Abstract
Background
Pulmonary rehabilitation is an effective intervention for people with chronic obstructive pulmonary disease (COPD). People with COPD undertake repeat programs, but synthesis of evidence regarding such practice has not been undertaken. The aim of this systematic review was to establish the effects of repeating pulmonary rehabilitation subsequent to an initial program in people with COPD.
Methods
Studies where participants with COPD undertook >1 pulmonary rehabilitation program were included, incorporating RCT (randomized controlled trial) and non-randomized studies. Electronic database searches were undertaken. Two authors independently undertook study identification, data extraction and risk of bias assessment. The primary outcome was health-related quality of life (HRQoL); secondary outcomes were exercise capacity, hospitalizations and exacerbations, adherence, mortality and adverse events. Narrative synthesis was undertaken for clinically heterogeneous trials. Data from RCTs and non-randomized studies were not combined for analysis.
Results
Ten included studies (2 RCTs) involved 907 participants with COPD (n=653 had undertaken >1 program). The majority of studies were at high risk of bias. One RCT (n=33) reported no difference in HRQol after a repeat program vs usual care following exacerbation (Chronic Respiratory Disease Questionnaire dyspnea domain score MD 0.4, 95% CI −0.5 to 3). In stable patients, clinically important and statistically significant improvements in HRQoL and exercise capacity were reported after repeat programs, but of a smaller magnitude than initial programs. There was evidence for reductions in exacerbations and hospitalizations, and shorter hospital length of stay for patients who repeated a program twice in 12 months compared to those who repeated once. No data for mortality or adverse events were available.
Conclusion
This systematic review provides limited evidence for benefits of repeating pulmonary rehabilitation in people with COPD, including improved HRQoL and exercise capacity, and reduced hospitalizations. However, most studies have high risk of bias, which reduces the certainty of these conclusions.
Study Registration
PROSPERO (CRD42020215093).
Introduction
Pulmonary rehabilitation is an effective intervention for people with chronic obstructive pulmonary disease (COPD) that is recommended in clinical guidelines worldwide.Citation1 Outpatient group programs commonly run twice a week for 7–8 weeks and encompass comprehensive assessment followed by patient-tailored therapies including exercise training, education and behavior change.Citation2 Level 1 evidence shows improvements in exercise capacity and symptomsCitation3 with a reduction in hospitalizations and length of stay in the 12 months following program completion.Citation4,Citation5
It is well documented that the initial beneficial effects diminish over time following pulmonary rehabilitationCitation2,Citation6 and that there is scant evidence for the efficacy of current models of maintenance programs.Citation7 In clinical practice, it is not uncommon for people with COPD to be re-referred to pulmonary rehabilitation on more than one occasion and international guidelines/statements acknowledge that additional pulmonary rehabilitation programs at some time following the initial program may provide further benefits.Citation2,Citation6 The timing of a repeat course of pulmonary rehabilitation may be prompted by a gradual decline in function or a rapid deterioration, such as may occur following an acute exacerbation of COPD. There have been calls for more information regarding the clinical benefits of repeat courses of pulmonary rehabilitationCitation8 and recommendations regarding frequency,Citation9 but synthesis of the available evidence to inform this practice has not been undertaken.
The aim of this systematic review is to establish the effects of repeating pulmonary rehabilitation subsequent to an initial program in people with COPD.
Methods
This systematic review was prospectively registered on PROSPERO (19 October 2020, CRD42020215093) and is presented according to the PRISMA guidelines.Citation10
Studies, Participants and Intervention
Studies where participants with COPD undertook a pulmonary rehabilitation program on more than one occasion were included, incorporating randomized controlled trials (RCTs) and non-randomized studies. A control group was not required for inclusion. Data for adults (18 years of age and over) with a diagnosis of COPD according to established criteria were included, regardless of disease severity.
All participants must have undertaken an initial pulmonary rehabilitation program of defined duration that included a component of physical rehabilitation incorporating whole-body exercise training (with or without resistance training) with or without any form of education and/or psychological support.Citation9 The repeat pulmonary rehabilitation program was in accordance with the definition for the initial intervention and undertaken at any time point after completion of an initial pulmonary rehabilitation program. Studies involving maintenance programs were excluded, where exercise training was delivered at a lower dose than the initial program and/or was an indefinite/ongoing program.Citation11 This was to ensure that any repeat program was delivered according to the same model and dose as the initial program. Studies not published in English were excluded.
Outcome Measures
The primary outcome was disease-specific health-related quality of life as measured with tools, eg, Chronic Respiratory Disease Questionnaire (CRQ, higher score = improvement), St George’s Respiratory Questionnaire (SGRQ, lower score = improvement). Secondary outcome measures were exercise capacity, hospitalization, mortality, adverse events and adherence. Measures of exercise capacity could reflect maximal capacity, peak capacity or functional exercise capacity measured by field walking tests including the 6-minute walk test (6MWT) or incremental shuttle walk test (ISWT). Where possible, the measure of effect was change from baseline value (post-program value – pre-program value). Post-program values were used if change values were not reported.
Search methods for Identification of Studies
Electronic searches of the following databases were undertaken: Cochrane Database of Systematic Reviews; MEDLINE; Embase; CINAHL (Cumulative Index to Nursing and Allied Health Literature); CENTRAL (Cochrane Central Register of Controlled Trials) and PEDro (Physiotherapy Evidence Database) (Supplemental Tables 1 and 2). Reference lists of included articles were reviewed. There were no limits on publication date prior to search execution on January 27, 2022 (English language only). Two co-authors (AB, MH) independently screened titles and abstracts, retrieved full-text publications and identified studies for inclusion. Discrepancies were resolved in consultation with a third co-author (AH).
Data Collection
Two co-authors (AB, CM) undertook independent data extraction including study characteristics (location and dates of data collection, design, inclusion and exclusion criteria, assessment timepoints), program features, participant characteristics and outcome data. Discrepancies were resolved in consultation with a third co-author (AH). Where necessary, data were extracted from published figures using https://automeris.io/WebPlotDigitizer/.
Assessment of Risk of Bias
Two co-authors (AB, CM) independently assessed risks of bias for each included RCT using the Cochrane Risk of Bias Tool.Citation12 Discrepancies were resolved in consultation with a third co-author (AH). We assessed risks of bias according to the following domains: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective outcome reporting; and other potential bias. Overall risk of bias was then determined for each study (low: all adequate; moderate: 1 inadequate or 2 unclear; high: >1 inadequate or >2 unclear).Citation12
Risk of bias was assessed for non-randomized studies using the Standard Quality Assessment Criteria.Citation13 These criteria incorporate study design, participant selection, allocation and blinding procedures, outcome measures, sample size, estimates of variance, confounding, reporting of results and evidence to support the conclusions. Each of 14 questions is scored according to criteria met (yes: 2 points; partial: 1 point; no: 0 points; N/A). The maximum score possible is 28, with scoring for each study calculated as a proportion of maximum possible accounting for the number of N/A items (28 minus [number of N/A × 2]). Two thresholds for study inclusion in systematic reviews have been proposed; 55% represents a liberal threshold and 75% represents a conservative threshold. We reported included studies against these thresholds, but due to the shortage of available data, thresholds were not used for study exclusion.
Where additional data were required to determine eligibility for inclusion or to facilitate analysis, the study authors were contacted.
Data Synthesis
If studies were clinical homogenous, then a pooled quantitative synthesis was to be undertaken. As the included studies were clinically heterogeneous, narrative synthesis was used. Data from RCTs and non-randomized studies were not combined for analysis. Data for the second program was analysed separately from that for subsequent programs. Where appropriate, the I2 statistic was to be used to measure heterogeneity (substantial statistical heterogeneity if I2 >50%).Citation14
Subgroup Analysis
Pulmonary rehabilitation programs commenced following an exacerbation were to be analysed separately to those commenced in a stable clinical state.
Statistical Analysis
Where data were able to be combined for analysis, data were entered into Review Manager 5 (RevMan Version 5.4: Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen) for calculation of mean differences (MD) and 95% confidence intervals (CI).
Results
Search results
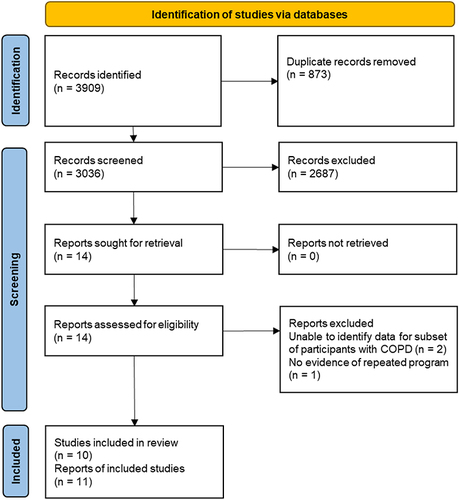
After removal of duplicates, 3036 records were screened, 14 records were reviewed in full text and 10 studies were included (11 reports). Two studies were excluded as data for the subset of participants with COPD were unavailableCitation15,Citation16 and one study had no evidence for repeated programCitation17 (). Meta-analysis was not possible due to study heterogeneity.
Figure 1 PRISMA flow diagram.
Study Characteristics
The 10 included studies collected data between 1996 and 2017. Three studies were undertaken in Australia,Citation18–20 two studies were undertaken in ItalyCitation21,Citation22 and one study each in Turkey,Citation23 Canada,Citation24 the United Kingdom,Citation25 the United StatesCitation26 and France.Citation27 Three studies were prospectiveCitation21,Citation22,Citation24 of which two were RCTsCitation22,Citation24 (Supplemental Table 3).
One RCT followed participants for 12 months after an initial (inpatient/outpatient) pulmonary rehabilitation program.Citation24 Participants were monitored to identify development of an exacerbation and any who experienced such events were subsequently randomized to a repeat program or usual care. The remaining nine studies involved repeat programs during periods of clinical stability. Of these, another prospective RCT employed an inpatient pulmonary rehabilitation model delivered twice at 6- and 12-month intervals after the first program or once at 12 months following the first program.Citation22 Seven studies involved outpatient programs,Citation18–21,Citation26 of which two were published as abstractsCitation23,Citation25 (Supplemental Table 4).
Of the eight observational studies, three studies reported outcomes for participants who had undertaken a first and second pulmonary rehabilitation program.Citation20,Citation23,Citation25 Two studies reported outcomes for participants who did and did not repeat programs following the initial program, as well as outcomes for participants following the secondCitation18,Citation26 and third programs.Citation18 Three other studies reported outcomes for participants who undertook threeCitation19,Citation27 and five programsCitation21 (Supplemental Table 5). Time between the first and subsequent programs in retrospective studies varied from mean 17 (SD 6) monthsCitation21 to 45 (24) monthsCitation26 ().
Table 1 Overview of Time Frame and Reported Outcome Measures
Participant Characteristics
The included studies involved 907 participants with COPD of whom 653 had undertaken more than one pulmonary rehabilitation program (). The mean age of participants varied from 68 to 70 years, FEV1 ranged from 36% to 58% predicted, and proportion of participants who were female varied from 12% to 64% (RCT: Supplemental Table 5; non-randomized studies: Supplemental Table 6).
Reported Outcome Measures
The primary outcome measure of health-related quality of life was reported by seven studies, with four studies using the SGRQCitation19,Citation21–23 and three studies using the CRQCitation18,Citation24,Citation25 ().
The secondary outcome measure of exercise capacity was reported by nine studies, with seven studies using the distance walked on the 6-minute walk test (6MWD),Citation18–20,Citation22,Citation24,Citation26,Citation27 two studies using the distance walked on the incremental shuttle walk test (ISWD)Citation23,Citation25 and one study reporting peak workload.Citation21 Hospitalization was reported by three studiesCitation21,Citation22,Citation24 and program adherence by two studies.Citation18,Citation26 No studies reported mortality or adverse events (). Additional data were sought and provided by corresponding authors for two studiesCitation19,Citation23 and in two instances original data were available from previous co-author publications.Citation18,Citation20
Risk of Bias
Both of the RCTs were assessed as being at high risk of bias, due to the number of domains assessed as unclear (Supplemental Table 7).Citation22,Citation24
Of the remaining eight non-randomized studies, six studies met the liberal threshold of 55%Citation18–21,Citation26,Citation27 reflecting a high risk of bias. Four studies that met the conservative threshold of 75%Citation18,Citation20,Citation21,Citation27 (Supplemental Table 8).
OUTCOME, Primary: Health-Related Quality of Life
RCTs
In patients recovering from an exacerbation, no overall group × time effect in health-related quality of life was demonstrated comparing those who repeated a program (outpatient program n=7, inpatient n=9) to those who did not (n=17) (post-rehabilitation CRQ domain scores: dyspnea MD 0.4, 95% CI −0.5 to 3; fatigue MD −0.1, 95% CI −0.9 to 0.7; emotional function MD 0.2, 95% CI −0.6 to 1.0; mastery 0.6, 95% CI −0.2 to 1.4; follow-up scores: dyspnea MD 0.8, 95% CI −0.3 to 1.9; fatigue MD 0.5, 95% CI −0.4 to 1.4; emotional function MD 1.0, 95% CI 0.3 to 1.7; mastery MD 1.2, 95% CI 0.5 to 1.9) (Supplemental Figure 1).Citation24
In stable patients, one RCT demonstrated significantly better health-related quality of life (SGRQ symptoms domain score MD −9, 95% CI −15 to −3) at 12 months in patients who had repeated pulmonary rehabilitation twice (n=14) compared to those who had repeated once (n=15); no between-group differences were demonstrated in SGRQ total (MD 1, 95% CI −4 to 6) or other domain scores (activity MD 0, 95% CI −7 to 7; impact MD −4 95% CI −10 to 2) (Supplemental Figure 2).Citation22
Non-Randomized Studies
One abstract (n=125) reported within-group improvements in CRQ total score following the first program (MD 16.4, 95% CI 19.9 to 12.9) and second program (MD 11.1, 95% CI 8.0 to 14.2). The observed improvement was greater following the first program (MD 5.3, 95% CI 0.92 to 9.6).Citation25 One study demonstrated that first (n=46) and second (n=38) programs results in similar improvements in CRQ domain scores, with more variability demonstrated following the third program (n=6) (Supplemental Figure 3).Citation18
Figure 3 Exercise capacity in non-randomized studies: Distance walked on the 6-minute walk test (5 studies). Data from [18–21,26].
![Figure 3 Exercise capacity in non-randomized studies: Distance walked on the 6-minute walk test (5 studies). Data from [18–21,26].](/cms/asset/1588a399-9ab6-4ed0-9bf9-2fe1fb24452a/dcop_a_12152888_f0003_c.jpg)
Three uncontrolled studies reported SGRQ before and after repeat programs ().Citation19,Citation21,Citation22 Clinically significant improvements were demonstrated for repeat programs, however two studies indicated larger gains following the first program when compared to subsequent programs.Citation19,Citation21 The proportion of patients attaining a clinically significant change in SGRQ score did not change across the study period: first program n=6 (12.5%); second program n=4 (8.3%); third program n=6 (12.5%); fourth program n=6 (12.5%); and fifth program n=7 (14.6%).Citation21 Changes in SGRQ domain scores following each program are presented in Supplemental Table 9.
OUTCOME, Secondary: Exercise Capacity
RCTs
In one RCT of patients recovering from an exacerbation, the difference in 6MWD was greater in those who repeated a program (outpatient program n=7, inpatient n=9) than those who did not (n=17) both immediately post program (MD 30m, 95% CI −36 to 96) and at follow-up (MD 56m (95% CI −21 to 133) (Supplemental Figure 4).Citation24
In one RCT of stable patients, the difference in 6MWD between those who repeated pulmonary rehabilitation once (n=15) and those who had repeated twice (n=14) was MD 4m (95% CI −58 to 66) at 12 months (Supplemental Figure 5).Citation22
Non-Randomized Studies
Six uncontrolled studies reported 6MWD for repeat programs.Citation18–21,Citation26,Citation27 One study presented the within-program change in 6MWD for the initial program (mean 65m, SD 30, n=190), for the second program (mean 44m, SD 20, n=190) and for the third program (mean 55m, SD 58, n=62).Citation27 Pre/post program 6MWD data are presented in for the remaining studies.Citation18–21,Citation26 All programs resulted in within-program improvements in 6MWD with smaller changes in 6MWD following repeat programs. Six studies reported differences in 6MWD outcomes between first and second programs ranging from no difference to 30 m less improvement on the second program.Citation18–21,Citation26,Citation27 Four studies reported differences in 6MWD outcomes between the first and third programs ranging from 2 to 65 m less improvement on the third program.Citation18,Citation19,Citation21,Citation27 One longitudinal study also reported less improvement in fourth and fifth programs compared to the first program (Supplemental Table 10).Citation21
Data for the proportion of participants who achieved the 6MWD MID following pulmonary rehabilitation programs were reported by five studies ().Citation18,Citation19,Citation21,Citation26,Citation27 A range of threshold definitions were used. Results for studies using 25–35m thresholds demonstrated consistent results across second programs, with 61–67% of the participants achieving the MID.Citation18,Citation19,Citation26,Citation27 One study demonstrated that of the 41/190 non-responders to the initial program, 23 (56%) did respond to the second program and of the 149/190 responders to the initial program, 44 (30%) became non-responders in the second program.Citation27 The study looking at five programs used a 54m threshold and demonstrated consistent results over the first three programs (31–33% achieving the MID) with a decrease evident in the final two programs (first vs fourth program 12.5%, p<0.01; first vs fifth program 14.6%, p=0.03).Citation21
Table 2 Exercise Capacity in Non-Randomized Studies: Proportion of Participants Who Achieved the Minimal Important Difference in the Distance Walked on the 6-Minute Walk Test (5 Studies)
Two abstracts reported change in ISWD.Citation23,Citation25 Clinically significant improvements were demonstrated for repeat programs (MD 46m, 95% CI 9 to 83, n=14; MD 46m, 95% CI 36 to 56, n=125).Citation23,Citation25 Comparisons demonstrated no statistically significant difference between programs in one abstract (p=0.864)Citation23 and a statistically significant decrease in change in ISWD following the second program in the other abstract (MD −18m, 95% CI −33 to −2)Citation25; however, this is less than the MID.Citation28
The study looking at five programs (n=48) demonstrated significant improvements in peak workload with each program with the exception of the fifth program (Supplemental Table 11).Citation21 No significant decreases in post-program peak workload comparing the first program with the second program (MD-1 watts, 95% CI −8 to 6), third program (MD −1 watts, 95% CI −8 to 6), or fourth program (MD −5 watts, 95% CI −12 to 2). Comparing the first and fifth programs, a significant decrease in post-program peak workload was demonstrated (MD −13 watts, 95% CI −53 to −9).Citation21
OUTCOME, Secondary: Hospitalizations and Non-Admitted Exacerbations
RCT
In the year following the initial program, there was no difference in the mean number of hospitalizations per participant for those who repeated pulmonary rehabilitation twice (at 6 and 12 months) compared to those who repeated once (at 12 months) (mean 1.0 (SD 0.8) vs 1.5 (1.1) hospitalizations, p=0.132).Citation22 Results were similar for hospital length of stay (mean 14 (SD 9) vs 9 (8) days, p=0.122).Citation22 Those who repeated once were significantly more likely to spend more than 10 days in hospital (n=12) than participants who repeated twice (n=5, p<0.001).Citation22
Non-Randomized Studies
The study looking at five programs (n=48) demonstrated a significant reduction in the number of exacerbations and hospitalizations following repeat programs compared to the year before the first program, as well as a significant increase in the number of participants free from exacerbations and hospitalizations per participant per year (exacerbation: episodes not requiring hospitalization but requiring a change of usual medication and prescription of systemic steroids and/or antibiotics; Supplemental Table 12).Citation21
OUTCOME, Secondary: Adherence
RCT
Nil data.
Non-Randomized Studies
Participants completed a similar number of sessions in the initial and subsequent programs. One study offering a 16-session program reported completion of median 12 (IQR 11 to 14) sessions in the initial program, median 13 (IQR 11 to 14) sessions in the first repeat program and median 14 (IQR 10 to 14) sessions in the second repeat program.Citation18 One study offering a 24-session program reported completion of mean 21 (SD 6) sessions in the initial program and mean 22 (SD 6) sessions in the first repeat program.Citation26
Discussion
Current practice may incorporate repeating pulmonary rehabilitation according to clinical indication and personal factorsCitation20,Citation29 but no systematic review of the effects of programs subsequent to an initial pulmonary rehabilitation program has previously been undertaken.
In patients following an exacerbation, a single RCT did not demonstrate any benefits of repeating pulmonary rehabilitation shortly after the exacerbation, in comparison to usual care.Citation24 For stable patients, clinically meaningful benefits of repeating pulmonary rehabilitation were demonstrated, with one RCT suggesting that more frequent programs might have greater benefit (repeating twice in 12 months vs once in 12 months).Citation22 Uncontrolled data suggest that the absolute magnitude of improvement in health-related quality of life may not be as large in repeat programs, as it is in the first, but benefits remain clinically meaningful. Important reductions in hospitalizations were also demonstrated with repeating pulmonary rehabilitation. Most studies were at high risk of bias which reduces certainty in these findings.
This review only identified one study that specifically assessed repeat programs following hospitalization.Citation24 International guidelines recommend referral to pulmonary rehabilitation following an exacerbationCitation2,Citation30,Citation31 with evidence for improvements in quality of life and exercise capacity as well as important reductions in hospital readmissions and mortality for patients who have had a hospitalization.Citation4,Citation32 Recent US data have further demonstrated that initiation of pulmonary rehabilitation within 3 months of hospital discharge was associated with significantly fewer hospital readmissions, shorter hospital stays and a lower mortality risk at 12 months.Citation33,Citation34 The well-documented benefits and the value placed on these benefits by people with COPDCitation35 highlight the need for more evidence to inform this element of COPD management.
The aim of the other recommendation regarding timing for re-referral is to prevent decline in pulmonary rehabilitation outcomes.Citation2 The data in this review reinforce that clinically important improvements can be achieved following repeat programs, even if the extent of improvement is less than that seen following the initial program. This review also identified important new preliminary evidence that people who were not identified as “responders” following the initial program were able to achieve clinically significant improvements following a second program.Citation27 One study had previously demonstrated that gains in the CRQ mastery domain were greater following a repeat program relative to the initial program, and suggested that more time may be required to achieve gains, in mastery in this instance, relative to other outcomes.Citation18 Therefore, patients who are not identified as “responders” following an initial program should be considered eligible for repeat programs.Citation6
Participants do not respond uniformly to pulmonary rehabilitation,Citation36 and the rate of decline in different outcomes following program completion varies.Citation37 Current guidelines state that re-referral may be considered from 12 months following pulmonary rehabilitationCitation1 but may be appropriate earlier in the case of clinical indication.Citation6 Included studies that sought to repeat pulmonary rehabilitation at scheduled intervals following initial programs (6- and 12-months,Citation22 12 months,Citation27 12 to 18 monthsCitation21) demonstrated benefits for health-related quality of life,Citation22 consistent proportions of 6MWD responders across the first three programsCitation21,Citation27 and ongoing benefits in terms of reducing both exacerbations and hospitalizations over five programs.Citation21 Whilst the amount and quality of data preclude firm conclusions, studies in this review do signal that there may be benefits to this approach.
A model of care to assist people with COPD to “maintain the gains” after they finish pulmonary rehabilitation remains elusive but important, particularly as people live for longer with COPD.Citation38 This review excluded studies providing maintenance programs (ie, exercise training undertaken at a lower dose than the initial program and/or of an indefinite/ongoing nature). International statements and two systematic review have been unable to recommend any model of maintenance due to insufficient evidence of benefit.Citation1,Citation7,Citation11,Citation39 Despite this, significant clinical resources are devoted to maintenance programs and some patients may find them useful to maintain motivation for physical activity, and to access support from peers and health professionals.Citation40 The data in this review illustrate the evolving body of evidence for an alternative approach of repeating pulmonary rehabilitation, and supports calls for access to repeat programs.Citation8 However, resource reallocation would require more evidence not only for clinical measures but also incorporate the impact of repeat programs on outcomes such as healthcare utilization, for which hospitalization forms the bulk of direct medical costs.Citation41 Reductions in total healthcare costs over 12 months have been associated with pulmonary rehabilitation completion,Citation42 but there are challenges to designing sufficiently large and long prospective studies to capture the long-term data that are required to assess the costs and benefits of repeat programs over many years.
Limitations to this review include the small number of eligible studies; as a result, we elected to include two abstracts, and this represents a variation from our published protocol. The lack of data from prospective studies (total of 62 participants in two RCTs) and the high risk of bias seen in many of the included studies does limit the capacity to draw strong conclusions. Only participants with COPD were included, so the findings may not be extrapolated to other disease groups commonly referred to pulmonary rehabilitation. Included studies mirrored the real-world heterogeneity in pulmonary rehabilitation program format, staffing and resources,Citation43 but this also precluded meaningful quantitative synthesis.
Conclusion
This systematic review provides limited evidence for benefits of repeating pulmonary rehabilitation in people with COPD, including improvements in health-related quality of life and exercise capacity, and reduced need for hospitalization. However, the majority of included studies were at high risk of bias. Future studies should investigate the optimal timing and frequency for repeat programs, and investigate the cost-effectiveness of this strategy.
Abbreviations
CI, confidence intervals; COI, conflict of interest; COPD, chronic obstructive pulmonary disease; CRQ, Chronic Respiratory Disease Questionnaire; 6MWD, distance walked on the 6-minute walk test; 6MWT, 6-minute walk test; FER, forced expiratory ratio; FEV1, forced expiratory volume in one second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HRQoL, health-related quality of life; IQR, interquartile range; ISWD, distance walked on incremental shuttle walk test; ISWT, incremental shuttle walk test; MD, mean difference; MCID, minimal clinically important difference; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PROSPERO, Prospective Register of Systematic Reviews; RCTs, randomized controlled trials; SD, standard deviation; SGRQ, St George’s Respiratory Questionnaire.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; Have drafted or written, or substantially revised or critically reviewed the article; Have agreed on the journal to which the article will be submitted; Reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage; Agree to take responsibility and be accountable for the contents of the article.
Disclosure
The authors report no conflicts of interests in this work.
Acknowledgments
The abstract of this paper was presented at the American Thoracic Society International Conference as a poster presentation with interim findings. The poster’s abstract was published in ‘Poster Abstracts’ in American Journal of Respiratory and Critical Care Medicine: https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2021.203.1_MeetingAbstracts.A4142
Additional information
Funding
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD; 2022. Available from: http://goldcopd.org. Accessed August 4, 2022.
- Spruit M, Singh S, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–64. doi:10.1164/rccm.201309-1634ST
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Syst Rev. 2015;23(2):CD003793. doi:10.1002/14651858.CD003793.pub3
- Puhan M, Gimeno-Santos E, Cates C, Troosters T. Pulmonary rehabilitation for people who have been in hospital with an exacerbation of chronic obstructive pulmonary disease. Cochrane Database of Syst Rev. 2016;12:CD005305. doi:10.1002/14651858.CD005305.pub4
- Griffiths T, Burr M, Campbell I, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet. 2000;355(9201):362–368. doi:10.1016/s0140-6736(99)07042-7
- Bolton C, Bevan-Smith E, Blakey J, et al. British Thoracic Society guideline on pulmonary rehabilitation in adults. Thorax. 2013;68(Suppl 2):ii1–30. doi:10.1136/thoraxjnl-2013-203808
- Alison J, McKeough Z, Johnston K, et al. Australian and New Zealand pulmonary rehabilitation guidelines. Respirology. 2017;22(4):800–819. doi:10.1111/resp.13025
- Rochester C, Vogiatzis I, Holland A, et al. An official American Thoracic Society/European Respiratory Society policy statement: enhancing implementation, use and delivery of pulmonary rehabilitation. Am J Respir Crit Care Med. 2015;192(11):1373–1386. doi:10.1164/rccm.201510-1966ST
- Vogiatzis I, Rochester C, Spruit M, Troosters T, Clini E. Increasing implementation and delivery of pulmonary rehabilitation: key messages from the new ATS/ERS policy statement. Eur Respir J. 2016;47(5):1336–1341. doi:10.1183/13993003.02151-2015
- Page M, McKenzie J, Bossuyt P, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
- Malaguti C, Dal Corso S, Janjua S, Holland A. Supervised maintenance programmes following pulmonary rehabilitation compared to usual care for chronic obstructive pulmonary disease. Cochrane Database of Syst Rev. 2021;8:CD013569. doi:10.1002/14651858.CD013569.pub2
- Higgins J, Altman D, Sterne J. Chapter 8: assessing risk of bias in included studies. In: Higgins J, Churchill R, Chandler J, Cumpston M, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0; 2017.
- Kmet L, Lee R, Cook L. Standard quality assessment criteria for evaluating primary research papers from a variety of fields. Edmonton, Alberta, Canada: Alberta Heritage Foundation for Medical Research; 2004.
- Deeks J, Higgins J, Altman D. Chapter 9: analysing data and undertaking meta analyses. In: Higgins J, Churchill R, Chandler J, Cumpston M, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0. The Cochrane Collaboration; 2017.
- Foglio K, Bianchi L, Ambrosino N. Is it really useful to repeat outpatient pulmonary rehabilitation programs in patients with chronic airway obstruction? A 2-year controlled study. Chest. 2001;119(6):1696–1704. doi:10.1378/chest.119.6.1696
- Hill K, Bansal V, Brooks D, Goldstein R. Repeat pulmonary rehabilitation programs confer similar increases in functional exercise capacity to initial programs. J Cardiopulm Rehabil Prev. 2008;28(6):410–414. doi:10.1097/HCR.0b013e31818c3c8d
- Camillo C, Langer D, Osadnik C, et al. Survival after pulmonary rehabilitation in patients with COPD: impact of functional exercise capacity and its changes. Int J COPD. 2016;11:2671–2679. doi:10.2147/COPD.S113450
- Heng H, Lee A, Holland A. Repeating pulmonary rehabilitation: prevalence, predictors and outcomes. Respirology. 2014;19(7):999–1005. doi:10.1111/resp.12365
- Sandoz J, Roberts M, Cho J, Wheatley J. Magnitude of exercise capacity and quality of life improvement following repeat pulmonary rehabilitation in patients with COPD. Int J COPD. 2017;12:1085–1091. doi:10.2147/COPD.S131778
- Storey S, Erbas B, Holland A. Why do people with chronic obstructive pulmonary disease repeat pulmonary rehabilitation? Perspectives of patients and health professionals. Chron Respir Dis. 2018;16:1–8. doi:10.1177/1479973118816420
- Foglio K, Bianchi L, Bruletti G, et al. Seven-year time course of lung function, symptoms, health-related quality of life, and exercise tolerance in COPD patients undergoing pulmonary rehabilitation programs. Respir Med. 2007;101(9):1961–1970. doi:10.1016/j.rmed.2007.04.007
- Romagnoli M, Dell’Orso D, Lorenzi C, et al. Repeated pulmonary rehabilitation in severe and disabled COPD patients. Respiration. 2006;73(6):769–776. doi:10.1159/000092953
- Yeşiloğlu E, Candemir I, Ergün P, Kaymaz D, Demir N, Şengül F. The efficiency of pulmonary re-rehabilitation [abstract]. Eur Respir J. 2018;52:PA4140. doi:10.1183/13993003.congress-2018.PA4140
- Carr S, Hill K, Brooks D, Goldstein R. Pulmonary rehabilitation after acute exacerbation of chronic obstructive pulmonary disease in patients who previously completed a pulmonary rehabilitation program. J Cardiopulm Rehabil Prev. 2009;29(5):318–324. doi:10.1097/HCR.0b013e3181ac7bb8
- Ingram K, Canavan J, Kon S, et al. Repeat pulmonary rehabilitation programmes: Are they beneficial in COPD? [abstract]. Eur Respir J. 2014;44:P610.
- Atabaki A, Fine J, Haggerty M, et al. Effectiveness of repeated courses of pulmonary rehabilitation on functional exercise capacity in patients with COPD. J Cardiopulm Rehabil Prev. 2015;35(4):272–277. doi:10.1097/HCR.0000000000000115
- Al Chikhanie Y, Bailly S, Amroussia I, Veale D, Herengt F, Verges S. Trajectories of COPD patients’ response to repeated pulmonary rehabilitation programs. Respir Med. 2021;190:106678. doi:10.1016/j.rmed.2021.106678
- Evans R, Singh S. Minimum important difference of the incremental shuttle walk test distance in patients with COPD. Thorax. 2019;74(10):994–995. doi:10.1136/thoraxjnl-2018-212725
- Spruit M, Rochester C, Pitta F, et al. Pulmonary rehabilitation, physical activity, respiratory failure and palliative respiratory care: state of the art review. Thorax. 2019;74(7):693–699. doi:10.1136/thoraxjnl-2018-212044
- Criner G, Bourbeau J, Diekemper R, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147(4):894–942. doi:10.1378/chest.14-1676
- Wedzicha J, Miravitlles M, Hurst J, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;49(3):1600791. doi:10.1183/13993003.00791-2016
- Ryrsø C, Godtfredsen N, Kofod L, et al. Lower mortality after early supervised pulmonary rehabilitation following COPD-exacerbations: a systematic review and meta-analysis. BMC Pulm Med. 2018;18(1):154. doi:10.1186/s12890-018-0718-1
- Lindenauer P, Stefan M, Pekow P, et al. Association between initiation of pulmonary rehabilitation after hospitalization for COPD and 1-year survival among Medicare beneficiaries. JAMA. 2020;323(18):1813–1823. doi:10.1001/jama.2020.4437
- Spitzer K, Stefan M, Priya A, et al. Participation in pulmonary rehabilitation after hospitalization for chronic obstructive pulmonary disease among Medicare beneficiaries. Ann Am Thorac Soc. 2019;16(1):99–106. doi:10.1513/AnnalsATS.201805-332OC
- Zhang Y, Morgan R, Alonso-Coello P, et al. A systematic review of how patients value COPD outcomes. Eur Respir J. 2018;52(1):180222. doi:10.1183/13993003.00222-2018
- Spruit M, Augustin I, Vanfleteren L, et al. Differential response to pulmonary rehabilitation in COPD: multidimensional profiling. Eur Respir J. 2015;46(6):1625–1635. doi:10.1183/13993003.00350-2015
- Güell R, Casan P, Belda J, et al. Long-term effects of outpatient rehabilitation in COPD: a randomised trial. Chest. 2000;117(4):976–983. doi:10.1378/chest.117.4.976
- James G, Donaldson G, Wedzicha J, Nazareth I. Trends in management and outcomes of COPD patients in primary care, 2000–2009; A retrospective cohort study. Prim Care Respir Med. 2014;24(1):14015. doi:10.1038/npjpcrm.2014.15
- Jenkins A, Gowler H, Curtis F, Holden N, Bridle C, Jones A. Efficacy of supervised maintenance exercise following pulmonary rehabilitation on health care use: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018;13:257–273. doi:10.2147/COPD.S150650
- Souto-Miranda S, Dias C, Jácome C, Melo E, Marques A. Long-term maintenance strategies after pulmonary rehabilitation: perspectives of people with chronic respiratory diseases, informal carers, and healthcare professionals. Healthcare. 2022;10(1):119. doi:10.3390/healthcare10010119
- European Respiratory Society. Chapter 2: the economic burden of lung disease. In: White Book; 2016.
- Burge A, Holland A, McDonald C, et al. Home-based pulmonary rehabilitation for COPD using minimal resources: an economic analysis. Respirology. 2020;25(2):183–190. doi:10.1111/resp.13667
- Desveaux L, Janaudis-Ferreira T, Goldstein R, Brooks D. An international comparison of pulmonary rehabilitation: a systematic review. COPD. 2015;12(2):144–153. doi:10.3109/15412555.2014.922066

![Figure 2 Health-related quality of life in non-randomized studies: Change in St George’s Respiratory Questionnaire total score (3 studies). Data from [19,21,23].](/cms/asset/c03e5c4a-1760-4d03-a6dc-4db7909f1b93/dcop_a_12152888_f0002_c.jpg)