Abstract
Objective
Chronic obstructive pulmonary disease (COPD) represents a burden on patients and health systems. Roflumilast, an oral, selective phosphodiesterase-4-inhibitor reduces exacerbations and improves lung function in severe/very severe COPD patients with a history of exacerbations. This study aimed to estimate the lifetime cost and outcomes of roflumilast added-on to commonly used COPD regimens in Switzerland.
Methods
A Markov cohort model was developed to simulate COPD progression in patients with disease states of severe, very severe COPD, and death. The exacerbation rate was assumed to be two per year in severe COPD. COPD progression rates were drawn from the published literature. Efficacy was expressed as relative ratios of exacerbation rates associated with roflumilast, derived from a mixed-treatment comparison. A cost-effectiveness analysis was conducted for roflumilast added to long-acting muscarinic antagonists (LAMA), long-acting β2-agonist/ inhaled corticosteroids (LABA/ICS), and LAMA + LABA/ICS. The analysis was conducted from the Swiss payer perspective, with costs and outcomes discounted at 2.5% annually. Parameter uncertainties were explored in one-way and probabilistic sensitivity analyses.
Results
In each of the comparator regimens mean life expectancy was 9.28 years and quality-adjusted life years (QALYs) gained were 6.19. Mean estimated lifetime costs per patient in the comparator arms were CHF 83,364 (LAMA), CHF 88,161 (LABA/ICS), and CHF 95,564 (LAMA + LABA/ICS) respectively. Adding roflumilast resulted in a mean cost per patient per lifetime of CHF 86,754 (LAMA + roflumilast), CHF 91,470 (LABA/ICS + roflumilast), and CHF 99,364 (LAMA + LABA/ICS + roflumilast), respectively. Life-expectancy and quality-adjusted life-expectancy were 9.63 years and 6.47 QALYs (LAMA + roflumilast), 9.64 years and 6.48 QALYs (LABA/ICS + roflumilast), and 9.63 years and 6.47 QALYs (LAMA + LABA/ ICS + roflumilast). Incremental cost-effectiveness ratios were CHF 12,313, CHF 11,456, and CHF 13,671 per QALY when roflumilast was added to the three regimens.
Conclusion
Treatment with roflumilast is estimated to reduce the health and economic burden of COPD exacerbations and represent a cost-effective treatment option for patients with frequent exacerbations in Switzerland.
Background and objective
Chronic obstructive pulmonary disease (COPD)
COPD is a chronic disease characterized by airflow limitation that is only partially reversible and progresses over time.Citation1 It is a major cause of disability and death worldwide. An international study, The Burden of Obstructive Lung Disease Program (BOLD) showed “higher levels and more advanced staging of spirometrically confrmed COPD than have typically been reported.”Citation2 The same study also showed a fairly high prevalence of stage II or more COPD in individuals who have never smoked.
The overall prevalence of COPD for Global Initiative for Chronic Obstructive Lung Disease (GOLD)Citation3 stage II or higher reported in this study was 10.1% in adults aged 40 years and older. Symptoms including activity limitation and exacerbations place a heavy burden on patients and impair their quality of life. Spirometry is currently used as the main method to confrm the diagnosis of COPD and to ascertain its severity by measuring post bronchodilator forced expiratory volume in 1 second (FEV1). COPD exacerbations represent a major concern as they are linked with disease severity as well as disease progression and therefore represent a substantial burden on health care systems.Citation4 The objective of COPD management, therefore, is to reduce the frequency of exacerbations and to limit disease progression. The importance of not only treating symptoms but of specifically reducing the risk of exacerbations has been recently emphasized in the 2011 update of the GOLD document, Global Strategy for the Diagnosis, Management, and Prevention of COPD.Citation3
Treatment regimens
Pharmacological management of COPD includes a stepwise escalation in treatment according to disease severity and symptoms. In Switzerland common therapy regimens include long-acting muscarinic antagonists (LAMA), and long-acting β2-agonist/inhaled corticosteroids (LABA/ICS) and LAMA + LABA/ICS administered concomitantly. None of the treatments for COPD have been shown conclusively to slow (or reverse) disease progression (ie, improve the long-term decline in lung function). Also until now, no therapy has been shown to treat the underlying inflammation found in COPD.
Roflumilast
Roflumilast is an oral, once-daily selective phosphodi-esterase-4 inhibitor with a broad range of action against inflammatory cells playing a major role in COPD. It was approved for Switzerland in November 2011 for use as concomitant maintenance treatment of severe COPD as a supplementary therapy to bronchodilators in patients with frequent exacerbations in the past.
As demonstrated in clinical trials,Citation5,Citation6 roflumilast reduced the rate of exacerbations in patients with severe airflow obstruction and a history of frequent exacerbations, whose COPD was associated with chronic bronchitis. Daxas® ([roflumilast] Takeda Pharma AG, Pfäffikon, Switzerland) was included in the Swiss positive list for reimbursement in February 2012.Citation7 The objective of this analysis is to estimate the lifetime cost and outcomes and the cost-effectiveness of roflumilast as a supplementary therapy to LAMA, LABA/ ICS, and LAMA + LABA/ICS from a health care payer perspective in Switzerland.
Materials and methods
Economic model
A state-transition, cohort-based Markov model was constructed to estimate the cost and health outcomes in a cohort of patients with COPD. The key features of the model were similar to that reported in a study published earlierCitation8 and included: (1) a lifetime simulation of the progression of COPD based on FEV1; (2) simulation of direct health care costs and health outcomes; (3) treatment effect represented by a reduction of exacerbations; and (4) modeling the impact of moderate (community-treated) and severe (hospital-treated) exacerbations on patients’ health-related quality of life, and the consequent reduction of in-hospital mortality due to severe exacerbations. In addition, the model has the ability to simulate lung function improvement, however in the base case no impact of treatment on lung function was assumed.
The structure of the Markov cohort model included three states: severe COPD (FEV1 30%–49%), very severe COPD (FEV1 30%) according to the classification of COPD severity by GOLD criteria,Citation3 and death (). In a recent revision of the GOLD guidelines, frequent exacerbations were added as a determinant of severity of disease. In this analysis, the rate of exacerbations in the comparator patient groups was assumed to be two exacerbations annually, reflecting the definition in the revised GOLD guidelines.
Figure 1 COPD cost-effectiveness model structure.
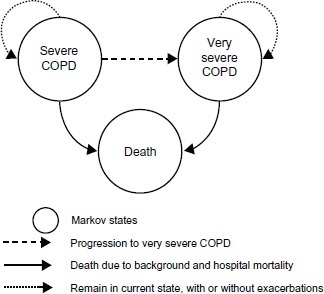
The framework of the analysis and the economic model are based on the best practice accepted in the COPD therapy areas, and results of the economic analysis are also in line with the economic evaluation for roflumilast reported earlier.Citation8
Patient cohort
The modeled cohort was characterized by a mean age at the start of the simulation, COPD severity (mean post-bronchodilator FEV1), proportion of males, patient height, and the combined rates of moderate and severe exacerbations in patients on comparator regimens (). Proportions of severe exacerbations in patients with severe and very severe COPD were based on the LABA subgroup in studies M2-124 and M2-125;Citation5,Citation6 the hospital case fatality rate due to severe exacerbations was drawn from a UK COPD audit report,Citation9 and was assumed to be similar in Switzerland.
Table 1 Demographic characteristic and outcomes in COPD cohort
COPD progression
Progression of patients with severe COPD to very severe COPD depends on the starting FEV1 value and the estimated annual lung volume decline in COPD patients. FEV1 naturally declines with age in the general healthy population,Citation10 but it declines more rapidly in patients with COPD (estimated at 52 mL a year).Citation11 Predictive equations for lung function for males and females in a general healthy population were taken from Crapo et al.Citation10 This allowed the estimation of the average time, T, required for lung function in the COPD cohort to reach the FEV1 threshold for very severe COPD (30% of post-bronchodilator FEV1 in a healthy population, according to the GOLD classification).Citation4 Subsequently, the monthly probability of progression from severe to very severe COPD was estimated as the reciprocal of the average time to progression, 1/T.
Exacerbations
Exacerbations in the model are either community-treated (moderate) or hospital-treated (severe); each is associated with costs and health consequences estimated as quality-adjusted life years (QALYs) lost per exacerbation event. The annual rates of exacerbations in each of the comparator treatments (ie, LAMA, LABA/ICS, and LAMA + LABA/ICS) were assumed to be equal to two in the severe COPD state representing patients with frequent exacerbations. Exacerbation rates in very severe COPD were estimated using the ratio of exacerbation frequency in patients with very severe COPD to that of patients with severe COPD obtained from a pooled analysis of data from the M2-124 and M2-125 studies (ie, 1.776/1.458 annually).
Therefore, using an assumption of two exacerbations per year per patient in the severe COPD state, the estimated rate of exacerbations in the comparator arms in the very severe COPD state would be 2.44 per year per patient.
A fixed proportion of all exacerbations in patients with severe and very severe COPD are hospital-treated, and the proportions applied in the model are drawn from the pooled analysis of the LABA subgroup in M2-124 and M2-125: 0.16 [95% confidence interval (CI): 0.133–0.178] in the severe COPD state, and 0.24 (95% CI: 0.208–0.280) in the very severe COPD state ().
Efficacy of roflumilast
The treatment effect of roflumilast in the analysis is determined using the relative ratios of exacerbation rates (RRRs). The RRRs for treatment regimens with roflumilast as an add-on, presented in , were calculated based on the results of a mixed treatment comparison (MTC) study,Citation12 and used in a similar manner to a recently-published analysis that conducted a fully incremental economic analysis comparing various treatment regimens used in the management of COPD in the UK.Citation8 Efficacy in terms of lung function improvement was not reported in the MTC, as the primary outcome of the study was event rates of exacerbations.
Table 2 Treatment efficacy – reduction of exacerbations
Mortality
Mortality in the modeled COPD cohort occurs both as a result of case fatality during a hospital-treated exacerbation and as background mortality.
Hospital mortality
The hospital case fatality rate due to a hospital-treated exacerbation was taken from the 2008 UK National COPD AuditCitation9 where hospital mortality was reported at 7.7% for a sample of 9716 admissions for exacerbations.
Background mortality and standardized mortality ratios (SMRs)
The background mortality is expressed via the age- and sex-specific risk of death in the general population and the SMR for background mortality that excludes hospital death, in the severe COPD and very severe COPD states. The background mortality and the hospital mortality combined represent the true all-cause mortality in severe and very severe COPD. The SMRs for COPD populations are not well known, and in the model the SMRs for severe and very severe COPD are assumed to be 1.5 and 2.0 respectively.Citation13 As the SMRs are uncertain parameters, their impact on the cost-effectiveness of roflumilast was tested in a sensitivity analysis.
Health-related utilities
Two types of health-related utility measurement are employed in the model (). The severe COPD and very severe COPD states are associated with the health-related utility values of 0.751 (95% CI:0.738–0.765) and 0.657 (95% CI:0.635–0.678), respectively.Citation5 The loss of utility due to hospital-treated exacerbations and community-treated exacerbations translates into a loss in QALYs. The loss of QALYs associated with an exacerbation was estimated based on the absolute reduction of utility of 0.01 (standard error [SE] = 0.007) and 0.042 (SE = 0.009) over a 1-year period for a community-treated exacerbation and a hospital-treated exacerbation respectively, as reported in Rutten-van Mölken et al.Citation14 In the model these values were applied to accrue the loss of QALYs within one model cycle when an exacerbation occurs, ie, monthly health utility reductions of 0.12 and 0.50 for moderate and severe exacerbations, respectively (see notes to ).
Table 3 Health-related utilities and utility decrements
Adverse events
The key roflumilast studies M2-124 and M2-125Citation5,Citation6 suggest that the majority of adverse events (AEs) in patients treated with roflumilast were mild-to-moderate, transient, occurred within the first month from the beginning of treatment, and would not have substantial impact on cost-effectiveness. For the treatment combinations used in this economic model there is no comparable data on AEs and no consideration of AEs were made.
Resource use and costs
Costs were estimated from a payer perspective in Switzerland. Total costs in the analysis included the on-going maintenance cost in severe and very severe COPD, the cost of COPD regimens, and the resource use estimates and cost of treatment of moderate and severe exacerbations, as shown in .
Table 4 Key model inputs: resource use and unit costs
Results
Base case
Total costs, life years, and QALYs were estimated over a lifetime horizon. shows the results for all three treatment comparator pairs. The results show similar patterns for each treatment comparator pair whereby the addition of roflumilast to each of the treatments results in a total lifetime incremental cost of CHF 3390, CHF 3308, and CHF 3799 and a QALY gain of 0.275, 0.289, and 0.278 in LAMA + roflumilast versus LAMA, LABA/ICS + roflumilast versus LABA/ICS, and LAMA + LABA/ICS + roflumilast versus LABA/ICS, respectively.
Table 5 Cost-effectiveness results and cost breakdown, base case
The additional costs and additional health gain result in incremental cost-effectiveness ratios (ICERs) of CHF 12,313, CHF 11,456, and CHF 13,671 per QALY gained (). The cost breakdown in suggests that the additional cost in the regimens with roflumilast added-on come from the cost of maintenance (which includes the cost of COPD drugs as well as the cost of ongoing maintenance), and are equal to CHF 10,376, CHF 10,638, and CHF 10,851, which is explained by roflumilast add-on therapy. These incremental costs are partly offset by cost savings due to the reduction of moderate exacerbations and most importantly severe exacerbations (see notes to ).
Sensitivity analysis
One-way sensitivity analysis and probabilistic sensitivity analysis were performed to identify the key determinants of cost-effectiveness in the Swiss setting and to assess the robustness of the model through testing the combined impact of all uncertainties in model input parameters on cost-effectiveness.
One-way sensitivity analysis
One-way sensitivity analyses for each treatment and comparator pairs are presented in (LABA/ICS + roflumilast vs LABA/ICS), (LAMA + LABA/ICS + rofumilast vs LAMA + LABA/ICS), and (LAMA + roflumilast vs LAMA).
Table 6 One-way sensitivity analysis: LABA/ICS + roflumilast vs LABA/ICS
Table 7 One-way sensitivity analysis: LAMA + LABA/ICS + roflumilast vs LAMA + LABA/ICS
Table 8 One-way sensitivity analysis: LAMA + roflumilast vs LAMA
The low and high estimates for the parameters used in the one-way sensitivity analysis were based where possible on the respective 95% CIs. The parameters with the largest impact on the ICER are treatment efficacy (RRR) and hospital case fatality rate. At the low estimates of each of these parameters roflumilast appears either dominant or highly cost-effective. On the other hand at the upper limits of the 95% CI for RRR and for hospital case fatality rate, the values of the ICERs increase. The cost of a hospital-treated exacerbation, background exacerbation rates in the comparator regimens, and the proportion of hospital treated exacerbations have a high impact on the ICER. On the other hand parameters such as the cost of underlying/background COPD therapies, or SMRs have a low impact on the ICER (–). The high impact on the ICER of discount rates on the cost and outcomes and the impact of the analysis time horizon, result from the survival benefit associated with the reduction of hospital exacerbations, and the subsequent decrease in hospital mortality.
Probabilistic sensitivity analysis
In a probabilistic sensitivity analysis relevant distributions were applied to all input parameters, based where pos-sible on their estimated 95% CIs. Although in Switzerland there is no established willingness-to-pay (WTP) threshold the commonly quoted WTP value is €60,000 per QALY (approximately CHF 72,000 at the current exchange rate).15,16 These also broadly correspond to the cost-effectiveness criteria recommended by the World Health Organization (1–3 × gross domestic product per capita).17 The probabilistic sensitivity analysis suggested that the likelihood of rofumilast being cost-effective for each of the three treatment-comparator pairs is 79%, 96%, and 96% at the WTP of CHF 70,000 per QALY when roflumilast is added to LAMA, LABA/ICS, or LAMA + LABA/ICS, respectively.
Discussion
The value of roflumilast as a supplementary regimen to the commonly used COPD treatments comes from the reduction of exacerbation rates, thus reducing the burden of exacerbations on COPD patients and health care providers. The additional cost of the treatment regimens with rofumilast is partly offset by the reduction of exacerbations. The economic effect of the reduction of exacerbation is particularly prominent in patients who experience frequent exacerbations despite current treatments and are mainly represented by patients with severe and very severe COPD. This assumption in the analysis is particularly relevant in light of the recent revision of the GOLD guidelines for the prevention, management, and treatment of COPD.Citation3
The limitations of the analysis mainly relate to (1) the uncertainty in the published MTC data on the efficacy of roflumilast, and (2) the lack of published data for the Swiss setting on such parameters as hospital case fatality rates. The uncertainty of the efficacy inputs result from the absence of studies directly comparing health outcomes for the treatment and comparators of interest and the need to use a mixed treatment comparison study to derive the required parameters. In the model it was also assumed that the reduction in the rate of exacerbations occurs over the entire duration of the simulation and that the reduction of all exacerbation rates results in a proportional reduction of severe (hospital-treated) exacerbations and subsequently, reduction of the hospital case fatality rate. In the absence of published data in Switzerland on hospital mortality due to severe COPD exacerbations, we used the results of a UK COPD audit.Citation9 As all of the above limitations of the study are related to the lack of, or uncertainty of input parameters, further country-specific research is required to narrow the uncertainties in future analyses.
Observational studies (ie, real-world evidence) would be a valuable source of such data. Despite the above limitations, this analysis represents a robust estimate of the cost- effectiveness of roflumilast in the Swiss setting using the best available evidence.
The most recently updated GOLD guidelinesCitation3 describe the importance of the frequency and severity of symptoms and exacerbations in the diagnosis and progression of disease severity. In the management of stable COPD, the goals for treatment include the reduction of symptoms (symptom relief, improvement in health status, and exercise tolerance) and the reduction of risk, through the prevention of disease progression, prevention and treatment of exacerbations, and the consequential reduction in mortality. Roflumilast (Daxas®) was approved for reimbursement in Switzerland in February 2012Citation7 for severe and very severe COPD patients with frequent exacerbations in the past and being pretreated with inadequate bronchodilator therapy. Roflumilast is included in the revised GOLD guidelinesCitation3 in the list of medications commonly used to treat COPD, and is recommended for use in reducing exacerbations for patients with chronic bronchitis, severe and very severe COPD, and with a history of frequent exacerbations.
The cost-effectiveness analysis presented in this paper suggests that roflumilast can be a cost-effective treatment option for COPD patients with frequent exacerbations in Switzerland.
Considering the results of this analysis in the context of the revised GOLD guidelines presents an opportunity for clinicians to decrease the number of exacerbations experienced by COPD patients, in a cost-effective manner. Additionally, decreasing the number of hospitalized exacerbations would also be sensible with respect to the budgetary efficiency of the Swiss health care system.
Conclusion
In Switzerland, treatment with roflumilast in combination with commonly used regimens such as bronchodilators with or without inhaled corticosteroids is estimated to reduce the health and economic burden of COPD exacerbations and represents a cost-effective treatment option for patients with frequent exacerbations.
Acknowledgments and disclosure
This study was sponsored by Takeda Pharma AG, Switzerland. All the authors participated in the study design, data analysis, interpretation of the results, writing and reviewing of the manuscript, and the decision to submit the manuscript for publication. Yevgeniy Samyshkin and Matthew Radford are employees of IMS Health, which was commissioned by Takeda Pharma AG, Switzerland to conduct this study and write the manuscript. Michael Schlunegger, Susan Haefliger, and Sabine Ledderhose are employees of Takeda Pharma AG, Switzerland. The authors report no other conflicts of interest in this work.
References
- ViegiGPistelliFSherrillDLMaioSBaldacciSCarrozziLDefnition, epidemiology and natural history of COPDEur Respir J2007305993101317978157
- BuistASMcBurnieMAVollmerWMInternational variation in the prevalence of COPD (the BOLD Study): a population-based prevalence studyLancet2007370958974175017765523
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease Revised 2011Global Initiative for Chronic Obstructive Lung Disease, Inc2011 Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2011_Feb21.pdfAccessed August 20, 2012
- SapeyEStockleyRACOPD exacerbations. 2: aetiologyThorax200661325025816517585
- CalverleyPMRabeK FGoehringUMRoflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trialsLancet2009374969168569419716960
- BatemanEDRabeKFCalverleyPMRoflumilast with longacting β2-agonists for COPD: infuence of exacerbation historyEur Respir J201138355356021737553
- Bundesamt für GesundheitBulletin 6/12BernBundesamt für Gesundheit BAG2012 Available from: http://www.bag.admin.ch/dokumentation/publikationen/01435/11505/12789/index.html?lang=de&sort=ta&superflex=0_0&filter_dms_thema=10&filter_dms_fx=327&flter_dms_jahre=Accessed November 11, 2012
- HertelNKotchieRWSamyshkinYRadfordMHumphreysSJamesonKCost-effectiveness of available treatment options for patients suffering from severe COPD in the UK: a fully incremental analysisInt J Chron Obstruct Pulmon Dis2012718319922500119
- Royal College of PhysiciansReport of the National Chronic Obstructive Pulmonary Disease Audit 2008: Clinical Audit of COPD Exacerbations Admitted to Acute NHS Units Across the UK 2008LondonRoyal College of Physicians2008 Available from: http://www.rcplondon.ac.uk/sites/default/files/report-of-the-national-copd-audit-2008-clinical-audit-of-copd-exacerbations-admitted-to-acute-nhs-units-across-the-uk.pdfAccessed August 15, 2012
- CrapoROMorrisAHGardnerRMReference spirometric values using techniques and equipment that meet ATS recommendationsAm Rev Respir Dis198112366596647271065
- ScanlonPDConnettJEWallerLAAltoseMDBaileyWCBuistASSmoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health StudyAm J Respir Crit Care Med20001612 Pt 138139010673175
- MillsEJDruytsEGhementIPuhanMAPharmacotherapies for chronic obstructive pulmonary disease: a multiple treatment comparison meta-analysisClin Epidemiol2011310712921487451
- SpencerMBriggsAHGrossmanR FRanceLDevelopment of an economic model to assess the cost effectiveness of treatment interventions for chronic obstructive pulmonary diseasePharmacoeconomics200523661963715960557
- Rutten-van MölkenM PHoogendoornMLamersLMHolistic preferences for 1-year health profiles describing fuctuations in health: the case of chronic obstructive pulmonary diseasePharmacoeconomics200927646547719640010
- Matter-WalstraK WDedesKJSchwenkglenksMBrauchliPSzucsTDPestalozziBCTrastuzumab beyond progression: a cost-utility analysisAnn Oncol201021112161216820444849
- Matter-WalstraKJoergerMKühnelUSzucsTPestalozziBSchwenkglenksMCost-effectiveness of maintenance pemetrexed in patients with advanced nonsquamous-cell lung cancer from the perspective of the Swiss health care systemValue Health2012151657122264973
- WHO. WHO-CHOICE [webpage on the Internet]CHOosing Interventions that are Cost Effective (WHO-CHOICE)GenevaWorld Health Organization2012Available from: http://www.who.int/choice/enAccessed July 2, 2012
- SchrammWHaakeDBrandtADie Wirtschaftlichkeit von Tiotropium bei chronisch obstruktiver Lungenerkrankung [Economic value of tiotropium in the treatment of chronic obstructive pulmonary disease]Praxis (Bern 1994)2005944618031810 German16329401
- OostenbrinkJBRutten-van MölkenMPMonzBUFitzGeraldJMProbabilistic Markov model to assess the cost-effectiveness of bronchodilator therapy in COPD patients in different countriesValue Health200581324615841892
- compendiumch [webpage on the Internet] medicines Compendium of Switzerland®BaselDocumed Ltd2012 Available from: http://www.kompendium.chAccessed July 2, 2012German, French, English.
- TARMED Suisse [homepage on the Internet]BernTARMED Suisse2011 Available from: http://www.tarmedsuisse.ch/Accessed July 2, 2012