Abstract
Purpose
Triple therapy comprising a long-acting muscarinic antagonist, long-acting β2-agonist and inhaled corticosteroid is recommended for patients with chronic obstructive pulmonary disease (COPD) who continue to experience frequent exacerbations or symptoms whilst receiving dual therapy. Adherence and persistence to multiple-inhaler triple therapy (MITT) is known to be poor. This study assessed comparative adherence to single-inhaler triple therapy (SITT) versus MITT in a real-world setting in England.
Patients and Methods
This was a retrospective cohort study using linked primary care (Clinical Practice Research Datalink Aurum) and secondary care (Hospital Episode Statistics [HES] Admitted Patient Care) data to identify patients with COPD who were newly initiated on SITT or MITT between November 2017 and June 2019. Eligible patients were aged ≥35 years and had a forced expiratory volume in 1 second/forced vital capacity <0.7, linkage to HES and continuous registration with a general practitioner for 12 months pre- and 6 months post-initiation. Inverse probability of treatment weighting was used to balance baseline characteristics between cohorts. Adherence was measured using the proportion of days covered by days’ supply of SITT or MITT prescriptions. Persistence was measured with a gap of >30 days between the end of a prescription and the following refill used to determine non-persistence.
Results
Overall, 4080 SITT and 6579 MITT users comprised the study cohort. After weighting, the baseline characteristics between the cohorts were comparable (absolute standardized mean difference <10%). SITT users had significantly higher adherence than MITT users at 6, 12, and 18 months post-initiation (p<0.001 for all comparisons). Median persistence was higher among SITT users than MITT users (5.09 months vs 0.99 months).
Conclusion
Patients with COPD in England initiating SITT had significantly better adherence and persistence compared with MITT initiators. These improvements continued at least 18 months following treatment initiation.
Introduction
Triple therapy comprising a long-acting muscarinic antagonist (LAMA), a long-acting β2-agonist (LABA) and an inhaled corticosteroid (ICS) is recommended for patients with chronic obstructive pulmonary disease (COPD) who continue to experience exacerbations or symptoms, whilst receiving dual therapy (LAMA/LABA or ICS/LABA).Citation1 Adherence and persistence to maintenance treatment are critical for achieving optimal clinical outcomes;Citation2,Citation3 however, adherence and persistence to multiple-inhaler triple therapy (MITT) is low in real-world settings.Citation4,Citation5 Dosing regimen complexity (ie the use of multiple inhalers at different daily frequencies and/or via differing inhalation techniques) is linked to lower treatment adherence and persistence observed with MITT.Citation5 This is important as low adherence to therapy is associated with higher healthcare resource utilization rates and costs, as well as reduced health-related quality of life.Citation6–8
In 2017, two single-inhaler triple therapies (SITT) were approved for the long-term maintenance treatment of adult patients with moderate-to-severe COPD in the United Kingdom (UK).Citation9,Citation10 The first comprises fluticasone furoate, umeclidinium, and vilanterol (FF/UMEC/VI) and requires once-daily dosing via an ELLIPTA dry powder inhaler.Citation9 The second comprises beclomethasone, formoterol, and glycopyrronium bromide (BDP/FOR/GB), and requires twice-daily dosing via a pressurized metered dose inhaler.Citation10
There is limited evidence comparing adherence and persistence with SITT versus MITT.Citation11 The objectives of this study were to compare medication adherence and persistence in patients with COPD initiating MITT to that in those initiating SITT in a real-world setting.
Materials and Methods
Study Design
This was a new-user, active comparator, retrospective cohort study using primary care data from the Clinical Practice Research Datalink (CPRD) Aurum and linked secondary care data from the Hospital Episode Statistics (HES) Admitted Patient Care database. The CPRD Aurum is a longitudinal, electronic health record database of primary care interactions in the UK. Data captured include demographic information, diagnoses, secondary care referrals, prescriptions issued in primary care, diagnostic testing, and lifestyle information.Citation12 CPRD Aurum is linked to the HES database, which contains data from a subset of patients registered with participating primary care practices in England only. Approximately 99% of CPRD primary care practices have consented to participate in the linkage scheme. Data captured include demographic information, date of hospital admission and discharge, diagnoses, specialists seen, and procedures undertaken.Citation13 Algorithms for ascertaining COPD diagnosis and COPD exacerbations in CPRD have previously been developed and validated against patient notes.Citation14–16
The first date of triple therapy initiation (single-inhaler FF/UMEC/VI or BDP/FOR/GB, or an overlapping supply of LAMA, LABA and ICS for MITT users; e-Figure 1) between November 15, 2017 and June 30, 2019 was defined as the index date (). Of note, the entire study period encompassing both baseline and follow-up was prior to the start of the COVID-19 pandemic, due to uncertainty in how patients were accessing healthcare during this period. Adherence and persistence were compared between the following triple therapy treatment cohorts: SITT versus MITT, FF/UMEC/VI versus MITT, and BDP/FOR/GB versus MITT. The comparisons were run independently and were not mutually exclusive (ie patients could be indexed on more than one triple therapy and thus could be included in multiple comparisons). Inverse probability of treatment weighting (IPTW) based on propensity scores (PS) was used when modelling to adjust for confounders by balancing baseline characteristics between treatment groups. An absolute standardized mean difference <10% between treatment groups being compared was considered indicative of adequate balance.Citation17 IPTW is commonly used in observational studies to allow estimation of the average treatment effect (ie the effect of treatment in the entire population) via creation of a pseudo-population such that the distribution of covariates in the population is independent of the treatment assigned.Citation18,Citation19 A benefit of IPTW compared with other propensity score-based methods is the ability to retain most individuals in the analysis; in other methods such as PS matching, unmatched individuals frequently in the tails of the PS distribution are discarded, thus decreasing the effective sample size.Citation20
Baseline covariates considered for inclusion in the PS model were: age; sex; year of index; geographic region and socioeconomic status at index; body mass index (most recent measure from any timepoint in a patients’ medical history prior to and including index); forced expiratory volume in 1 second (FEV1), and Medical Research Council score (taken as the most recent measure in the 24 months prior to and including index); current asthma diagnosis (ie asthma diagnosis in the 24 months prior to and including index); comorbidities at any time in a patient’s medical history prior to index; number of moderate-to-severe exacerbations of COPD in the 12 months prior to index; and respiratory medications in the 12 months prior to index. A new PS model was generated for each different treatment comparison.
Study Population
Eligible patients were required to have: ≥1 diagnosis of COPD at age 35 years or older;Citation21 a FEV1/forced vital capacity <0.7 at any time in their patient history either prior to or on the index date; patient records linked to HES; and continuous registration with a general practitioner for ≥1 year prior to and ≥6 months following the index date. Patients with at least one diagnosis of any medical condition related to lung or bronchial developmental anomalies, degenerative processes (cystic fibrosis, pulmonary fibrosis), pulmonary resection or other significant respiratory disorders (other than COPD) which may have made ascertainment of a COPD diagnosis using electronic medical record data difficult, or substantially change the natural history of the disease were excluded. Patients with record of concomitant asthma were not excluded from the study population to reflect actual treatment/management of COPD in clinical practice. Additional inclusion and exclusion criteria applied for each of the treatment comparisons are shown in .
Table 1 Additional Patient Inclusion and Exclusion Criteria for Individual Analyses
Study Outcomes
The primary outcome was medication adherence (binary categorical) at 6, 12, and 18 months following index date. Patients with a proportion of days covered (PDC) ≥80% were considered to be adherent. The proportion of adherent patients was compared between cohorts, and 95% confidence intervals (CIs) and p-values generated from a logistic regression model. Patients were considered 'covered' for days on which they had a valid prescription for SITT or all three components of MITT (see e-appendix and e-Figure 2 for further details regarding calculation of PDC). A sensitivity analysis, using PDC ≥50% as the definition of adherence was carried out.
The secondary outcome was medication persistence (time from treatment initiation at index to discontinuation, or end of follow-up, whichever was earliest). Discontinuation was defined as a gap >30 days between the end of a prescription and the following refill (this applied to any component of triple therapy for MITT users). All prescriptions were assumed to be 30 days in length. Sensitivity analyses, including the use of a longer allowable gap (45 and 60 days) to define triple therapy discontinuation were carried out. Exploratory outcomes included treatment adherence (continuous) as measured by PDC at 6, 12, and 18 months; mean PDC was compared between cohorts, with 95% CIs and p-values generated from a generalized linear model. For patients who underwent a treatment switch from MITT to SITT during the study period, adherence measured by mean PDC was evaluated in the 6 months prior to the treatment switch and the 6 months following the treatment switch.
Results
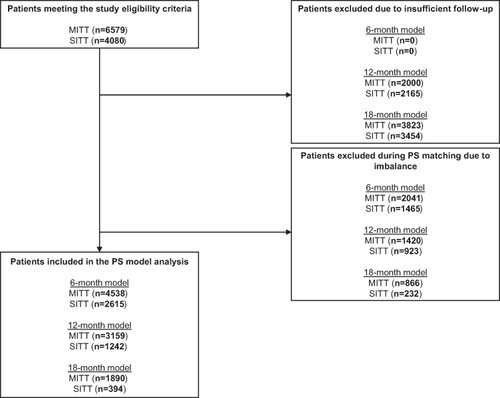
In total, 4080 SITT users and 6579 MITT users met the eligibility criteria and were included in the analysis. The unweighted baseline characteristics of all patients are shown in . Overall, 52% of SITT users and 53% of MITT users were male, and the mean (standard deviation [SD]) age was 71 (11) years and 69 (11) years among SITT users and MITT users, respectively. In total, 48% of SITT users were current smokers and 50% were former smokers; 50% of MITT users were current smokers and 46% were former smokers. The mean (SD) FEV1% predicted in the baseline period was 55 (19) among SITT users and 59 (19) among MITT users. The mean number of moderate and severe exacerbations in the 12 months prior to index were 0.6 and 0.3 among SITT users, and 0.5 and 0.2 among MITT users, respectively.
Table 2 Patient Baseline Characteristics
Individual components of MITT regimens for patients included in the MITT arm (n=6579) are presented in e-Table 1. The three most common combinations were beclometasone dipropionate/formoterol fumarate dihydrate+tiotropium bromide (n=1544), budesonide/formoterol fumarate dihydrate+tiotropium bromide (n=580) and fluticasone propionate/salmeterol xinafoate+tiotropium bromide (n=549).
SITT versus MITT
Patient attrition for the weighted analyses is outlined in . The baseline characteristics of the weighted and unweighted 6-, 12-, and 18-month SITT and MITT cohorts are shown in e-Tables 2-4, respectively. Prior to weighting, there were some imbalances between cohorts, particularly in terms of age and number of respiratory medicines in the 12 months prior to indexing. After weighting, the baseline characteristics between the two cohorts were comparable. All data reported are from the weighted cohorts (unless otherwise stated).
Figure 2 Patient attrition (SITT versus MITT).
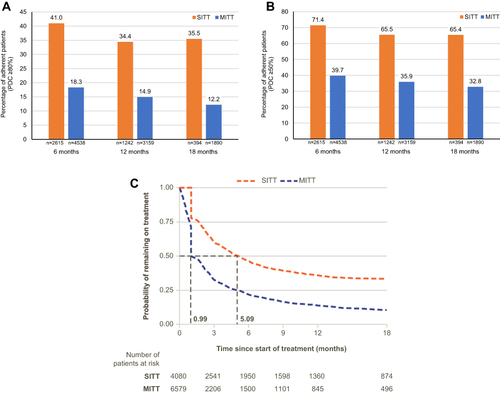
Patients initiating SITT were 124% more likely to be adherent (PDC ≥80%) at 6 months (p<0.001), 131% more likely to be adherent at 12 months (p<0.001) and 192% more likely to be adherent at 18 months (p<0.001) compared with MITT initiators (). Mean PDC was higher among SITT users than MITT users at all timepoints (0.66 vs 0.44 at 6 months, 0.61 vs 0.39 at 12 months, 0.59 vs 0.37 at 18 months). When defining adherence as PDC ≥50%, patients initiating SITT were 80% more likely to be adherent at 6 months (p<0.001), 82% more likely to be adherent at 12 months (p<0.001), and 100% more likely to be adherent at 18 months (p<0.001) compared with MITT initiators (). Compared with MITT initiators, patients initiating SITT were 110% more likely to stay on treatment for at least 6 months. Median persistence was 5.09 months for SITT users and 0.99 months for MITT users (). Median persistence remained higher for SITT users compared with MITT users, using both a 45-day and a 60-day gap to define non-persistence (e-Figure 3).
Figure 3 (A) SITT versus MITT adherence at 6, 12, and 18 months (PDC ≥80%), (B) SITT versus MITT adherence at 6, 12, and 18 months (PDC ≥50%), and (C) SITT versus MITT persistence.
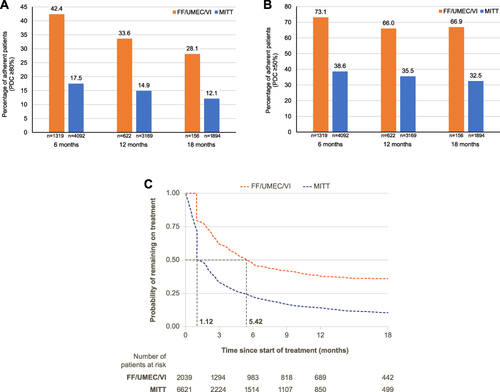
FF/UMEC/VI versus MITT
Patient attrition for the weighted analyses is outlined in e-Figure 4. The baseline characteristics of the weighted and unweighted 6-, 12-, and 18-month FF/UMEC/VI and MITT cohorts are shown in e-Tables 5-7, respectively. Patients initiating FF/UMEC/VI were 142% more likely to be adherent at 6 months (p<0.001), 125% more likely to be adherent at 12 months (p<0.001), and 132% more likely to be adherent at 18 months (p<0.001), compared with MITT initiators (). Mean PDC was higher among FF/UMEC/VI users compared with MITT users at all timepoints (0.67 vs 0.43 at 6 months, 0.61 vs 0.39 at 12 months, and 0.58 vs 0.36 at 18 months). When defining adherence as PDC ≥50%, patients initiating FF/UMEC/VI were 89% more likely to be adherent at 6 months (p<0.001), 86% more likely to be adherent at 12 months (p<0.001), and 106% more likely to be adherent at 18 months (p<0.001) compared with MITT initiators (). Compared with MITT initiators, patients initiating FF/UMEC/VI were 110% more likely to stay on treatment for at least 6 months. Median persistence was 5.42 months for FF/UMEC/VI users and 1.12 months for MITT users (). Median persistence remained higher for FF/UMEC/VI users compared with MITT users, using both a 45-day and a 60-day gap to define non-persistence (e-Figure 5).
Figure 4 (A) FF/UMEC/VI versus MITT adherence at 6, 12, and 18 months (PDC ≥80%), (B) FF/UMEC/VI versus MITT adherence at 6, 12, and 18 months (PDC ≥50%), and (C) FF/UMEC/VI versus MITT persistence.
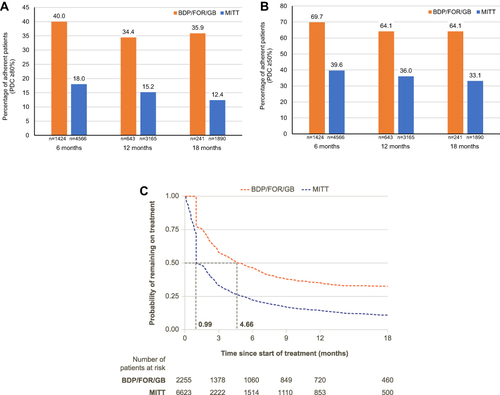
BDP/FOR/GB versus MITT
Patient attrition for the weighted analyses is outlined in e-Figure 6. The baseline characteristics of the weighted and unweighted 6-, 12-, and 18-month BDP/FOR/GB and MITT cohorts are shown in e-Tables 8-10, respectively. Patients initiating BDP/FOR/GB were 122% more likely to be adherent (PDC ≥80%) at 6 months (p<0.001), 127% more likely to be adherent at 12 months (p<0.001) and 190% more likely to be adherent at 18 months (p<0.001), compared with MITT initiators (). Mean PDC was higher among BDP/FOR/GB users compared with MITT users at all timepoints (0.66 vs 0.43 at 6 months, 0.61 vs 0.38 at 12 months, and 0.60 vs 0.35 at 18 months). When defining adherence as PDC ≥50%, patients initiating BDP/FOR/GB were 76% more likely to be adherent at 6 months (p<0.001), 78% more likely to be adherent at 12 months (p<0.001), and 94% more likely to be adherent at 18 months (p=0.001) compared with MITT initiators (). Compared with MITT initiators, patients initiating BDP/FOR/GB were 105% more likely to stay on treatment for at least 6 months. Median persistence was 4.66 months for BDP/FOR/GB users and 0.99 months for MITT users (). Median persistence remained higher for BDP/FOR/GB users compared with MITT users, using both a 45-day and a 60-day gap to define non-persistence (e-Figure 7).
Figure 5 (A) BDP/FOR/GB versus MITT adherence at 6, 12, and 18 months (PDC ≥80%), (B) BDP/FOR/GB versus MITT adherence at 6, 12, and 18 months (PDC ≥50%), and (C) BDP/FOR/GB versus MITT persistence.
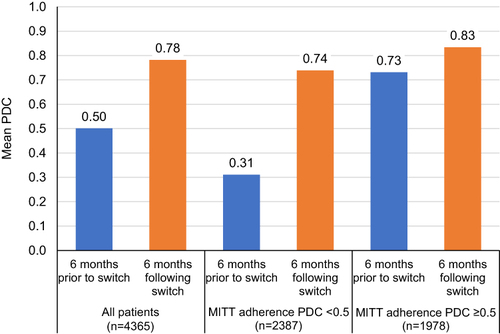
Adherence Following a Switch from MITT to SITT
Patients with COPD who switched from MITT to SITT had higher adherence following the switch (). Mean PDC improved from 0.50 pre-switch to 0.78 post-switch among the overall cohort. Among patients with low MITT adherence (PDC <0.50 prior to switch), mean PDC improved from 0.31 pre-switch to 0.74 post-switch. Among patients with high MITT adherence (PDC ≥0.50 prior to switch), mean PDC still improved from 0.73 pre-switch to 0.83 post-switch.
Figure 6 Adherence prior to, and following, MITT to SITT switch.
Discussion
In England, patients with COPD initiating SITT had significantly better adherence and persistence compared with patients initiating MITT. The findings were robust when applying sensitivity analyses including the use of PDC ≥50% as the definition of adherence and allowable gaps for persistence of up to 60 days. Similar levels of improved adherence and persistence were observed for both FF/UMEC/VI and BDP/FOR/GB compared with MITT; these improvements continued at least 18 months from index, which may have important clinical implications. The findings of the present study are consistent with a recent retrospective analysis in the United States, which found that patients receiving SITT with FF/UMEC/VI had significantly higher medication adherence compared with patients receiving MITT, and FF/UMEC/VI users were almost twice as likely to be persistent with their therapy at 12 months (212139 study; submitted to Respiratory Medicine).
A recent real-world evidence study demonstrated that SITT with FF/UMEC/VI resulted in greater improvements in lung function and quality of life compared with MITT.Citation22 A real-world non-interventional effectiveness study in Austria assessing the efficacy of BDP/FOR/GB in patients with moderate-to-severe COPD, reported that following 52 weeks of therapy, significant improvements in lung function (mean FEV1 and FVC) and symptoms were observed among the entire population compared with baseline. A significant reduction in moderate and severe exacerbations was also reported in patients who were classified as Global Initiative for Chronic Obstructive Lung Disease group B or D. After 52 weeks, 93.7% of patients continued their treatment and the authors noted that clinical improvements may be related to patients’ compliance with therapy.Citation23 Increased medication adherence has previously been associated with a lower risk of exacerbation and symptoms in patients with COPD and patients with asthma. In a Canadian observational study of >23,000 patients with COPD, good adherence (medication possession ratio ≥80%) to LAMA or ICS/LAMA therapies was associated with a significantly reduced risk of exacerbation and a reduced burden of disease.Citation2 In a Spanish cluster-randomized controlled trial of >1000 patients with hypertension, asthma, or COPD, a medication adherence management intervention was found to increase adherence among the total cohort, and a clinically significant improvement in Clinical COPD Questionnaire score was observed among patients with COPD (n=299).Citation24
In the current study, patients who switched therapy from MITT to SITT had higher adherence following the switch, and this difference was higher for particularly non-adherent patients on MITT. A third SITT with formoterol, glycopyrronium bromide and budesonide was approved for use in adults with moderate-to-severe COPD in the UK in 2020;Citation25 however, as this therapy was approved after the indexing period for the current study, no data from this SITT were available for inclusion in this analysis.
Future studies should explore the link between medication adherence and outcomes. As patients receiving SITT have been found to have better adherence and persistence to therapy compared with patients receiving MITT, increasing the use of SITT in the UK may result in less treatment discontinuation and improved health-related quality of life for patients with COPD. Despite the increased adherence observed using SITT, there is still scope for further improvement; additional work is needed with regard to other interventions, eg behavioral, which may improve adherence even further. A first-in-kind study is currently ongoing in the UK, which aims to assess how adherence technology measures (eg add-on inhaler sensor devices and mobile applications) may affect time-to-treatment failure and other clinical outcomes in patients with COPD who have historic poor treatment adherence.Citation26
Strengths of this analysis include the use of real-world data from the CPRD and HES, which allows more broadly representative results compared with those obtained from randomized controlled trials, which often include a more strictly defined patient population. Also, the use of IPTW based on propensity scores to balance characteristics between treatment groups; we observed similar results from both the weighted and the unweighted analyses, suggestive that substantial confounding between groups is unlikely for variables adjusted for in the model, and minimal unmeasured confounding is likely when using linked CPRD/HES data. Finally, adherence and persistence were measured over a longer period in the current study compared with previous studies.Citation27
Limitations of the current analysis include those common to database studies, eg inaccurate coding of COPD in the database and missing data. However, this is reflective of varying clinical documentation seen in real-world practice,Citation28 and COPD diagnoses and COPD exacerbations were defined based on a previous study, which validated definitions against patient notes in CPRD.Citation14–16 The criteria of overlap used to define MITT may have erroneously misclassified patients who were not intended to continue all three components as MITT users; this may have led to underestimation of adherence and persistence to MITT. Whilst prescriptions allow for an indirect measure of adherence, the dispensing of a medication does not guarantee that the medication was taken as prescribed by the patient. Also, in the persistence analysis it is not possible to distinguish between discontinuation of treatment due to advice from a clinician (possibly due to an adverse event or a lack of efficacy) versus a patient simply stopping treatment. Very non-adherent patients (ie patients who remain on treatment, but with very big treatment gaps) are also a consideration; despite remaining on treatment, these patients will appear to be non-persistent in the analysis. Nevertheless, the data from the persistence analysis suggest that patients persist on SITT longer than MITT. In addition, patients with concomitant asthma were included in this analysis; although this is reflective of real-world practice, it is possible that these patients may get greater symptomatic relief from treatment, which may therefore affect adherence and persistence. Also, although IPTW was used to balance baseline characteristics between the two groups, and substantial confounding is unlikely, unmeasured confounding is still possible; however, the similar results observed between the weighted and the unweighted cohorts is suggestive of limited unobserved confounders. Finally, this analysis was conducted using data collected prior to the emergence of COVID-19; it is possible that patient behavior and patient care have changed over the course of the pandemic. Patients may be more likely to be adherent to their therapy due to fears of their disease getting worse at a time when accessing care is more difficult.Citation29
Conclusions
Patients with COPD in England initiating SITT with FF/UMEC/VI or BDP/FOR/GB had significantly better adherence and persistence compared with patients initiating MITT. These improvements persisted for at least 18 months following treatment initiation. These findings highlight an overall benefit of SITT in patients with COPD and suggest the need for further investigation into the clinical and economic benefits of SITT treatments in clinical practice.
Abbreviations
BDP, beclomethasone; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CPRD, Clinical Practice Research Datalink; FEV1, forced expiratory volume in 1 second; FF, fluticasone furoate; FOR, formoterol; GB, glycopyrronium bromide; HES, Hospital Episode Statistics; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; MITT, multiple-inhaler triple therapy; PDC, proportion of days covered; PS, propensity score; SD, standard deviation; SITT, single-inhaler triple therapy; UMEC, umeclidinium; VI, vilanterol.
Data Sharing Statement
The data analyzed in this publication are derived from the Clinical Practice Research Datalink (www.cprd.com) and Hospital Episode Statistics database (https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics). Authors had access to the study data for the purposes of this work only. Data were accessed through an existing GSK license to address the prespecified research questions only. Therefore, the data cannot be broadly disclosed or made publicly available at this time. Access to each database can be requested via the respective websites.
Ethics Approval and Informed Consent
This study complied with all applicable laws regarding patient privacy. No direct patient contact or primary collection of individual patient data occurred; therefore, informed patient consent was not required. This study was approved by CPRD (study number 20_000121). Generic ethical approval for observational research approved by CPRD has been granted by a Health Research Authority Research Ethics Committee (East Midlands-Derby, UK; REC reference number 05/MRE04/87).
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the manuscript; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Kieran J Rothnie, Alexandrosz Czira, Chris Compton, Kirill Nikitin, Raj Sharma, and Afisi S Ismaila are employees of and/or hold stocks/shares in GSK. Neil Snowise is a previous employee of GSK and holds GSK stocks/shares. Victoria Banks, Robert Wood, Theo Tritton, Olivia Massey, and Rosie Wild are employees of Adelphi Real World. Adelphi Real World received funding from GSK to conduct the study. David MG Halpin reports personal fees from AstraZeneca, personal fees and non-financial support from Boehringer Ingelheim, personal fees from Aerogen, Chiesi, CSL Behring, and GSK, personal fees and non-financial support from Novartis, and personal fees from Pfizer and Sanofi. Claus F Vogelmeier has received research grants from AstraZeneca, Boehringer Ingelheim, CSL Behring, Chiesi, GSK, and Grifols, and has given presentations at symposia and/or served on scientific advisory boards sponsored by Aerogen, AstraZeneca, Boehringer Ingelheim, CSL Behring, Chiesi, GSK, Grifols, Menarini, Novartis, and Nuvaira. The authors report no other conflicts of interest in this work.
Acknowledgments
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing, and referencing) was provided by Rebecca Cunningham of Aura, a division of Spirit Medical Communications Group Limited, and was funded by GSK.
This study is based in part on data from the Clinical Practice Research Datalink obtained under license from the UK Medicines and Healthcare products Regulatory Agency. The data is provided by patients and collected by the NHS as part of their care and support.
Data from this study have previously been presented in abstract/conference presentation form at:
The 2021 European Respiratory Society – 31st Annual congress. The abstract was published in European Respiratory Journal. 2021;58(65):OA2947. Available from https://erj.ersjournals.com/content/58/suppl_65/OA2947 DOI:10.1183/13993003.congress-2021.OA2947
The 2021 British Thoracic Society Winter Meeting. The abstract was published in Thorax 2021;76:A20-A21. Available from: https://thorax.bmj.com/content/thoraxjnl/76/Suppl_2/A20.2.full.pdf
Additional information
Funding
References
- GOLD. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease - 2021 report; 2021. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf. Accessed January 17, 2022.
- Ismaila A, Corriveau D, Vaillancourt J, et al. Impact of adherence to treatment with tiotropium and fluticasone propionate/salmeterol in chronic obstructive pulmonary diseases patients. Curr Med Res Opin. 2014;30(7):1427–1436. doi:10.1185/03007995.2014.908828
- Vestbo J, Anderson JA, Calverley PM, et al. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax. 2009;64(11):939–943. doi:10.1136/thx.2009.113662
- Yu AP, Guérin A, Ponce de Leon D, et al. Therapy persistence and adherence in patients with chronic obstructive pulmonary disease: multiple versus single long-acting maintenance inhalers. J Med Econ. 2011;14(4):486–496. doi:10.3111/13696998.2011.594123
- Bogart M, Stanford RH, Laliberté F, Germain G, Wu JW, Duh MS. Medication adherence and persistence in chronic obstructive pulmonary disease patients receiving triple therapy in a USA commercially insured population. Int J Chron Obstruct Pulmon Dis. 2019;14:343. doi:10.2147/COPD.S184653
- Lareau SC, Yawn BP. Improving adherence with inhaler therapy in COPD. Int J Chron Obstruct Pulmon Dis. 2010;5:401–406. doi:10.2147/COPD.S14715
- Mäkelä MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107(10):1481–1490. doi:10.1016/j.rmed.2013.04.005
- Sanduzzi A, Balbo P, Candoli P, et al. COPD: adherence to therapy. Multidiscip Respir Med. 2014;9(1):60. doi:10.1186/2049-6958-9-60
- EMA. European Medicines Agency - Trelegy Ellipta summary of product characteristics; 2017. Available from: https://www.ema.europa.eu/en/documents/product-information/trelegy-ellipta-epar-product-information_en.pdf. Accessed January 17, 2022.
- EMA. European Medicines Agency - Trimbow summary of product characteristics; 2017. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/trimbow. Accessed January 17, 2022.
- Bogart M, Wu B, Germain G, et al. Real-world adherence to single-inhaler vs multiple-inhaler triple therapy among patients with COPD in a commercially insured US population. CHEST. 2020;158(4):A1773–A1774. doi:10.1016/j.chest.2020.08.1548
- Wolf A, Dedman D, Campbell J, et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Aurum. Int J Epidemiol. 2019;48(6):1740–1740g. doi:10.1093/ije/dyz034
- NHS digital. Hospital Episode Statistics (HES); 2021. Available from: https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics. Accessed January 17, 2022.
- Quint JK, Müllerova H, DiSantostefano RL, et al. Validation of chronic obstructive pulmonary disease recording in the Clinical Practice Research Datalink (CPRD-GOLD). BMJ Open. 2014;4(7):e005540. doi:10.1136/bmjopen-2014-005540
- Rothnie KJ, Müllerová H, Hurst JR, et al. Validation of the recording of acute exacerbations of COPD in UK primary care electronic healthcare records. PLoS One. 2016;11(3):e0151357. doi:10.1371/journal.pone.0151357
- Rothnie KJ, Müllerová H, Thomas SL, et al. Recording of hospitalizations for acute exacerbations of COPD in UK electronic health care records. Clin Epidemiol. 2016;8:771–782. doi:10.2147/CLEP.S117867
- Austin PC. Goodness-of-fit diagnostics for the propensity score model when estimating treatment effects using covariate adjustment with the propensity score. Pharmacoepidemiol Drug Saf. 2008;17(12):1202–1217. doi:10.1002/pds.1673
- Allan V, Ramagopalan SV, Mardekian J, et al. Propensity score matching and inverse probability of treatment weighting to address confounding by indication in comparative effectiveness research of oral anticoagulants. J Comp Eff Res. 2020;9(9):603–614. doi:10.2217/cer-2020-0013
- Borah BJ, Moriarty JP, Crown WH, Doshi JA. Applications of propensity score methods in observational comparative effectiveness and safety research: where have we come and where should we go? J Comp Eff Res. 2014;3(1):63–78. doi:10.2217/cer.13.89
- Chesnaye NC, Stel VS, Tripepi G, et al. An introduction to inverse probability of treatment weighting in observational research. Clin Kidney J. 2021;15(1):14–20. doi:10.1093/ckj/sfab158
- NICE. Chronic obstructive pulmonary disease in over 16s: diagnosis and management; 2019. Available from: https://www.nice.org.uk/guidance/ng115/chapter/Recommendations#diagnosing-copd. Accessed January 17, 2022.
- Halpin DMG, Worsley S, Ismaila AS, et al. INTREPID: single- versus multiple-inhaler triple therapy for COPD in usual clinical practice. ERJ Open Res. 2021;7:2. doi:10.1183/23120541.00950-2020
- Marth K, Renner A, Pohl W. TRICOP - A real-world effectiveness study with a single-inhaler extrafine triple therapy over 52 weeks in Austrian patients with COPD. Respir Med. 2021;182:106398. doi:10.1016/j.rmed.2021.106398
- Torres-Robles A, Benrimoj SI, Gastelurrutia MA, et al. Effectiveness of a medication adherence management intervention in a community pharmacy setting: a cluster randomised controlled trial. BMJ Qual Saf. 2021;2:bmjqs-2020-011671. doi:10.1136/bmjqs-2020-011671
- EMC. Electronic medicines compendium - Trixeo Aerosphere 5 micrograms/7.2 micrograms/160 micrograms pressurised inhalation, suspension; 2020. Available from: https://www.medicines.org.uk/emc/product/12028/smpc#gref. Accessed January 17, 2022.
- Price D, Jones R, Pfister P, et al. Maximizing adherence and gaining new information for your chronic obstructive pulmonary disease (MAGNIFY COPD): study protocol for the pragmatic, cluster randomized trial evaluating the impact of dual bronchodilator with add-on sensor and electronic monitoring on clinical outcomes. Pragmat Obs Res. 2021;12:25–35. doi:10.2147/POR.S302809
- Mannino D, Bogart M, Wu B, et al. Adherence and persistence to once-daily single-inhaler versus multiple-inhaler triple therapy among patients with chronic obstructive pulmonary disease in the USA: a real-world study. Respir Med. 2022;197:106807. doi:10.1016/j.rmed.2022.106807
- Wells BJ, Chagin KM, Nowacki AS, Kattan MW. Strategies for handling missing data in electronic health record derived data. EGEMS. 2013;1(3):1035. doi:10.13063/2327-9214.1035
- Kaye L, Theye B, Smeenk I, Gondalia R, Barrett MA, Stempel DA. Changes in medication adherence among patients with asthma and COPD during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8(7):2384–2385. doi:10.1016/j.jaip.2020.04.053