Abstract
Background
The role of inducible costimulator (ICOS) signaling in chronic obstructive pulmonary disease (COPD) has not been fully elucidated.
Methods
We compared the percentages of ICOS+ T cells and ICOS+ regulatory T (Treg) cells in CD4+ T cells and CD4+CD25+FOXP3+ Tregs, respectively, in the peripheral blood of smokers with or without COPD to those in healthy controls. We further characterized their phenotypes using flow cytometry. To investigate the influence of ICOS signaling on C-X-C motif chemokine receptor 3 (CXCR3) expression in COPD, we evaluated the expression levels of ICOS and CXCR3 in vivo and in vitro.
Results
ICOS expression was elevated on peripheral CD4+ T cells and CD4+ Tregs of COPD patients, which positively correlated with the severity of lung function impairment in patients with stable COPD (SCOPD), but not in patients with acute exacerbation of COPD (AECOPD). ICOS+CD4+ Tregs in patients with SCOPD expressed higher levels of coinhibitors, programmed cell death protein 1 (PD-1) and T-cell immunoreceptor with Ig and ITIM domains (TIGIT), than ICOS−CD4+ Tregs, whereas ICOS+CD4+ T cells mostly exhibited a central memory (CD45RA−CCR7+) or effector memory (CD45RA−CCR7−) phenotype, ensuring their superior potential to respond potently and quickly to pathogen invasion. Furthermore, increased percentages of CXCR3+CD4+ T cells and CXCR3+CD4+ Tregs were observed in the peripheral blood of patients with SCOPD, and the expression level of CXCR3 was higher in ICOS+CD4+ T cells than in ICOS−CD4+ T cells. The percentage of CXCR3+CD4+ T cells was even higher in the bronchoalveolar lavage fluid than in matched peripheral blood in SCOPD group. Lastly, in vitro experiments showed that ICOS induced CXCR3 expression on CD4+ T cells.
Conclusions
ICOS signaling is upregulated in COPD, which induces CXCR3 expression. This may contribute to increased numbers of CXCR3+ Th1 cells in the lungs of patients with COPD, causing inflammation and tissue damage.
Introduction
Chronic obstructive pulmonary disease (COPD) is a smoking-related disease characterized by progressive airflow limitation and irreversible damage to lung function,Citation1 which causes a major health care burden worldwide and is the third leading cause of death.Citation2 Despite advances in controlling symptoms and precautions against acute exacerbations, the cellular and molecular mechanisms involved in the development of COPD are poorly understood.Citation3 Thus, there remains a compelling need to identify optimal biomarkers for disease development and potential intervention targets for this complex disease. The contribution of immune disorders to COPD pathogenesis has been a research topic of great interest.
Growing evidence has identified the role of autoimmunity in the development of COPD, with significant participation by the adaptive immune responses.Citation4 Increased proportions of T helper (Th)1/cytotoxic T (Tc)1, Th17/Tc17 cells, as well as proinflammatory subsets of regulatory T (Treg) cells have been verified in the lung tissues and peripheral blood of patients with COPD.Citation5–8 The development of lung lymphoid follicles in spirometrically severe stages of COPD, is another critical immunological change in COPD.Citation4,Citation9 These lymphoid follicles consist of large aggregates of B cells, scattered follicular dendritic cells and a small number of T cells, including a key type of lymphoid tissue inducer cells called CD4+ T follicular helper (Tfh) cells. In this study, we focused on a classical activation marker, inducible costimulator (ICOS), which is highly involved in regulating Th1/2/17 cell-mediated immune responses and Tfh cell function.Citation10 Immunotherapy targeting this costimulatory receptor has been developed as a new strategy for cancer treatment and some autoimmune diseases.Citation11,Citation12
ICOS, discovered by Andreas Hutloff in 1999, is a homodimer with a relative molecular mass of 55,000±60,000 Da and is expressed on the surface of activated T cells.Citation13 It belongs to the CD28 superfamily, along with CD28, Cytotoxic T-Lymphocyte Associated Protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and B- and T-Lymphocyte Attenuator (BTLA).Citation14 ICOS has been identified to have functions in regulating T cell activation, differentiation, effector function, and tissue residency.Citation15,Citation16 Furthermore, numerous studies have confirmed that ICOS has a crucial role in Tfh cell differentiation and maintenance, as well as in T-B cell responses and germinal center formation.Citation17 In addition, ICOS widely participates in innate immunity. For example, ICOS is highly expressed on the surface of innate lymphoid type 2 cells and natural killer T cells and is required for their function and homeostasis.Citation18,Citation19 Notably, ICOS can also be expressed on some types of regulatory cells, including Tregs and follicular regulatory T cells, and a series of studies have shown that ICOS+ Tregs have greater inhibitory effect on T cell proliferation than ICOS− Tregs, owing to their ability to produce more interleukin (IL)-10.Citation20,Citation21 Therefore, ICOS activation could mediate a paradoxical function. The holistic role of ICOS is difficult to estimate due to the broad distribution of ICOS on the immune cells, and previous evidence indicates that it should be evaluated in a context-dependent manner.Citation10 For example, Icos−/− C57BL/6 mice infected systemically with Salmonella enterica serovar Typhimurium exhibited a significant decrease in interferon-γ (IFN-γ) production mediated by CD4+ T cells and reduced antigen-specific CD8 response, resulting in delayed clearance of Salmonella.Citation22 However, Icos−/− mice showed increased IFN-γ-producing CD4+ T cells and reduced Treg numbers in the last chronic stage of Mycobacterium tuberculosis infection, thereby accelerating control of M.tuberculosis burden in the spleen.Citation23 To date, the role of ICOS signaling in COPD has not been reported, and clarification of this issue will help in providing new biotargets for immunotherapy of COPD.
C-X-C motif chemokine receptor 3 (CXCR3), a member of the superfamily of G-protein-coupled receptors, is a major chemokine receptor of Th1/Tc1 cells – two predominant immune cell types in COPD development.Citation24 CXCR3 can recruit Th1 or Tc1 cells to inflamed sites.Citation6,Citation25 By producing granzyme B and perforin or secreting proinflammatory cytokines, these Th1/Tc1 cells cause pathological lesions in the lung.Citation26 According to one study of cancer immunology, CD4+ T cells require ICOS-mediated phosphoinositide 3-kinase (PI3K)-protein kinase B (Akt) signaling to increase T-bet expression,Citation27 where T-bet can serve as a critical transcription factor for Cxcr3 mRNA induction in both Th1 cells and Tregs.Citation28–30 Therefore, we speculate that ICOS may also play a role in CXCR3 expression in COPD, thus promoting CXCR3+ Th1 cell induction and accumulation in the lung.
In this study, we analyzed the expression of ICOS and CXCR3 on CD4+ T cells and CD4+CD25+FOXP3+ Tregs in the peripheral blood of patients with COPD and further characterized the phenotypes of ICOS+CD4+ T cells and ICOS+CD4+ Tregs in COPD. Increased ICOS and CXCR3 expression were observed on both CD4+ T cells and CD4+ Tregs in patients with COPD compared with those in healthy controls. In addition, the expression of ICOS was positively correlated with the severity of lung function impairment in the SCOPD group, suggesting that ICOS activation is associated with poor prognosis in COPD. Finally, we demonstrated that enhanced ICOS signaling could induce CXCR3 expression in CD4+ T cells, thus promoting the aggregation of CXCR3+ Th1 cells in the lungs of patients with COPD.
Materials and Methods
Participants
Four different groups of people were enrolled according to the diagnostic criteria for COPD of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines: 15 healthy controls (HC), 11 asymptomatic healthy smokers (HS), 22 patients with stable COPD (SCOPD), and 14 patients with acute exacerbation of COPD (AECOPD) (). People with a smoking history of more than 10 packs/year and a normal lung function were considered as asymptomatic healthy smokers. The SCOPD group included first-time outpatients with a smoking history and COPD diagnosis but did not have an exacerbation within four weeks and did not use corticosteroids for any purpose. Patients with AECOPD required no new treatment 72 h before admission. Patients with allergic diseases, autoimmune diseases, neoplasms, coronary heart disease, liver and kidney diseases, or diabetes were excluded. The study was approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (#2019/S877) and was performed following the Declaration of Helsinki. All the patients and volunteers signed a consent form.
Table 1 Characteristics of All Subjects
Preparation of PBMCs
Five milliliters of peripheral blood was collected from each participant in the four groups. Peripheral blood of patients with SCOPD who made an appointment for bronchoscopy was obtained 30 min before the procedure. Peripheral blood mononuclear cells (PBMCs) were isolated via density gradient centrifugation using a human lymphocyte separation medium (DaYou, Shenzhen, China). PBMCs were used for flow cytometry analysis.
Bronchoalveolar Lavage Fluid (BALF) Collection and Processing of BALF Cells
Bronchoalveolar lavage fluid (BALF) was obtained from four patients with SCOPD who had never received any treatment after diagnosis. Demographic and clinical data are listed in . Bronchoscopy was performed after overnight fasting, with a routine blood examination, assessment of four coagulation indices, chest X-ray/computed tomography, and hepatitis B surface antigen examination performed prior to the procedure. After aerosol inhalation with 2% lignocaine 10 min prior to the investigation, the bronchoscope was inserted nasally, followed by endotracheal anesthesia with lignocaine. Five aliquots of 20 ml sterile physiological saline at 37°C were instilled into the right middle lobe, and BALF was gently suctioned back at a negative pressure of less than 100 mmHg. At least 30 mL BALF was obtained, placed on ice, and immediately sent to the laboratory. BALF was filtered sequentially using 100- and 40-µM pore size cell strainers (BD Falcon, USA). The cells were pelleted via centrifugation at 1500 rpm for 6 min and washed again with phosphate buffer saline (PBS). The collected cells were used for flow cytometry analysis.
Table 2 Demographic and Clinical Data of Patients Who Undertook Bronchoscopy
Functional Assays
Naïve CD4+ T cells were isolated from 30 mL fresh peripheral blood obtained from healthy volunteers by magnetic cell sorting using a human naïve CD4+ T cell isolation kit II (Miltenyi Biotec, USA). The purity of naïve CD4+ T cells was greater than 95%, as determined by flow cytometry. Then, the isolated naïve CD4+ T cells were resuspended in RPMI 1640 medium containing 10% fetal bovine serum (Gibco, USA) and diluted to 5×105 cells/mL. The cells were cultured at 37°C in 48-well plates and stimulated with plate-bound anti-human CD3 mAb (OKT3; 5 µg/mL; eBioscience, USA) and anti-human CD28 mAb (5 µg/mL; eBioscience, USA) in the presence of IL-2 (5 ng/mL; PeproTech, USA) for four days. To assess the influence of ICOS signaling on CXCR3 expression, anti-ICOS mAb (ISA-3; 5 µg/mL; eBioscience, 16–9948-82, USA) was added to the culture medium.
Flow Cytometric Analyses
Immune cells were washed twice with PBS and stained directly with fluorochrome-conjugated Abs against surface markers, which are listed as follows: anti-human CD4-FITC (clone: RPA-T4, BD Biosciences, USA), anti-human CD4-APC (clone: RPA-T4, BD Biosciences, USA), anti-human CD4-BV605 (clone: RPA-T4, BD Biosciences, USA), anti-human CD25-PE-Cy7 (clone: M-A251, BD Biosciences, USA), anti-human ICOS-BV421 (clone: DX29, BD Biosciences, USA), anti-human CXCR3-APC (clone: 1C6, BD Biosciences, USA), anti-human CXCR3-APC-Cy7 (clone: G025H7, BioLegend, USA), anti-human T-cell immunoreceptor with Ig and ITIM domains (TIGIT)-APC (clone: MBSA43, eBioscience, USA), anti-human PD-1-BV605 (clone: EH12.1, BD Biosciences, USA), anti-human CD45RA-PE-Cy7 (clone: HI100, BD Biosciences, USA), anti-human CD62L-BV510 (clone: DREG-56, BioLegend, USA), anti-human CCR7-PerCP-Cy5.5 (clone: 150503, BD Biosciences, USA), anti-human CD27-APC (clone: M-T271, BD Biosciences, USA), anti-human CD28-APC-R700 (clone: CD28.2, BD Biosciences, USA), anti-human CD69-PE (clone: FN50, BD Biosciences, USA), anti-human CD57-PE594 (clone: NK-1, BD Biosciences, USA), and anti-human killer cell lectin-like receptor subfamily G member 1 (KLRG-1)-PE (clone: SA231A2, BioLegend, USA). Then, the samples were washed with PBS after incubation for 30 min at 4°C in the dark. Intracellular staining was performed after the cells were fixed and permeabilized with a fixation/permeabilization solution (eBioscience, USA) according to the manufacturer’s instructions. The intracellular proteins forkhead box protein 3 (FOXP3), Ki-67, and IKAROS family zinc finger 2 (Helios) were stained with a PE-conjugated anti-human FOXP3 Ab (clone: 236A/E7, eBioscience, USA), an APC-conjugated anti-human Ki-67 Ab (clone: 20Raj1, eBioscience, USA), and an APC-conjugated anti-human Helios Ab (clone: 22F6, eBioscience, USA), respectively. Flow cytometry was performed on a BD LSRFortessa X-20, and the data were analyzed using FlowJo V10 software.
Statistical Analyses
Data are expressed as mean ± standard error of mean (SEM) or mean (range). Comparisons between two groups were performed using paired Student’s t-test. Differences among the four groups were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test unless otherwise noted. Correlations were determined using Pearson’s correlation analysis. Data analyses were performed using GraphPad Prism 8 software (GraphPad Software, La Jolla, California, USA), and P-values less than 0.05 were considered statistically significant.
Results
Elevated Expression of ICOS on Both CD4+ T Cells and CD4+CD25+FOXP3+ Tregs of Patients with COPD
ICOS expression was evaluated on both CD4+ T cells and CD4+CD25+FOXP3+ Tregs from peripheral blood obtained from four groups of people (HC, HS, SCOPD and AECOPD) (). As expected, the percentage of ICOS+CD4+ T cells among CD4+ T cells was higher in patients with SCOPD than in HC group (). Furthermore, a significant increase was observed in acute exacerbation cases compared to that in the other three groups, indicating that ICOS was intensely upregulated by acute inflammation (). Similarly, we observed an enhanced ICOS expression level on CD4+CD25+FOXP3+ Tregs in patients with SCOPD compared to that in HC group (). The frequency of ICOS+ Tregs was also sharply elevated in the AECOPD group compared to that in the other three groups and was approximately two times higher than that in the HC group (). However, no significant increase was observed in the percentage of ICOS+CD4+ T cells or ICOS+CD4+ Tregs in HS group compared with that in HC group ( and ). Notably, ICOS was expressed in a larger percentage on CD4+CD25+FOXP3+ Tregs than on CD4+ T cells (17.14±1.68% vs 4.47±0.46% in the HC group and 24.26±1.65% vs 8.76±0.80% in SCOPD group) ( and ). This could reflect that the T cell receptors of Tregs have a high affinity for major histocompatibility complex/autoantigen peptides and that Tregs can obtain ICOS expression during the recognition of autoantigens in their development in the thymus.Citation20,Citation24 In addition, the percentage of ICOS+ T cells in CD4+ T cells and the proportion of ICOS+ Tregs in CD4+ Tregs were negatively correlated with forced expiratory volume in one second/forced vital capacity (FEV1/FVC) and FEV1 (% predicted) in SCOPD group (). Correlations in the other groups could not be detected (data not shown).
Figure 1 Expression of inducible costimulator (ICOS) on CD4+ T cells and CD4+ regulatory T (Treg) cells in the peripheral blood of four groups of people. (A) Representative flow cytometric dot plots of ICOS expression on CD4+ T cells and CD4+CD25+FOXP3+ Tregs of patients with stable chronic obstructive pulmonary disease (SCOPD). Comparisons of the percentage of ICOS+ T cells within CD4+ T cells (B) and CD4+CD25+FOXP3+ Tregs (C) in the peripheral blood from 15 healthy controls (HC), 11 healthy smokers (HS), 22 patients with SCOPD, and 14 patients with acute exacerbation of COPD (AECOPD). Correlations of the frequencies of ICOS+CD4+ T cells (D and E) and ICOS+CD4+CD25+FOXP3+ Tregs (F and G) with forced expiratory volume in one second/forced vital capacity (FEV1/FVC) and FEV1 (% predicted), respectively (n = 22). *p<0.05, **p<0.01, ****p<0.0001.
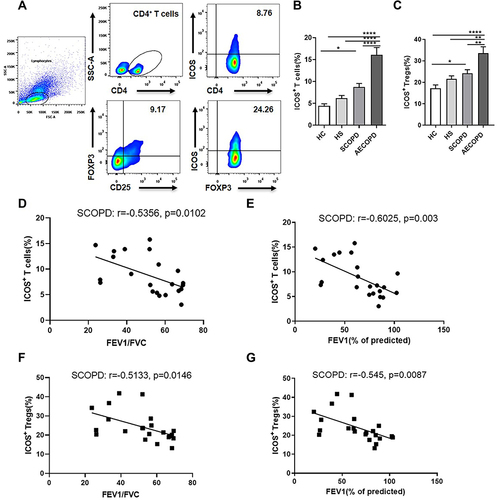
Phenotypic Characteristics of ICOS+CD4+CD25+FOXP3+ Tregs
To assess the overall role of ICOS signaling in COPD, we first investigated the phenotypic characteristics of ICOS+ Tregs in the peripheral blood of patients with SCOPD. As shown in , ICOS+ Tregs expressed higher levels of Helios than ICOS− Tregs, which is a marker of the thymic origin of Tregs. The proliferation index, Ki-67, was also higher in ICOS+ Tregs than in ICOS− Tregs, with up to 82.2% of ICOS+ Tregs and only 66.8% of ICOS− Tregs being positive for Ki-67. Furthermore, we assessed the expression of two coinhibitory receptors on Tregs, TIGIT and PD-1, which are regarded as major negative regulatory molecules of Tregs. Both coinhibitors displayed an increased expression pattern on ICOS+ Tregs compared with ICOS− Tregs.
Figure 2 Characteristics of ICOS+ or ICOS− Tregs and the relationship between inducible costimulator (ICOS) and forkhead box protein 3 (FOXP3). (A) Comparisons of the expression levels of Ki-67, IKAROS family zinc finger 2 (Helios), programmed cell death protein 1 (PD-1), and T-cell immunoreceptor with Ig and ITIM domains (TIGIT) in ICOS+CD4+CD25+FOXP3+ Tregs and ICOS−CD4+CD25+FOXP3+ Tregs in the peripheral blood from patients with stable chronic obstructive pulmonary disease (SCOPD, n = 6). Representative flow cytometric plots of the expression level of FOXP3 in four subsets of CD4+ T cells gated by CD25 and ICOS in healthy controls (HC, n = 15), healthy smokers (HS, n = 11), and patients with SCOPD (n = 22) (B) and the corresponding histograms (C). (D) Quantification of FOXP3 expressed by ICOS+CD4+CD25+FOXP3+ Tregs or ICOS−CD4+CD25+FOXP3+ Tregs of HC (n = 15), HS (n = 11) and patients with SCOPD (n = 22). Significance was determined by two-way ANOVA analysis followed by Tukey’s multiple comparisons test (D). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
In addition, we examined the expression level of FOXP3 in four subgroups of CD4+ T cells, which were divided according to the positive or negative expression of CD25 and ICOS. FOXP3 expression was higher in all subsets except ICOS−CD25+ T cell subset of patients with SCOPD than in the HC groups, and it was significantly increased in ICOS+CD25− T cell subset of HS group compared with that in HC group ( and ). Furthermore, ICOS+CD25+ T cells expressed the highest level of FOXP3 in all three groups, with up to 70% of ICOS+CD25+ T cells expressing FOXP3 in SCOPD group, and ICOS−CD25+ T cells exhibited the second highest FOXP3 expression ( and ). In addition, the mean fluorescence intensity (MFI) of FOXP3 was significantly higher in ICOS+ Tregs than in ICOS− Tregs in both HC group and patients with SCOPD (). Moreover, the MFI of FOXP3 in ICOS+ Tregs was higher in patients with SCOPD than in the HC and HS groups (). These results indicated that ICOS could favor FOXP3 expression.
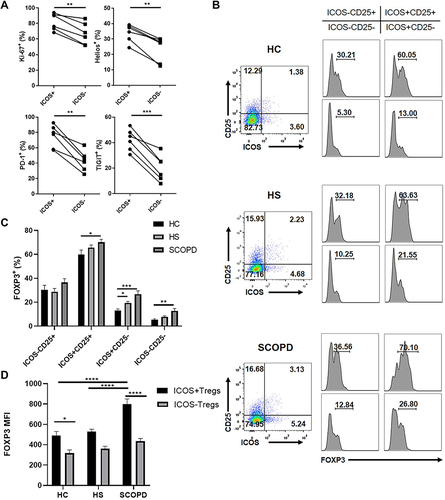
Phenotypic Characteristics of ICOS+CD4+ T Cells
The phenotype of ICOS+CD4+ T cells was also evaluated in the peripheral blood of patients with SCOPD. First, we divided CD4+ T cells, with or without ICOS expression, into distinct naïve/memory T cell subsets based on CD45RA and CCR7 expression (). Most ICOS+ T cells exhibited a CD45RA−CCR7+ central memory T cell (TCM) phenotype or CD45RA−CCR7− effector memory T cell (TEM) phenotype, whereas CD45RA+CCR7+ naïve T cells accounted for a higher percentage of ICOS− T cells than ICOS+ T cells. Unexpectedly, increased expression of CD62L and decreased expression of CCR7 were observed on ICOS+ T cells compared with ICOS− T cells (), which led to an increased percentage of the CD62L+CCR7− T cell subset in ICOS+CD45RA− T cells compared with ICOS−CD45RA− T cells (data not shown). Next, we examined several markers that have been suggested as hallmarks for activation or senescence of T cells (). The expression levels of CD27 and CD28 were slightly but significantly higher on ICOS+ T cells than on ICOS− T cells. We also detected a significant difference between ICOS+ T cells and ICOS− T cells with respect to the expression level of another early activation marker, CD69. CD57+ or KLRG-1+ T cells, which represent senescent T cells, were observed to account for a lower percentage of ICOS+ T cells than ICOS− T cells. Furthermore, several inhibitory receptors, such as PD-1 and TIGIT, which could be upregulated after TCR engagement, showed enhanced expression on ICOS+ T cells compared to ICOS− T cells. Collectively, because ICOS+ T cells are mainly composed of TCM and TEM cells, we suggest that they represent a functional subset that not only has a higher sensitivity to antigen stimulation but also exerts a rapid effector function after TCR engagement compared with ICOS− T cells, which contain higher percentages of naïve T cells and senescent T cells.
Figure 3 Comparisons of the characteristics of ICOS+CD4+ T cells and ICOS−CD4+ T cells in the peripheral blood of patients with stable chronic obstructive pulmonary disease (SCOPD). (A) The proportions of naïve T cells (CD45RA+CCR7+), TCM cells (CD45RA−CCR7+), TEM cells (CD45RA−CCR7−) and TEMRA cells (CD45RA+CCR7−) in ICOS+CD4+ T cells and ICOS−CD4+ T cells of 6 patients with SCOPD. (B) Representative flow cytometric dot plots of the expression of CD45RA, CD62L, CCR7, CD27, CD28, CD69, CD57, killer cell lectin-like receptor subfamily G member 1 (KLRG-1), programmed cell death protein 1 (PD-1), and T-cell immunoreceptor with Ig and ITIM domains (TIGIT) on ICOS+CD4+ T cells and ICOS−CD4+ T cells and the corresponding histograms (n = 6). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Increased Expression of CXCR3 on Both CD4+ T Cells and CD4+CD25+FOXP3+ Tregs of Patients with COPD
We next explored the possible mechanism of ICOS signaling that contributes to the pathogenesis of COPD. We analyzed the expression level of CXCR3 on CD4+ T cells and CD4+CD25+FOXP3+ Tregs from the peripheral blood of the four groups of people (). Similar to the ICOS expression pattern, the percentages of CXCR3+CD4+ T cells among CD4+ T cells and CXCR3+CD4+ Tregs among CD4+ Tregs were higher in patients with SCOPD than in the HC group, but not in the HS group ( and ). We also observed that the frequencies of CXCR3+ T cells and CXCR3+ Tregs were significantly higher in the AECOPD group than in the other three groups ( and ). We did not observe a significant correlation between the frequency of ICOS+ T cells and CXCR3+ T cells (data not shown). However, we detected a higher expression level of CXCR3 on ICOS+ T cells than on ICOS− T cells in the peripheral blood of patients with SCOPD ().
Figure 4 Inducible costimulator (ICOS) signaling induces C-X-C motif chemokine receptor 3 (CXCR3) expression in vivo and in vitro. (A) Representative flow cytometric dot plots of the frequencies of CXCR3+CD4+ T cells and CXCR3+CD4+CD25+FOXP3+ Tregs within patients with stable chronic obstructive pulmonary disease (SCOPD). Comparisons of the percentage of CXCR3+ T cells within CD4+ T cells (B) and CD4+CD25+FOXP3+ Tregs (C) in the peripheral blood from 15 healthy controls (HC), 11 healthy smokers (HS), 22 patients with SCOPD (SCOPD), and 14 patients with acute exacerbation of COPD (AECOPD). (D) Representative gating of CXCR3+ICOS+CD4+ T cells and CXCR3+ICOS−CD4+ T cells and the corresponding histograms (n = 22). (E) Naïve CD4+ T cells obtained from the peripheral blood of healthy people were cultured with plate-bound anti-CD3/28 mAbs in the presence of IL-2 with or without anti-ICOS mAb (ISA-3) for four days. The frequency of CXCR3+CD4+ T cells was analyzed with flow cytometry. Comparable results were obtained from six independent experiments. (F) The percentage of CXCR3+CD4+ T cells in the peripheral blood and corresponding BALF of four patients with SCOPD. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
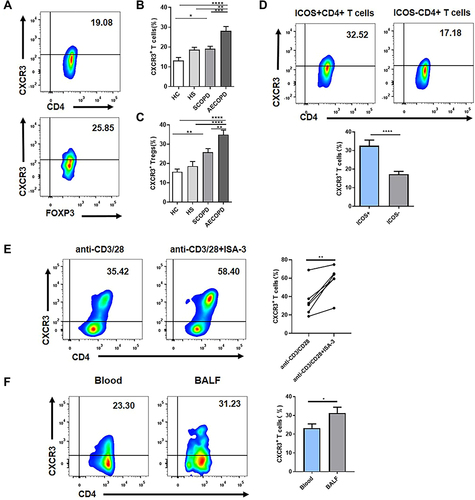
ICOS Activation Could Induce CXCR3 Expression in vitro
To determine whether ICOS plays a role in CXCR3 expression, we examined the relationship between ICOS and CXCR3 in vitro. Naïve CD4+ T cells were cultured with plate-bound anti-CD3/28 mAbs in the presence of IL-2 for four days. Anti-ICOS mAb was added to activate ICOS signaling in T cells. An increased frequency of CXCR3+ T cells was observed in CD4+ T cells after ICOS engagement ().
Higher Percentage of CXCR3+CD4+ T Cells Exists in the Airways Than in the Peripheral Blood
As the earlier findings were all obtained from peripheral blood, we next evaluated the expression level of CXCR3 in the lungs of patients with SCOPD. As expected, CXCR3+ T cells in BALF obtained from SCOPD patients occupied a higher percentage in CD4+ T cells than in matched peripheral blood (). Collectively, these results indicate that ICOS activation could upregulate CXCR3 expression on T cells, which may result in CXCR3+ Th1 cell accumulation in COPD lungs.
Discussion
Microbes and endogenous molecules released after cell damage due to cigarette smoke exposure lead to continuous activation of the immune system in COPD, among which T helper cells and Tregs are two important participators in the abnormal adaptive immune responses.Citation26 Past experience suggested that the holistic role of ICOS is divergent in different disease models, which depends on the interplay of various immune cells in a context-dependent manner.Citation10 Under various circumstances, ICOS can either enhance or decrease Th1 and Th2 immune responses. One reason for this confusing result may be the involvement of ICOS-dependent Treg induction.Citation22,Citation31–33 In this work, we investigated the role of ICOS signaling in COPD for the first time to our knowledge. We observed an increased expression level of ICOS on both CD4+ T cells and CD4+CD25+FOXP3+ Tregs of the peripheral blood from patients with COPD, implying a complex role of ICOS in immune responses. The sharp increase in ICOS expression in patients with AECOPD suggests a relationship between ICOS expression and inflammatory activity, as acute exacerbation in the AECOPD group was mainly triggered by pathogen infection. However, we found only a slight but not significant increase in the percentages of ICOS+CD4+ T cells and ICOS+CD4+ Tregs in the HS group compared to that in the HC group, perhaps limited by the sample size. Furthermore, we observed a correlation between a higher percentage of ICOS+ T cells or ICOS+ Tregs and worse lung function in patients with SCOPD, indicating a poor outcome of COPD with high ICOS expression. However, we did not detect a correlation between the frequency of ICOS+ T cells or ICOS+ Tregs and spirometry parameters in other groups, perhaps because of the small sample size of enrolled people. The expression of ICOS could be affected by the stress of acute inflammation or long-term medication in the AECOPD group, which may result in discrepancies with the spirometry parameters.
To understand the role of ICOS signaling in COPD, we first explored the phenotype of ICOS+CD4+ Tregs. We observed that ICOS+CD4+ Tregs in the peripheral blood of patients with COPD demonstrated a highly proliferative state with upregulated expression of the inhibitory coreceptors PD-1 and TIGIT. These ICOS+CD4+ Tregs were mostly Helios-positive, implying that they were generated by the expansion of naturally occurring Tregs, which is consistent with the results of Vocanson et al, who observed the same phenomenon in a 2,4-dinitrofluorobenzene-sensitized contact hypersensitivity mouse model.Citation34 In addition, the higher FOXP3 expression in ICOS+CD25+ T cells and stronger FOXP3 MFI in ICOS+ Tregs than in ICOS− Tregs were observed, indicating that ICOS favors FOXP3 expression. This observation is in line with the previously published data.Citation35 This crucial role of ICOS in Tregs can be mediated by facilitating the combination of FOXP3 with the nuclear factor of activated T cells that upregulates FOXP3 downstream genes or by promoting the demethylation of Foxp3 conserved noncoding sequence 2.Citation36,Citation37
Numerous studies have suggested a superior suppressive ability of ICOS+ Tregs. Compared to their ICOS− counterparts, ICOS+ Tregs display higher surface expression of CTLA-4 and secrete more IL-10.Citation20,Citation35 CD8+ responder cells show insufficient proliferation when co-cultured with ICOS+CD4+CD25+ cells sorted from tumor-infiltrating lymphocytes of patients with gastric cancer compared to co-culturing with ICOS−CD4+CD25+ cells.Citation38 Moreover, higher expression levels of ICOS on CD4+FOXP3+ tumor-infiltrating lymphocytes have been observed to be associated with tumor progression.Citation38 In addition, ICOS+ Tregs accumulating in the islets could delay the onset of type 1 diabetes in nonobese diabetic mice,Citation39 and low-dose IL-2 administration can reverse type 1 diabetes onset in non-obese diabetic mice by specifically expanding ICOS-positive pancreatic Tregs.Citation40
However, it should be noted that FOXP3 induction relies on TCR signaling, which can be transiently induced when CD4+CD25− T cells are activated under TCR stimulation.Citation41,Citation42 We have also shown dynamic changes in CD25 and FOXP3 in peripheral CD4+ T cells of patients with COPD, whose expression levels are influenced by the inflammatory activity in these patients.Citation43 Therefore, the existence of some FOXP3+ activated T cells within the Tregs identified by CD25 and FOXP3 expression cannot be excluded. In addition, the higher expression of coinhibitory receptors in ICOS+ Tregs can also be a reflection of T cell activation.Citation44 Furthermore, as we have described, ICOS+ Tregs represent a highly proliferative subset. Notably, activated Tregs can be less stable than naïve Tregs in their maintenance of FOXP3, thus favoring ex-Tregs, especially in inflammatory conditions.Citation45,Citation46 Enhanced PI3K signaling mediated by ICOS is one of the contributors to Treg instability.Citation46 In addition, Duhen et al and Vocanson et al successively discovered IFN-γ/IL-17 expression in ICOS+ Tregs in homeostasis and inflammatory settings.Citation24,Citation34 Despite the remaining suppressive function, these IFN-γ/IL-17+ Tregs are considered an intermediate state of Tregs transforming into proinflammatory Th1/Th17 cells under inflammatory conditions in several studies.Citation47,Citation48 Hence, the stability and suppressive function of ICOS+ Tregs in COPD requires further investigation. The positive correlation between the frequency of ICOS+ Tregs and the severity of lung function impairment also led us to hypothesize that the increased percentage of ICOS+ Tregs in COPD could be more than a response to inflammatory T cells; these cells could be proinflammatory Tregs, similar to what Liu et al suggested in systemic lupus erythematosus.Citation49 In other words, these ICOS+ Tregs may have impaired inhibitory ability or are originally activated proinflammatory T cells. This hypothesis should be further verified.
In addition, ICOS plays a well-documented role in T cell activation and effector function,Citation15 thereby promoting pathogen clearance, enhancing anti-tumor efficacy or causing immune damage. To explore whether ICOS signaling has a proinflammatory effect on COPD development, we further examined the phenotype of ICOS+CD4+ T cells in patients with SCOPD. These ICOS+ T cells were mainly TCM and TEM cells and exhibited an exhausted but not a senescent phenotype, as they expressed high levels of exhausted markers, including PD-1 and TIGIT, but senescent T cells exhibit downregulation of CD27 and CD28 and upregulation of CD57 and KLRG-1, contrary to phenotypes observed in ICOS+ T cells.Citation44 The central memory or effector memory phenotype of ICOS+ T cells ensures that they produce a faster and more potent immune response to pathogen invasion.Citation50 Considering the positive correlation between the percentage of ICOS+CD4+ T cells and the severity of lung function impairment in the SCOPD group, the activated ICOS signaling may play a deleterious role in lung tissue inflammation and damage in COPD. Intriguingly, there was inconsistency when dividing TCM and TEM cells based on CD45RA and CD62L expression or CD45RA and CCR7 expression. ICOS+ T cells had a higher proportion of the CR45RA−CCR7−CD62L+ T cell subset. Previous studies also reported heterogeneous expression of CD62L on CD45RA−CCR7− TEM cells, whereas the subtle role of this unique subset is still unknown.Citation51
Finally, we explored the underlying mechanisms of ICOS signaling in COPD. We observed that CXCR3 expression was increased on both CD4+ T cells and CD4+ Tregs in COPD compared to that in the HC group, which was consistent with the expression pattern of ICOS. Furthermore, we observed higher levels of CXCR3 on ICOS+ T cells than on ICOS− T cells in patients with SCOPD and increased expression of CXCR3 on CD4+ T cells in the presence of the anti-ICOS mAb during the induction and differentiation of naïve T cells in vitro, indicating that ICOS could favor CXCR3 expression.
CXCR3 has been shown to play an indispensable role in the pathogenesis of COPD.Citation52 Increased percentages of CXCR3+CD3+ T cells and/or CXCR3+CD8+ T cells have been observed in the peripheral blood and BALF of patients with COPD compared to those in healthy non-smokers and smokers.Citation53,Citation54 However, other researchers found no difference in the proportion of CXCR3+CD4+ T cells or CXCR3+CD8+T cells in the peripheral blood between patients with COPD and smokers or non-smokers,Citation55 which is inconsistent with our results. This difference may be caused by several reasons, such as different subject selection criteria. For example, we included first-visit SCOPD patients who had not received any drug therapy, such as bronchodilators or inhaled corticosteroids, which may have decreased CXCR3 expression.Citation56 No difference in the proportion of CXCR3+CD4+ T cells between HC and HS group was found by us or other researchers. Furthermore, a significant increase in CXCR3+ cells in the bronchiolar epithelium and submucosa was found in smokers with COPD compared to that in healthy non-smokers, with the majority of these cells being CD8+ and IFN-γ-producing.Citation25 Increased numbers of CXCR3+CD8+ T cells in the airways and parenchyma have also been observed in cigarette smoke-exposed rats compared to that in controls.Citation57 Moreover, CXCR3 is highly expressed on CD4+/CD8+ T cells and B cells in lung lymphoid follicles, and its expression level reportedly increases with worsening COPD.Citation58 In addition, the concentrations of the CXCR3 chemokines CXCL9/10/11 have also been observed to be increased in the sputum of patients with COPD, implying a potential role of the CXCL9/10/11-CXCR3 axis in COPD pathogenesis.Citation59 By releasing multiple inflammatory mediators, redundant CXCR3+ T cells in the lung amplify inflammation, leading to the destruction of lung tissues.Citation26
In this study, we observed a significantly higher percentage of CXCR3+CD4+ T cells in BALF than in blood samples of COPD patients. We suggest that the increased expression of CXCR3 result from elevated ICOS signaling. ICOS signaling promotes CXCR3+ Th1 cell differentiation, which may contribute to abnormal Th1 cell infiltration in the lung tissues of patients with COPD, causing tissue inflammation and damage. However, we could not distinguish whether these CXCR3+ T cells came from the periphery or were just derived from the expansion of T cells in local lymphoid. In contrast, other researchers have confirmed that PBMCs from patients with COPD patients have a stronger migratory ability towards CXCR3 chemokines than cells from smokers and nonsmokers.Citation55 In addition, the ICOS effect on chemotactic responses in COPD should be verified further. A previous study demonstrated that ICOS could downregulate CCR7 and CD62L in CD4+ T cells, thereby reducing recirculation of T cells to the lymph nodes and promoting the migration of these activated T cells to the lungs.Citation60 This study indicates a potential chemotactic ability of ICOS+ T cells from a different perspective. Moreover, we observed increased expression of other chemokine receptors, such as CCR4 and CXCR5, in ISA-3-treated CD4+ T cells (data not show). The chemotactic effect of ICOS on CD4+ T cells in COPD may not be restricted to the recruitment of CXCR3+ Th1 cells to the lungs.
Our study has several limitations. Due to the inability to obtain sufficient blood from patients with COPD, we could not identify the actual suppressive function of ICOS± Tregs through co-culture experiments. The function of ICOS+CD4+ T cells and ICOS−CD4+ T cells can be evaluated in more detail by proinflammatory cytokine secretion and chemotactic responses. Moreover, further work should be performed to verify the effect of overall ICOS signaling on the development of COPD in experimental animal models. In addition, several anti-ICOS/ICOSL antibodies have been under clinical trials, some of which have been verified to be therapeutic for several types of tumors.Citation11 These antibodies were also preliminarily tested in several autoimmune diseases, such as systemic lupus erythematosus and active lupus arthritis.Citation61,Citation62 Therefore, regulating ICOS signaling could be a possible way to adjust the unbalanced immune environment and ameliorate immunopathological injury caused by accumulated CXCR3+ Tc1/Th1 cells in COPD. Whether these monoclonal antibodies could benefit patients with COPD remains to be examined, and further efforts should be made to verify this hypothesis.
Conclusions
In this study, we observed upregulated ICOS signaling in COPD, which was correlated with the severity of lung function impairment in patients with SCOPD. In vivo and in vitro experiments showed that ICOS signaling induces CXCR3+ Th1 cell differentiation, which may promote Th1 cell aggregation in COPD lungs and cause excess inflammation and tissue damage.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors thank Pang-Pang Chang, Yi-Ting Xu, Wen Yang, and Zhen Yu for their technical assistance. We also thank all patients and volunteers for their contributions to this research. Dan-Yang Li and Long Chen contributed equally to this work and share first authorship.
Additional information
Funding
References
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49(3):1700214. doi:10.1183/13993003.00214-2017
- GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585–596. doi:10.1016/S2213-2600(20)30105-3
- Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389(10082):1931–1940. doi:10.1016/S0140-6736(17)31222-9
- Caramori G, Ruggeri P, Di Stefano A, et al. Autoimmunity and COPD: clinical implications. Chest. 2018;153(6):1424–1431. doi:10.1016/j.chest.2017.10.033
- Zhang MQ, Wan Y, Jin Y, et al. Cigarette smoking promotes inflammation in patients with COPD by affecting the polarization and survival of Th/Tregs through up-regulation of muscarinic receptor 3 and 5 expression. PLoS One. 2014;9(11):e112350. doi:10.1371/journal.pone.0112350
- Grumelli S, Corry DB, Song LZ, et al. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med. 2004;1(1):e8. doi:10.1371/journal.pmed.0010008
- Di Stefano A, Caramori G, Gnemmi I, et al. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol. 2009;157(2):316–324. doi:10.1111/j.1365-2249.2009.03965.x
- Hou J, Sun Y, Hao Y, et al. Imbalance between subpopulations of regulatory T cells in COPD. Thorax. 2013;68(12):1131–1139. doi:10.1136/thoraxjnl-2012-201956
- Caramori G, Casolari P, Barczyk A, Durham AL, Di Stefano A, Adcock I. COPD immunopathology. Semin Immunopathol. 2016;38(4):497–515. doi:10.1007/s00281-016-0561-5
- Wikenheiser DJ, Stumhofer JS. ICOS co-stimulation: friend or foe? Front Immunol. 2016;7:304. doi:10.3389/fimmu.2016.00304
- Amatore F, Gorvel L, Olive D. Role of Inducible Co-Stimulator (ICOS) in cancer immunotherapy. Expert Opin Biol Ther. 2020;20(2):141–150. doi:10.1080/14712598.2020.1693540
- Ahearne MJ, Allchin RL, Fox CP, Wagner SD. Follicular helper T-cells: expanding roles in T-cell lymphoma and targets for treatment. Br J Haematol. 2014;166(3):326–335. doi:10.1111/bjh.12941
- Hutloff A, Dittrich AM, Beier KC, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397(6716):263–266. doi:10.1038/16717
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23(1):515–548. doi:10.1146/annurev.immunol.23.021704.115611
- Simpson TR, Quezada SA, Allison JP. Regulation of CD4 T cell activation and effector function by inducible costimulator (ICOS). Curr Opin Immunol. 2010;22(3):326–332. doi:10.1016/j.coi.2010.01.001
- Peng C, Huggins MA, Wanhainen KM, et al. Engagement of the costimulatory molecule ICOS in tissues promotes establishment of CD8(+) tissue-resident memory T cells. Immunity. 2022;55(1):98–114.e115. doi:10.1016/j.immuni.2021.11.017
- Panneton V, Chang J, Witalis M, Li J, Suh WK. Inducible T-cell co-stimulator: signaling mechanisms in T follicular helper cells and beyond. Immunol Rev. 2019;291(1):91–103. doi:10.1111/imr.12771
- Maazi H, Patel N, Sankaranarayanan I, et al. ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity. 2015;42(3):538–551. doi:10.1016/j.immuni.2015.02.007
- Kadri N, Korpos E, Gupta S, et al. CD4(+) type II NKT cells mediate ICOS and programmed death-1-dependent regulation of type 1 diabetes. J Immunol. 2012;188(7):3138–3149. doi:10.4049/jimmunol.1101390
- Ito T, Hanabuchi S, Wang YH, et al. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28(6):870–880. doi:10.1016/j.immuni.2008.03.018
- Fonseca VR, Ribeiro F, Graca L. T follicular regulatory (Tfr) cells: dissecting the complexity of Tfr-cell compartments. Immunol Rev. 2019;288(1):112–127. doi:10.1111/imr.12739
- Vidric M, Bladt AT, Dianzani U, Watts TH. Role for inducible costimulator in control of Salmonella enterica serovar Typhimurium infection in mice. Infect Immun. 2006;74(2):1050–1061. doi:10.1128/IAI.74.2.1050-1061.2006
- Nouailles G, Day TA, Kuhlmann S, et al. Impact of inducible co-stimulatory molecule (ICOS) on T-cell responses and protection against Mycobacterium tuberculosis infection. Eur J Immunol. 2011;41(4):981–991. doi:10.1002/eji.201040608
- Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood. 2012;119(19):4430–4440. doi:10.1182/blood-2011-11-392324
- Saetta M, Mariani M, Panina-Bordignon P, et al. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165(10):1404–1409. doi:10.1164/rccm.2107139
- Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011;378(9795):1015–1026. doi:10.1016/S0140-6736(11)60988-4
- Chen H, Fu T, Suh WK, et al. CD4 T cells require ICOS-mediated PI3K signaling to increase T-Bet expression in the setting of anti-CTLA-4 therapy. Cancer Immunol Res. 2014;2(2):167–176. doi:10.1158/2326-6066.CIR-13-0155
- Lord GM, Rao RM, Choe H, et al. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood. 2005;106(10):3432–3439. doi:10.1182/blood-2005-04-1393
- Beima KM, Miazgowicz MM, Lewis MD, Yan PS, Huang TH, Weinmann AS. T-bet binding to newly identified target gene promoters is cell type-independent but results in variable context-dependent functional effects. J Biol Chem. 2006;281(17):11992–12000. doi:10.1074/jbc.M513613200
- Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10(6):595–602. doi:10.1038/ni.1731
- Wikenheiser DJ, Ghosh D, Kennedy B, Stumhofer JS. The costimulatory molecule ICOS regulates host Th1 and follicular Th cell differentiation in response to Plasmodium chabaudi chabaudi AS infection. J Immunol. 2016;196(2):778–791. doi:10.4049/jimmunol.1403206
- Clay BS, Shilling RA, Bandukwala HS, et al. Inducible costimulator expression regulates the magnitude of Th2-mediated airway inflammation by regulating the number of Th2 cells. PLoS One. 2009;4(11):e7525. doi:10.1371/journal.pone.0007525
- Redpath SA, van der Werf N, Cervera AM, et al. ICOS controls Foxp3(+) regulatory T-cell expansion, maintenance and IL-10 production during helminth infection. Eur J Immunol. 2013;43(3):705–715. doi:10.1002/eji.201242794
- Vocanson M, Rozieres A, Hennino A, et al. Inducible costimulator (ICOS) is a marker for highly suppressive antigen-specific T cells sharing features of TH17/TH1 and regulatory T cells. J Allergy Clin Immunol. 2010;126(2):280–289, 289 e281–287. doi:10.1016/j.jaci.2010.05.022
- Zheng J, Chan PL, Liu Y, et al. ICOS regulates the generation and function of human CD4+ Treg in a CTLA-4 dependent manner. PLoS One. 2013;8(12):e82203. doi:10.1371/journal.pone.0082203
- Chen Q, Mo L, Cai X, et al. ICOS signal facilitates Foxp3 transcription to favor suppressive function of regulatory T cells. Int J Med Sci. 2018;15(7):666–673. doi:10.7150/ijms.23940
- Landuyt AE, Klocke BJ, Colvin TB, Schoeb TR, Maynard CL. Cutting edge: ICOS-deficient Regulatory T cells display normal induction of Il10 but readily downregulate expression of Foxp3. J Immunol. 2019;202(4):1039–1044. doi:10.4049/jimmunol.1801266
- Nagase H, Takeoka T, Urakawa S, et al. ICOS(+) Foxp3(+) TILs in gastric cancer are prognostic markers and effector regulatory T cells associated with Helicobacter pylori. Int J Cancer. 2017;140(3):686–695. doi:10.1002/ijc.30475
- Kornete M, Sgouroudis E, Piccirillo CA. ICOS-dependent homeostasis and function of Foxp3+ regulatory T cells in islets of nonobese diabetic mice. J Immunol. 2012;188(3):1064–1074. doi:10.4049/jimmunol.1101303
- Grinberg-Bleyer Y, Baeyens A, You S, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med. 2010;207(9):1871–1878. doi:10.1084/jem.20100209
- Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37(1):129–138. doi:10.1002/eji.200636435
- Ziegler SF. FOXP3: not just for regulatory T cells anymore. Eur J Immunol. 2007;37(1):21-23. doi:10.1002/eji.200636929
- Meng ZJ, Wu JH, Zhou M, et al. Peripheral blood CD4+ T cell populations by CD25 and Foxp3 expression as a potential biomarker: reflecting inflammatory activity in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2019;14:1669–1680. doi:10.2147/COPD.S208977
- ElTanbouly MA, Noelle RJ. Rethinking peripheral T cell tolerance: checkpoints across a T cell’s journey. Nat Rev Immunol. 2021;21(4):257–267. doi:10.1038/s41577-020-00454-2
- Zhou X, Bailey-Bucktrout SL, Jeker LT, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10(9):1000–1007. doi:10.1038/ni.1774
- Zhang Z, Zhang W, Guo J, Gu Q, Zhu X, Zhou X. Activation and functional specialization of Regulatory T cells lead to the generation of Foxp3 instability. J Immunol. 2017;198(7):2612–2625. doi:10.4049/jimmunol.1601409
- Feng T, Cao AT, Weaver CT, Elson CO, Cong Y. Interleukin-12 converts Foxp3+ regulatory T cells to interferon-γ-producing Foxp3+ T cells that inhibit colitis. Gastroenterology. 2011;140(7):2031–2043. doi:10.1053/j.gastro.2011.03.009
- Bovenschen HJ, van de Kerkhof PC, van Erp PE, Woestenenk R, Joosten I, Koenen HJ. Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin. J Invest Dermatol. 2011;131(9):1853–1860. doi:10.1038/jid.2011.139
- Liu Y, Zhu T, Cai G, et al. Elevated circulating CD4+ ICOS+ Foxp3+ T cells contribute to overproduction of IL-10 and are correlated with disease severity in patients with systemic lupus erythematosus. Lupus. 2011;20(6):620–627. doi:10.1177/0961203310392431
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi:10.1146/annurev.immunol.22.012703.104702
- van den Broek T, Borghans JAM, van Wijk F. The full spectrum of human naive T cells. Nat Rev Immunol. 2018;18(6):363–373. doi:10.1038/s41577-018-0001-y
- Li L, Liu Y, Chiu C, et al. A regulatory role of chemokine receptor CXCR3 in the pathogenesis of chronic obstructive pulmonary disease and emphysema. Inflammation. 2021;44(3):985–998. doi:10.1007/s10753-020-01393-9
- Brozyna S, Ahern J, Hodge G, et al. Chemotactic mediators of Th1 T-cell trafficking in smokers and COPD patients. COPD. 2009;6(1):4–16. doi:10.1080/15412550902724164
- Smyth LJ, Starkey C, Gordon FS, Vestbo J, Singh D. CD8 chemokine receptors in chronic obstructive pulmonary disease. Clin Exp Immunol. 2008;154(1):56–63. doi:10.1111/j.1365-2249.2008.03729.x
- Costa C, Traves SL, Tudhope SJ, et al. Enhanced monocyte migration to CXCR3 and CCR5 chemokines in COPD. Eur Respir J. 2016;47(4):1093–1102. doi:10.1183/13993003.01642-2015
- Wang W, Yang X, Luo L, et al. The effects of glucocorticoids and theophylline on CXCR3 expression of peripheral blood T cell in COPD patients (in Chinese). Chinese J Geriatr. 2016;35(11):1196–1200. doi:10.3760/cma.j.issn.0254-9026.2016.11.014
- Li Q, Sun J, Cao Y, et al. Bu-Shen-Fang-Chuan formula attenuates T-lymphocytes recruitment in the lung of rats with COPD through suppressing CXCL9/CXCL10/CXCL11-CXCR3 axis. Biomed Pharmacother. 2020;123:109735. doi:10.1016/j.biopha.2019.109735
- Kelsen SG, Aksoy MO, Georgy M, et al. Lymphoid follicle cells in chronic obstructive pulmonary disease overexpress the chemokine receptor CXCR3. Am J Respir Crit Care Med. 2009;179(9):799–805. doi:10.1164/rccm.200807-1089OC
- Costa C, Rufino R, Traves SL, Lapa ESJR, Barnes PJ, Donnelly LE. CXCR3 and CCR5 chemokines in induced sputum from patients with COPD. Chest. 2008;133(1):26–33. doi:10.1378/chest.07-0393
- Moore TV, Clay BS, Cannon JL, Histed A, Shilling RA, Sperling AI. Inducible costimulator controls migration of T cells to the lungs via down-regulation of CCR7 and CD62L. Am J Respir Cell Mol Biol. 2011;45(4):843–850. doi:10.1165/rcmb.2010-0466OC
- Cheng LE, Amoura Z, Cheah B, et al. Brief report: a randomized, double-blind, parallel-group, placebo-controlled, multiple-dose study to evaluate AMG 557 in patients with systemic lupus erythematosus and active lupus arthritis. Arthritis Rheumatol. 2018;70(7):1071–1076. doi:10.1002/art.40479
- Sullivan BA, Tsuji W, Kivitz A, et al. Inducible T-cell co-stimulator ligand (ICOSL) blockade leads to selective inhibition of anti-KLH IgG responses in subjects with systemic lupus erythematosus. Lupus Sci Med. 2016;3(1):e000146. doi:10.1136/lupus-2016-000146
