korenblat P, et al. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with moderate-to-severe COPD over 52 weeks: The GLOW2 study. Eur Respir J. July 26, 2012.Citation33
Abbreviations: FEV1, forced expiratory volume in one second; GLOW, GLycopyrronium bromide in COPD airWays; LSM, least squares means.
Dyspnea and health status
In GLOW1, glycopyrronium significantly improved the TDI focal score at Week 26, with a treatment difference of 1.04 (P < 0.001),Citation32 which exceeded the one-point treatment difference considered as clinically important ().Citation34 The percentage of patients achieving a clinically significant improvement in TDI focal score was 61.3% for glycopyrronium and 48.3% for placebo (). Thus, a clinically meaningful improvement in TDI focal score was 1.7-fold more likely for glycopyrronium compared with placebo (odds ratio [OR] 1.74, 95% confidence interval [CI] 1.25–2.42; P = 0.001, ).Citation32 In GLOW2, glycopyrronium and tiotropium significantly improved TDI focal scores compared with placebo at Week 26 (), with treatment differences of 0.81 and 0.94 (P = 0.002 for both active treatments).Citation33 The percentage of patients achieving a clinically meaningful improvement in TDI score was significantly higher with glycopyrronium and tiotropium compared with placebo (). For glycopyrronium, the OR was 1.58 with 95% CI 1.12–2.45 (P = 0.001) and for tiotropium the OR was 1.54 with 95% CI 1.04–2.30 (P = 0.032).Citation33
Figure 5 Improvements in (A and B) dyspnea and (C and D) health-related quality of life with glycopyrronium versus placebo in GLOW1.
Reprinted from D’Urzo A, Ferguson GT, van Noord JA, et al. Efficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trial. Respir Res. 2011;12:156.Citation32
Note: Data are LSM ± SE.
Abbreviations: HRQoL, health-related quality of life; GLOW, GLycopyrronium bromide in COPD airWays; LSM, least squares means; SE, standard error; TDI, Transition Dyspnea Index; MCID, minimal clinically important differences; OR, odds ratio; CI, confidence interval; SGRQ, St George’s Respiratory Questionnaire.
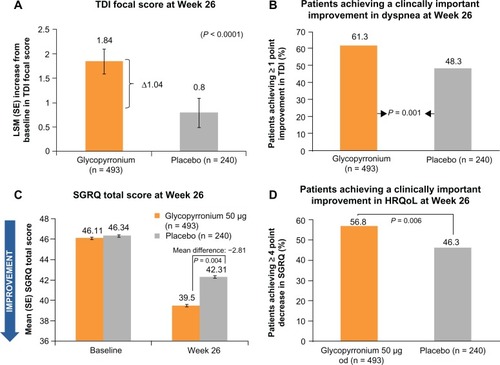
Figure 6 Improvements in (A and B) dyspnea and (C and D) health-related quality of life with glycopyrronium versus placebo in GLOW2. Reprinted from Kerwin E, Hébert J, Korenblat P, et al. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with moderate-to-severe COPD over 52 weeks: The GLOW2 study. Eur Respir J. July 26, 2012.Citation33
Abbreviations: HRQoL, health-related quality of life; GLOW, GLycopyrronium bromide in COPD airWays; LSM, least squares means; CI, confidence interval; TDI, Transition Dyspnea Index; od, once daily; OR, odds ratio; SGRQ, St George’s Respiratory Questionnaire.
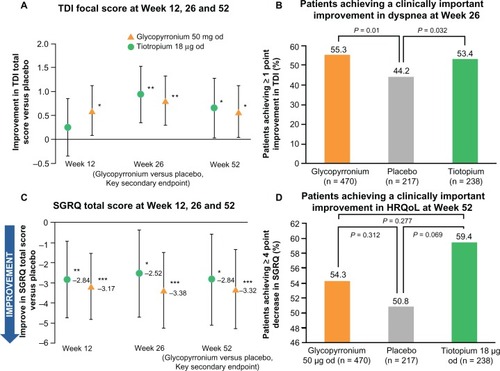
In GLOW1, the total SGRQ score was significantly better (lower) in patients receiving glycopyrronium compared with placebo (P = 0.004) at Week 26 (), with a treatment difference of −2.81, which was statistically significant, but not clinically meaningful (≥4-point reduction).Citation35 However, a significantly higher percentage of patients achieved a clinically meaningful improvement in SGRQ with glycopyrronium compared with placebo (OR 1.58, 95% CI 1.14–2.20; P = 0.006, ).Citation32 At Week 52 in GLOW2, the total SGRQ score was significantly improved in patients receiving glycopyrronium and tiotropium versus placebo, with treatment differences of −3.32 (P < 0.001) and −2.84 (P = 0.014, ).Citation33 A numerically higher proportion of patients receiving glycopyrronium or tiotropium achieved clinically meaningful improvements in SGRQ compared with placebo ().Citation33
Exacerbations and rescue medication
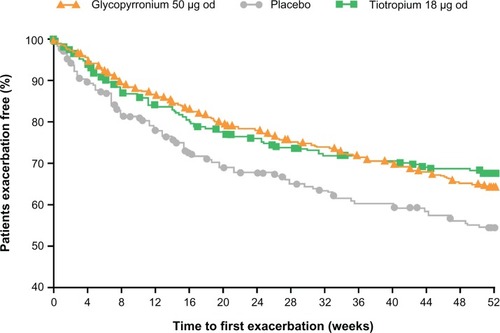
Glycopyrronium significantly prolonged the time to first moderate or severe exacerbation by 31% and 34% compared with placebo over 26 weeks (GLOW1; P = 0.023) and 52 weeks (GLOW2; P < 0.001; ).Citation32,Citation33 Furthermore, in the GLOW2 study, glycopyrronium demonstrated similar results to tiotropium, providing a 39% risk reduction versus placebo ( and ).Citation33
Table 1 Effect of active drug versus placebo on moderate to severe exacerbations and rescue medication use outcomes over 26 weeks (GLOW1) and 52 weeks (GLOW2). Adapted from D’Urzo A, Ferguson GT, van Noord JA, et al. Efficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trial. Respir Res. 2011;12:15632 and Kerwin E, Hébert J, Korenblat P, et al. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with moderate-to-severe COPD over 52 weeks: The GLOW2 study. Eur Respir J. July 26, 2012.Citation33
Figure 7 Glycopyrronium and tiotropium prolong time to first moderate to severe COPD exacerbation compared with placebo over 52 weeks in GLOW2. Reprinted from Kerwin E, Hébert J, Korenblat P, et al. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with moderate-to-severe COPD over 52 weeks: The GLOW2 study. Eur Respir J. July 26, 2012.Citation33
Abbreviations: COPD, chronic obstructive pulmonary disease; GLOW, GLycopyrronium bromide in COPD airWays; od, once daily.
During the 26-week GLOW1 study, treatment with glycopyrronium resulted in a significant reduction in the risk of severe COPD exacerbations leading to hospitalization (hazard ratio 0.35, 95% CI 0.141–0.857; P = 0.022), percentage of hospitalizations due to COPD exacerbations (1.7% versus 4.2%, OR 0.34, 95% CI 0.129–0.868; P = 0.024), and a numerical reduction in the rate of moderate or severe exacerbations versus placebo (0.43 versus 0.59/year; rate ratio 0.72; P = 0.071).Citation32 During the 52 weeks of the GLOW2 study, glycopyrronium and tiotropium were comparable and were both superior to placebo in reducing moderate or severe exacerbations needing systemic corticosteroids (glycopyrronium/placebo OR 0.61, 95% CI 0.434–0.870; P = 0.006; tiotropium/placebo OR 0.62, 95% CI 0.413–0.930; P = 0.021) and exacerbations requiring treatment with antibiotics (glycopyrronium/placebo OR 0.69, 95% CI 0.495–0.957; P = 0.026; tiotropium/placebo OR 0.65, 95% CI 0.438–0.949; P = 0.026).Citation33 In both GLOW1 and GLOW2, the use of daily rescue medication was significantly lower in patients receiving glycopyrronium compared with placebo ().Citation32,Citation33
Exercise endurance and exertional dyspnea
As demonstrated in the GLOW3 study after 3 weeks of treatment, glycopyrronium was significantly superior to placebo with respect to patients’ exercise endurance time; the LSM difference at Day 21 between treatment arms was 88.93 seconds (P < 0.001) corresponding to an approximately 21% difference ().Citation36
Figure 8 Improvements in (A) exercise tolerance, (B) lung function during exercise, and (C) exertional dyspnea with glycopyrronium versus placebo in GLOW3. Reprinted from Beeh KM, Singh D, Di Scala L, Drollmann A. Once-daily NVA237 improves exercise tolerance from the first dose in patients with COPD: the GLOW3 trial. Int J Chron Obstruct Pulmon Dis. 2012;7:503–513.Citation36
Abbreviations: GLOW, GLycopyrronium bromide in COPD airWays; LSM, least squares means; SE, standard error; CI, confidence interval; IC, inspiratory capacity.
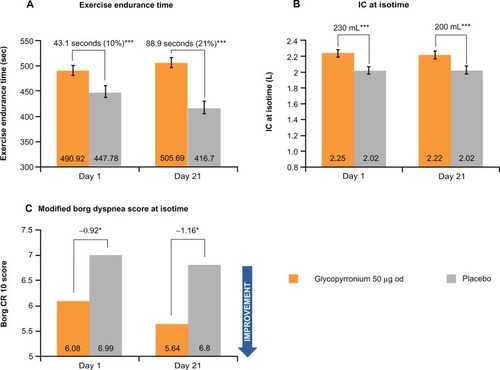
Dynamic hyperinflation is an important factor which limits exercise capacity in COPD, and results in a reduction of IC during exercise.Citation37,Citation38 Glycopyrronium produced a statistically significant increase in IC at isotime on Day 1 and Day 21 versus placebo (treatment difference of 230 mL and 200 mL, respectively; both P < 0.001), therefore reducing lung hyperinflation. A significantly greater improvement in IC was also seen in the long-term GLOW1 and GLOW2 studies.
Glycopyrronium was found to be superior to placebo for exertional dyspnea on Day 1 and Day 21 at isotime ().Citation36 On Day 1, the modified Borg dyspnea score improved by 13% with glycopyrronium versus placebo (treatment difference −0.92; P < 0.05), while on Day 21 the score improved by 20% (treatment difference −1.16; P < 0.05, ).Citation36
COPD symptoms collected via diary data
In the GLOW1 and GLOW2 studies, all patients were provided with an electronic patient diary to record daily morning and evening clinical symptoms, ie, cough, wheezing, shortness of breath, sputum volume and color, night-time awakenings, and rescue medication (salbutamol/albuterol) use. The diary allowed patients to keep a note of details of any change in symptoms, and any changes in concomitant medication throughout the study. Patients were required to score their symptoms in the diary in the morning (premedication) and the evening, on a scale of 0–3, with 0 representing no symptoms, no night-time awakening due to symptoms, no sputum produced, and no breathlessness or breathlessness only when running, and 3 representing severe symptoms, symptoms which completely stopped the patient from performing usual daily activities, more than 25 mL of sputum produced, green-colored sputum, and breathlessness at rest. Baseline scores were taken during the run-in period. The daily total symptom score was the sum of the worst of the morning and evening assessment for each symptom, and the daily individual symptom scores were the worst of the morning and evening assessments for the individual symptom.
In the GLOW1 study, the percentage of days when patients were able to perform usual daily activities was significantly greater in the glycopyrronium group compared with the placebo group (mean difference 5.13; P = 0.013; ). In the GLOW2 study, the percentage of nights with “no night-time awakenings” and “no daytime symptoms” over the 52-week treatment period was significantly higher than in placebo (mean differences 5.21 and 2.73, P < 0.05, respectively; ). In both the GLOW1 and GLOW2 studies, the change from baseline in the mean daily total symptom score was significantly greater in patients in the glycopyrronium group than in those on placebo; in the GLOW2 study, the changes seen with glycopyrronium versus placebo were comparable with those seen with tiotropium.
Table 2 Effect of glycopyrronium on COPD symptoms collected via patient diaries, compared with placebo
Safety
The overall data for glycopyrronium support an acceptable safety profile. Pooled data for GLOW1 and GLOW2 demonstrate that glycopyrronium was well tolerated, with a low frequency of cardiac and antimuscarinic side effects which was comparable with that of placebo and tiotropium 18 μg once daily.Citation32,Citation33 The incidence of adverse events for glycopyrronium (67%) was comparable with placebo (71%) and tiotropium (74%), with a higher frequency of COPD worsening (most common adverse event) in the placebo group ().Citation32,Citation33 Serious adverse events occurred with a slightly lower frequency in the glycopyrronium treatment group (11%) compared with placebo (13%) and the tiotropium group (15%, ).Citation32,Citation33
Table 3 Most frequent adverse events (≥5% in any treatment group); SAEs occurring in ≥5 patients in any treatment group, deaths, discontinuations due to adverse events and electrocardiographic abnormalities; pooled data from GLOW1 and GLOW2. Adapted from D’Urzo A, Ferguson GT, van Noord JA, et al. Efficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trial. Respir Res. 2011;12:156Citation32 and Kerwin E, Hébert J, Korenblat P, et al. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with moderate-to-severe COPD over 52 weeks: The GLOW2 study. Eur Respir J. July 26, 2012.Citation33
Discontinuations due to adverse events were 10% in the placebo group and 8% in the glycopyrronium and tiotropium groups. Antimuscarinic side effects, such as dry mouth, gastrointestinal disturbances, urinary retention, and urinary tract infections, occurred with a low frequency in the glycopyrronium, placebo, and tiotropium treatment groups.Citation32,Citation33
The percentage of patients with newly occurring or worsening clinically notable QTcF values (QT interval with Fridericia’s correction) was low across all treatment groups (). The number of deaths reported in each group was low and similar across treatments (), and none were considered to be related to the study medication.Citation32,Citation33
Discussion
Bronchodilators are central to the management of COPD. Because tiotropium is the only once-daily LAMA currently available for patients with COPD, there is a growing need to develop further LAMA treatment options. The new GOLD 2011 strategy recommends the use of LAMA monotherapy as one of the first choice treatments in groups B−D, and as a second choice in group A.Citation1 This highlights the pronounced role of LAMAs in the COPD treatment algorithm and the pressing need for the development of a therapeutic alternative to tiotropium.
An ideal LAMA for the treatment of COPD should improve lung function, exercise tolerance, and health status, provide relief from symptoms, and reduce the risk of exacerbation.Citation39 Data from Phase III clinical trials suggest that glycopyrronium can meet these needs and is as effective as tiotropium, the current gold-standard treatment for COPD. The improvement in lung function provided by glycopyrronium 50 μg once daily was shown to be sustained over long-term study (up to 52 weeks).Citation32,Citation33 This is particularly important in chronic conditions, such as COPD.
Improvement in exercise tolerance is an important therapeutic target in COPD.Citation1,Citation40 Glycopyrronium 50 μg once daily produced immediate and significant improvement in exercise tolerance from Day 1, which was accompanied by sustained reductions in lung hyperinflation (indicated by sustained and significant improvements in IC at isotime).Citation36 One of the most frequent activity-limiting symptoms in patients with COPD is reported to be breathlessness.Citation41 Glycopyrronium significantly improved exertional dyspnea and reduced breathlessness as measured by the TDI score.Citation34
One of the most important features of a bronchodilator for the treatment of COPD is a rapid onset of action. Rapid relief from symptoms may provide reassurance of effect and help improve compliance with medication.Citation9,Citation42 Furthermore, a rapid onset of action has important implications in COPD, as it provides immediate relief from early morning symptoms, which are considered by patients to be the most problematic.Citation2 This results in improvement in the ability to perform morning activities and reduction in the use of rescue medication.Citation2 Although tiotropium is effective and generally well tolerated, studies in patients with stable COPD have shown that it has a relatively slow onset of action, taking up to 3 hours to achieve optimal bronchodilation.Citation43 In contrast, the new LAMA glycopyrronium, which is being developed as a once-daily treatment for COPD, has a rapid onset of action. In the GLOW1 and GLOW2 studies, glycopyrronium-treated patients achieved significantly higher bronchodilation within 5 and 15 minutes of receiving the first dose on Day 1 versus placebo and tiotropium.Citation33 This rapid onset of effect, in conjunction with the sustained 24-hour bronchodilation demonstrated by glycopyrronium throughout the duration of the studies, indicate that it has the potential to have a significant impact on morning routines and daily life activities of patients with COPD. Moreover, once-daily morning inhalation of glycopyrronium 50 μg was shown to improve dyspnea and health status, and reduce rescue medication use compared with placebo.Citation33 Significant symptom improvements, captured in the patient diaries, were also seen in patients on glycopyrronium compared with placebo, both in the GLOW1 and GLOW2 studies.
Reducing the risk of exacerbation is an important aspect in the management of COPD; the most recent GOLD guidelines highlight the importance of treating exacerbations effectively and preventing future exacerbations.Citation1 This is based on the evidence that exacerbation reduces patient health status,Citation44,Citation45 and increases the risk of hospitalization and death.Citation46,Citation47 Furthermore, the frequency of exacerbations contributes to a long-term decline in lung function.Citation48 Glycopyrronium 50 μg once daily was shown to reduce the risk of exacerbation,Citation33 which should reduce the negative impact of COPD on patients’ quality of life.Citation46 Notably, the GLOW2 study was not powered to evaluate reductions in the risk of exacerbation, because only <28% of the patients had a baseline history of exacerbations. Therefore, the improvement seen in COPD exacerbations suggests that the beneficial effects of glycopyrronium could apply across a broad COPD population, not just patients with severe COPD or a history of frequent exacerbations.Citation33
Finally, an ideal LAMA should also have an acceptable safety and tolerability profile. Although generally well tolerated, LAMAs are associated with side effects common to all antimuscarinics, such as dry mouth, gastrointestinal disturbances, and cardiovascular effects.Citation19 Several studies with tiotropium have shown that dry mouth was the only event that was significantly higher in the treatment group than placebo, while there was no difference between treatment groups for other adverse events.Citation43,Citation49,Citation50 Recent clinical evidence for both glycopyrronium and tiotropium has demonstrated a low incidence of antimuscarinic side effects, including cardiac effects.Citation32,Citation33,Citation51 In addition to all the characteristics described above, an ideal LAMA should be delivered by a system with low internal resistance to allow effective and reproducible dose delivery.Citation24 This may increase adherence with treatment. Recent studies have shown that older patients and those with moderate-to-severe COPD have difficulty generating sufficient inspiratory flow for correct use of the dry-powder inhalersCitation52,Citation53 as inspiratory flow is inversely proportional to inhaler resistance, inhalers with low resistance, such as the Breezhaler® device, are suitable for the vast majority of patients with COPD, irrespective of age and symptom severity.Citation24
In summary, once-daily glycopyrronium provided significant improvements in lung function from the first dose, as well as improvements in dyspnea and health status, and reductions in the risk of exacerbations and use of rescue medication compared with placebo,Citation32,Citation33 with results similar to those of tiotropium.Citation33 Glycopyrronium also produced immediate and significant improvement in exercise toleranceCitation34 and had a similar safety profile to tiotropium with no unexpected adverse events.Citation32,Citation33 The clinical evidence to date suggests that glycopyrronium could be an alternative choice to tiotropium, which represents the current gold standard for patients with COPD.
The patient population in the Phase III clinical trials for glycopyrronium had moderate to severe COPD, classified as GOLD Stage II and III. The results of the GLOW studies indicate that glycopyrronium is efficacious and safe in patients with moderate COPD, where treatment may be more meaningful, as well as in those with severe disease. Long-acting bronchodilators, including LAMAs, are well represented in the treatment matrix recommended by the GOLD 2011 strategy as first choice in groups B–D, and second choice in symptomatic patients in Group A.
Current guidelines recommend the combined use of LABA and LAMA to maximize bronchodilation if symptoms are not improved by a single agent.Citation1 Therefore, glycopyrronium could be used as a monotherapy instead of tiotropium or as a combination therapy. The impact of LABA-LAMA combinations on FEV1 has been established.Citation54 There are currently a number of LABA-LAMA fixed-dose combinations in development for COPD, including QVA149, an inhaled dual bronchodilator containing a fixed-dose combination of glycopyrronium and another 24-hour agent, the LABA indacaterol (Onbrez® Breezehaler, Novartis Pharma AG, Basel, Switzerland).Citation55 In Phase II studies, QVA149 significantly improved lung function (P < 0.05 at all time points) and had a rapid onset of action compared with placebo and indacaterol, as well as producing sustained bronchodilation over 24 hours.Citation56,Citation57 QVA149 is undergoing Phase III investigation in the ongoing IGNITE clinical trial program, involving more than 7000 patients with COPD.Citation58 Data from four studies in the IGNITE program have recently been announced.Citation59 In SHINE, which enrolled more than 2100 patients, QVA149 showed superior improvement in trough FEV1 (P < 0.001) compared with once-daily indacaterol, glycopyrronium, and tiotropium. The ILLUMINATE study showed that once-daily QVA149 provided significant improvements in lung function compared with twice-daily salmeterol-fluticasone in symptomatic patients with COPD. The results of BRIGHT showed that patients achieved significantly better exercise tolerance compared with placebo (P = 0.006), while ENLIGHTEN demonstrated that QVA149 was well tolerated with a safety and tolerability profile similar to that of placebo. In addition, at a press briefing during the European Respiratory Society meeting in 2012, Novartis stated that the results of SPARK, which is also part of the IGNITE program and involved 2224 patients over a period of 64 weeks, have shown that QVA149 reduced the overall risk of exacerbations compared with glycopyrronium.
Conclusion
Glycopyrronium delivered once daily via the Breezhaler device is an effective and well tolerated treatment for the symptoms of COPD. It has a rapid onset of action with sustained bronchodilation and benefits on important outcomes in COPD, such as dyspnea, exacerbations, and health status, which are comparable with the current gold standard treatment for COPD.
Disclosure
RB has received reimbursement for attending scientific conferences, and/or fees for lecturing and/or consulting from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, and Nycomed. The Pulmonary Department at Mainz University Hospital received financial compensation for services performed during participation in single and multicenter clinical Phase I–IV trials organized by various pharmaceutical companies. DB is an employee of Novartis Pharmaceuticals Corporation. The authors were assisted in the preparation of the manuscript by Roberta Sottocornola, a professional medical writer contracted to CircleScience (Macclesfield, UK), and Mark J Fedele (Novartis). Writing support was funded by Novartis Pharma AG.
References
- Global Initiative for Obstructive Lung DiseaseGlobal strategy for the diagnosis, management and prevention of chronic obstructive pulmonary diseaseGOLD2011 Available from: http://www.goldcopd.com. Accessed July 11, 2012
- PartridgeMRKarlssonNSmallIRPatient insight into the impact of chronic obstructive pulmonary disease in the morning: an Internet surveyCurr Med Res Opin20092582043204819569976
- PartridgeMRMiravitllesMStåhlEKarlssonNSvenssonKWelteTDevelopment and validation of the Capacity of Daily Living during the Morning questionnaire and the Global Chest Symptoms Questionnaire in COPDEur Respir J20103619610419897551
- KesslerRPartridgeMRMiravitllesMSymptom variability in patients with severe COPD: a pan-European cross-sectional studyEur Respir J201137226427221115606
- HigginsVBroomfieldSSmallMSymptoms, consultations and comorbidities in real-world COPD patients classified by the GOLD 2011 strategyAbstract accepted at the COPD8 International Conference on Chronic Obstructive Pulmonary Disease2012
- CelliBRCoteCGLareauSCMeekPMPredictors of survival in COPD: more than just the FEV1Respir Med2008102Suppl 1S27S3518582794
- BeehKMBeierJThe short, the long and the “ultra-long”: why duration of bronchodilator action matters in chronic obstructive pulmonary diseaseAdv Ther201027315015920411368
- TashkinDPIs a long-acting inhaled bronchodilator the first agent to use in stable chronic obstructive pulmonary disease?Curr Opin Pulm Med200511212112815699783
- BourbeauJBartlettSJPatient adherence in COPDThorax200863983183818728206
- TamuraGOhtaKAdherence to treatment by patients with asthma or COPD: comparison between inhaled drugs and transdermal patchRespir Med200710191895190217587559
- ToyELBeaulieuNUMcHaleJMTreatment of COPD: relationships between daily dosing frequency, adherence, resource use, and costsRespir Med2011105343544120880687
- UK Medicines Information New Drugs OnlineGlycopyrrolate2012 Available from: http://www.ukmi.nhs.uk/applications/ndo/record_view_open.asp?newDrugID=4793. Accessed July 11, 2012
- Vectura press release. Novartis files NDA for NVA237 as a treatment for chronic obstructive pulmonary disease (COPD) in Japan; 2011. Available from: http://www.vectura.com/news/releases/2011/25-nov-2011.aspx. Accessed July 11, 2012.
- SimsMWPanettieriRAJrProfile of aclidinium bromide in the treatment of chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis2011645746622003291
- UK Medicines Information New Drugs OnlineAclidinium bromide2012 Available from: http://www.ukmi.nhs.uk/applications/ndo/record_view_open.asp?newDrugID=4515. Accessed August 15, 2012
- VogelmeierCBanerjiDNVA237, a long-acting muscarinic antagonist, as an emerging therapy for chronic obstructive pulmonary diseaseTher Adv Respir Dis20115316317321511677
- Ali-MelkkilaTKantoJIisaloEPharmacokinetics and related pharmacodynamics of anticholinergic drugsActa Anaesthesiol Scand19933776336428249551
- EglenRMReddyHWatsonNChallissRAMuscarinic acetylcholine receptor subtypes in smooth muscleTrends Pharmacol Sci19941541141198016895
- AbramsPAnderssonKEBuccafuscoJJMuscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladderBr J Pharmacol2006148556557816751797
- TrifilieffACopeNBohacekBThe inhaled muscarinic receptor antagonist, glycopyrrolate, has a favourable side-effect profile in a brown Norway rat lung function model when compared with tiotropiumChest20071324530a
- CooperNWalkerIKnowlesINVA237 has similar efficacy as tiotropium bromide against methacholine-induced bronchoconstriction and less systemic effect on cardiovascular variables in an anaesthetized rabbit model [Abstract]Eur Respir J200628Suppl 50P2544
- IshiiMKurachiYMuscarinic acetylcholine receptorsCurr Pharm Des200612283573358117073660
- SykesDADowlingMRLeighton-DaviesJThe influence of receptor kinetics on the onset and duration of action, and the therapeutic index of NVA237 and tiotropiumJ Pharmacol Exp Ther812012 [Epub ahead of print.]
- ChapmanKRFogartyCMPeckittCDelivery characteristics and patients’ handling of two single-dose dry-powder inhalers used in COPDInt J Chron Obstruct Pulmon Dis2011635336321760722
- PavkovRMuellerSFiebichKCharacteristics of a capsule based dry powder inhaler for the delivery of indacaterolCurr Med Res Opin201026112527253320843166
- WieshammerSDreyhauptJDry powder inhalers: which factors determine the frequency of handling errors?Respiration2008751182517911976
- TerzanoCDry powder inhalers and the risk of errorRespiration2008751141518185025
- ArievichHOverendTRenardDA novel model-based approach for dose determination of NVA237 in COPDRespir Res Submitted 2012
- FogartyCHattersleyHDi ScalaLDrollmannABronchodilatory effects of NVA237, a once daily long-acting muscarinic antagonist, in COPD patientsRespir Med2011105333734221144724
- VerkindreCFukuchiYFlémaleASustained 24-h efficacy of NVA237, a once-daily long-acting muscarinic antagonist, in COPD patientsRespir Med2010104101482148920541381
- VogelmeierCVerkindreCCheungDSafety and tolerability of NVA237, a once-daily long-acting muscarinic antagonist, in COPD patientsPulm Pharmacol Ther201023543844420416390
- D’UrzoAFergusonGTvan NoordJAEfficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trialRespir Res20111215622151296
- KerwinEHébertJKorenblatPEfficacy and safety of NVA237 versus placebo and tiotropium in patients with moderate-to-severe COPD over 52 weeks: The GLOW2 studyEur Respir J7262012 [Epub ahead of print.]
- WitekTJJrMahlerDAMinimal important difference of the transition dyspnoea index in a multinational clinical trialEur Respir J200321226727212608440
- JonesPLareauSMahlerDAMeasuring the effects of COPD on the patientRespir Med200599Suppl BS11S1816236492
- BeehKMSinghDDi ScalaLDrollmannAOnce-daily NVA237 improves exercise tolerance from the first dose in patients with COPD: the GLOW3 trialInt J Chron Obstruct Pulmon Dis2012750351322973092
- O’DonnellDEWebbKAThe major limitation to exercise performance in COPD is dynamic hyperinflationJ Appl Physiol2008105275375518678624
- GuenetteJAWebbKAO’DonnellDEDoes dynamic hyperinflation contribute to dyspnoea during exercise in patients with COPD?Eur Respir J201240232232922183485
- Global Initiative for Obstructive Lung DiseaseGlobal strategy for diagnosis, management and prevention of COPD2009 Available from: http://www.goldcopd.com. Accessed July 11, 2012
- CooperCBAirflow obstruction and exerciseRespir Med2009103332533419071004
- VogiatzisIStrategies of muscle training in very severe COPD patientsEur Respir J201138497197521737548
- Breekveldt-PostmaNSKoerselmanJErkensJALammersJWHeringsRMEnhanced persistence with tiotropium compared with other respiratory drugs in COPDRespir Med200710171398140517368011
- CasaburiRBriggsDDJrDonohueJFSerbyCWMenjogeSSWitekTJJrThe spirometric efficacy of once-daily dosing with tiotropium in stable COPD: a 13-week multicenter trial. The US Tiotropium Study GroupChest200011851294130211083677
- SpencerSCalverleyPMBurgePSJonesPWImpact of preventing exacerbations on deterioration of health status in COPDEur Respir J200423569870215176682
- MiravitllesMFerrerMPontAEffect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up studyThorax200459538739515115864
- AnzuetoAImpact of exacerbations on COPDEur Respir Rev20101911611311820956179
- WedzichaJASeemungalTACOPD exacerbations: defining their cause and preventionLancet2007370958978679617765528
- DonaldsonGCSeemungalTABhowmikAWedzichaJARelationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax2002571084785212324669
- DonohueJFvan NoordJABatemanEDA 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterolChest20021221475512114338
- BrusascoVHodderRMiravitllesMHealth outcomes following treatment for six months with once-daily tiotropium compared with twice daily salmeterol in patients with COPDThorax200358539940412728159
- CasaburiRMahlerDAJonesPWA long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary diseaseEur Respir J200219221722411866001
- Al-ShowairRATarsinWYAssiKHPearsonSBChrystynHCan all patients with COPD use the correct inhalation flow with all inhalers and does training help?Respir Med2007101112395240117629471
- JanssensWVan den BrandePHardemanEInspiratory flow rates at different levels of resistance in elderly COPD patientsEur Respir J2008311788317898020
- CazzolaMMolimardMThe scientific rationale for combining long-acting beta2-agonists and muscarinic antagonists in COPDPulm Pharmacol Ther201023425726720381630
- van der MolenTCazzolaMBeyond lung function in COPD management: effectiveness of LABA/LAMA combination therapy on patient-centred outcomesPrim Care Respir J201221110111822222945
- van NoordJABuhlRLaforceCQVA149 demonstrates superior bronchodilation compared with indacaterol or placebo in patients with chronic obstructive pulmonary diseaseThorax201065121086109120978028
- van de MaeleBFabbriLMMartinCHortonRDolkerMOverendTCardiovascular safety of QVA149, a combination of indacaterol and NVA237, in COPD patientsCOPD20107641842721166630
- BanerjiDChenHPatalanoFThe QVA149 IGNITE programme: dual bronchodilation as the future of COPD managementProceedings of the 6th International Primary Care and Respiratory Group (IPCRG) World Conference [Abstract]April 25–28, 2012Edinburgh, UK Abstract 179
- Vectura press release. Available from: http://www.vectura.com/news/releases/2012/24-apr-2012.aspx. Accessed August 15, 2012